Lower ATLG doses significantly accelerate posttransplant immune reconstitution.
The day of transplant can be scheduled according to a target serum level of ATLG following first order kinetics independent from dosing.
Visual Abstract
Anti–T lymphocyte globulin (ATLG) significantly reduces the risk of engraftment failure in allogeneic hematopoietic stem cell transplant (HSCT) but hampers posttransplant immune reconstitution. We hypothesized that in patients receiving haploidentical CD3/CD19-depleted grafts, these double-edged effects could be better balanced by attaining high ATLG serum concentrations before transplant but as low as possible on the day of transplant. Therefore, we moved the start of ATLG application to day −12 and determined serum concentrations of T-cell–specific ATLG in pediatric patients treated with 3 established dosing regimens (15, 30, or 60 mg/kg). Corresponding mean T-cell–specific ATLG serum concentrations at day 0 were 1.14, 2.99, or 12.10 μg/mL, respectively. Higher ATLG doses correlated with higher peak levels at days −8 and −7 and reduced graft rejection, whereas lower ATLG doses correlated with significantly faster posttransplant recovery of T and natural killer cells. The rate of graft-versus-host disease remained low, independent of ATLG doses. Moreover, in vitro assays showed that ATLG concentrations of 2.0 μg/mL and lower only slightly reduced the activity of natural killer cells, and therefore, the function of such effector cells might be preserved in the grafts. Pharmacokinetic analysis, compatible with linear first-order kinetics, revealed similar half-life values, independent of ATLG doses. Hence, the day on which a desired ATLG serum level is reached can be calculated before HSCT. Our retrospective study demonstrates the relevance of dosing and time of administration of ATLG on engraftment and immune recovery in ex vivo CD3/CD19-depleted haploidentical HSCT.
Introduction
Allogeneic hematopoietic stem cell transplant (HSCT) is a well-established procedure with curative potential for a broad spectrum of malignant and nonmalignant hematologic diseases. If HLA-matched sibling or unrelated donors are not available, it is a widely accepted option to use a haploidentical stem cell source.1 However, there are 2 clinically relevant obstacles that have to be overcome particularly in the haploidentical setting: graft-versus-host disease (GVHD) and graft rejection.
To reduce the risk of engraftment failure and of GVHD, polyclonal antibody preparations, directed against T-cell associated and other antigens, are usually added to the conditioning regimen. One of these preparations is rabbit anti–T lymphocyte globulin (ATLG; Grafalon; ATLG Neovii), which consists of antibodies directed against the human leukemic T-cell line Jurkat and reacts against both donor and host lymphocytes.2 Additionally, T-cell depletion (TCD) for GVHD prophylaxis can be accomplished by ex vivo TCRαβ/CD19 or CD3/CD19 depletion techniques.
Graft rejection is supposed to be mainly due to recipient T cells that have survived despite conditioning.3 Using T-cell–depleted grafts, persisting recipient T lymphocytes cannot be eliminated by donor T cells, resulting in host-versus-graft reaction and, thus, in primary graft rejection or secondary graft failure.4 The incidence of rejection can be decreased by either using a “megadose” of purified CD34+ stem cells5 and by intensive conditioning regimens that include serotherapy, such as ATLG.6 However, besides its advantageous effect on graft rejection and, in part, on GVHD, the use of ATLG hampers posttransplant immune recovery and limits potential antileukemic activity of natural killer (NK) and γδ T cells. Consequently, the risk of posttransplant infectious complications and of relapse may be increased.7-10
Here, we set out and investigated whether, by modifying scheduling and dosing of ATLG, posttransplant immune reconstitution can be optimized in patients having received CD3/CD19-depleted haploidentical stem cell grafts without increasing the rate of rejection. ATLG serum levels in patients treated with 3 common doses of ATLG were measured serially, allowing for us to establish a pharmacokinetic (PK) model for ATLG (Grafalon) for this population. In parallel, we tested the impact of ATLG on the viability and function of NK cells in vitro.
Materials and methods
Cytotoxicity assays
NK cells were isolated from healthy donors using column-based magnetic cell sorting by CD56+ microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) after previous CD3+ TCD of PBMCs by CD3+ dynabeads (Life Technologies, CA) according to the manufacturers’ protocols. NK cells were seeded in 96-well round-bottomed microplates at different effector to target ratios (E:T ratio) and incubated for 22 hours with 0, 2, or 1000 μg/mL ATLG. K562 target cells were labeled with BATDA (PerkinElmer, MA) and, after 3 washing steps, cocultured with ATLG-preincubated NK cells for 2 hours. Fluorescence of Europium-TDA chelates of supernatants was quantified using a VICTOR multilabel reader (Wallac, Finland).
Flow cytometric analysis of γδ T cells and apoptosis in NK cells
Activation of γδ T cells was analyzed by fluorescence-activated cell sorting (FACS) by CD69 (BioLegend, CA) and CD107a (BioLegend) staining. To determine necrosis and induction of apoptosis by ATLG, isolated NK cells were incubated with the indicated concentrations of ATLG (range, 0-1000 μg/mL) for 0 to 24 hours. After incubation, NK cells were stained with FITC-Annexin V and propidium iodide (PI) (BD Pharmingen, CA) and analyzed by FACS. Apoptotic cells were defined as annexin V single positive (early apoptosis) or annexin V/PI double positive (late apoptosis) and necrotic cells as PI single positive. Samples were acquired on FACSCalibur or on LSR II (BD Biosciences, NJ) and analyzed using FlowJo software V7.6.5 (FlowJo LLC, OR).
Detection and quantification of T-cell–specific rabbit IgG
For detection of T-cell–specific rabbit immunoglobulin G (IgG), mirroring rabbit ATLG in patients’ sera, Jurkat cells were incubated with a dilution series of human serum samples. The cell-bound rabbit IgG representing the T-cell–specific ATLG fraction was then detected by a FITC-labeled goat anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA) and quantified by FACS.
For calibration, a dilution series of an ATLG standard (5.000-0.078 μg/mL) was performed, and a calibration curve (second order polynomial) was generated. The concentration of T-cell–specific rabbit IgG was determined via the ATLG standard curve (LLOQ: 0.078 μg/mL). Analyses of T-cell–specific ATLG serum levels were performed at Neovii Biotech (formerly Fresenius, Graefelfing/Munich, Germany). For further details, see supplemental Data.
Patient characteristics and procedures
A cohort of 51 patients with different malignant or nonmalignant diseases and haploidentical donors was consecutively analyzed in 3 centers between February 2011 and June 2017.
All patients received a CD3/CD19-depleted stem cell graft from a parental donor after myeloablative conditioning (melphalan-based, n = 50; treosulfan-based, n = 1). In case of residual CD3+ T cells >2.5 × 10e4/kg, a short course (30 days) of mycophenolate mofetil was recommended. ATLG (Neovii Biotech) was administered in all patients on days −12 until −9 at 3 different body weight–based doses established in the allogeneic setting (15, 30, or 60 mg/kg). Chosen ATLG dose depended on standards of each transplant center. For further details, see Table 1.
Within our trial, ATLG serum levels were analyzed daily during the conditioning regimen and at various time points after transplant. ATLG dosing was correlated with clinical parameters (engraftment, GVHD, and immune recovery). The trial was approved and registered (number 115/2011BO2) by the ethics committee of the university of Tuebingen. Informed consent was obtained from each participant or his/her legal representative. All procedures were in accordance to the Declaration of Helsinki, the German legislation, and the local ethics committee.
PK analysis
PK parameters were estimated from measured ATLG serum concentrations by a professional institution (SocraTec Pharma, Germany). Compartmental and noncompartmental methods were used for analysis.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9.1.2 (GraphPad Software Inc, CA). To assess significance in Europium release assays and to compare the influence of different ATLG concentrations in apoptosis assays, 1-way analysis of variance (Tukey multiple comparisons) and Dunnett T3 test were used. Effect of complement and immune reconstitution were analyzed by Kruskal-Wallis test, and graft rejection was by Fisher exact test. Linear regression analysis was performed after transforming both variables (ATLG serum level; immune cell count) to logarithmic values. P values are given as ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Results
Viability and cytotoxic function of NK cells are compromised by ATLG in a dose dependent manner in vitro
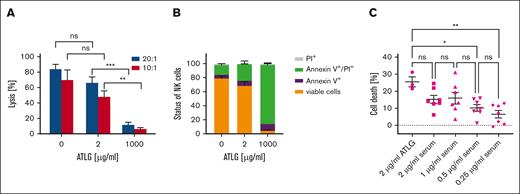
To assess whether donor NK cell functions could be preserved by applying lower doses of ATLG to patients receiving CD3/CD19-depleted grafts, we investigated the cytotoxicity and viability of isolated NK cells in the presence of different ATLG doses in vitro. Therefore, NK cell cytotoxicity against K562 cells was determined after incubation of isolated NK cells with low (2 μg/mL) and high (1000 μg/mL) doses of ATLG for 24 hours (Figure 1A).
Effect of ATLG on NK cell function and viability in vitro. (A) Lysis of K562 cells by NK cells. After preincubation of isolated NK cells with 0, 2, or 1000 μg/mL ATLG for 24 hours, NK cell–mediated lysis of K562 cells was quantified using the Europium release assay. E:T ratios varied (20:1 and 10:1). Shown are means of 5 independent assays performed in triplicates, bars indicate standard error of the mean (SEM). For analysis of significance, 1-way analysis of variance (ANOVA) test was performed. (B) Effect of ATLG on NK cell viability. Isolated NK cells were incubated for 24 hours with 0, 2, or 1000 μg/mL ATLG, then stained with annexin V/PI and analyzed by FACS. Columns show means of 4 independent assays, and bars show SEM. (C) Effect of sera obtained from ATLG-treated patients on NK cell viability. Isolated NK cells from 3 healthy donors were incubated for 24 hours with sera from 3 patients after treatment with ATLG, then analyzed by annexin V/PI staining. Sera were obtained on day –12 (before first ATLG dose) and on day −8 (after ATLG application). Patients had not received any chemotherapeutic agents until day –8. ATLG serum levels were adjusted to concentrations ranging from 0.25 to 2 μg/mL in in vitro assays. No significant difference of overall cell death rates (annexin V+/ PI+/annexin V+ and PI+) was seen in the presence of ex vivo reconstituted ATLG and patients’ sera, both adjusted to 2 μg/mL ATLG (1-way ANOVA, Tukey multiple comparisons). All experiments were performed in duplicates. Serum from 1 of 3 patients was tested only once. Shown are means with SEM.
Effect of ATLG on NK cell function and viability in vitro. (A) Lysis of K562 cells by NK cells. After preincubation of isolated NK cells with 0, 2, or 1000 μg/mL ATLG for 24 hours, NK cell–mediated lysis of K562 cells was quantified using the Europium release assay. E:T ratios varied (20:1 and 10:1). Shown are means of 5 independent assays performed in triplicates, bars indicate standard error of the mean (SEM). For analysis of significance, 1-way analysis of variance (ANOVA) test was performed. (B) Effect of ATLG on NK cell viability. Isolated NK cells were incubated for 24 hours with 0, 2, or 1000 μg/mL ATLG, then stained with annexin V/PI and analyzed by FACS. Columns show means of 4 independent assays, and bars show SEM. (C) Effect of sera obtained from ATLG-treated patients on NK cell viability. Isolated NK cells from 3 healthy donors were incubated for 24 hours with sera from 3 patients after treatment with ATLG, then analyzed by annexin V/PI staining. Sera were obtained on day –12 (before first ATLG dose) and on day −8 (after ATLG application). Patients had not received any chemotherapeutic agents until day –8. ATLG serum levels were adjusted to concentrations ranging from 0.25 to 2 μg/mL in in vitro assays. No significant difference of overall cell death rates (annexin V+/ PI+/annexin V+ and PI+) was seen in the presence of ex vivo reconstituted ATLG and patients’ sera, both adjusted to 2 μg/mL ATLG (1-way ANOVA, Tukey multiple comparisons). All experiments were performed in duplicates. Serum from 1 of 3 patients was tested only once. Shown are means with SEM.
In the absence of ATLG, mean lysis of K562 cells by NK cells was 84% at an E:T ratio of 20:1. Preincubation of NK cells with 2 μg/mL ATLG reduced mean lysis to 66% (Figure 1A; E:T = 20:1), not differing significantly to lysis mediated by untreated NK cells (1-way analysis of variance test). This means that donor NK cells are not relevantly functionally compromised if low ATLG levels are reached on day 0. In the presence of 1000 μg/mL ATLG, serving as a positive control, mean NK cell–mediated lysis declined to 11% (E:T = 20:1).
Regarding viability, in the mean, 69% of NK cells remained viable after preincubation with 2 μg/mL ATLG compared with 80% in the absence of ATLG. By contrast, viability was compromised distinctively to 4% by preincubation with 1000 μg/mL ATLG (Figure 1B).
To ensure that the mild effect of “low dose” ATLG is not a sole in vitro phenomenon, NK cells were incubated for 24 hours in parallel with freshly reconstituted ATLG as well as with sera taken from patients who received ATLG intravenously. For this purpose, serum samples were used to create ATLG concentrations of 0.25, 0.5, 1, and 2 μg/mL in the assay, respectively. Subsequent viability assays revealed no significant difference of cell death rates independent of whether ATLG from patients’ sera (1.0; 2.0 μg/mL) or ATLG off-the-shelf (2.0 μg/mL) was used (Figure 1C).
ATLG affects NK cell viability in a time-dependent manner in vitro
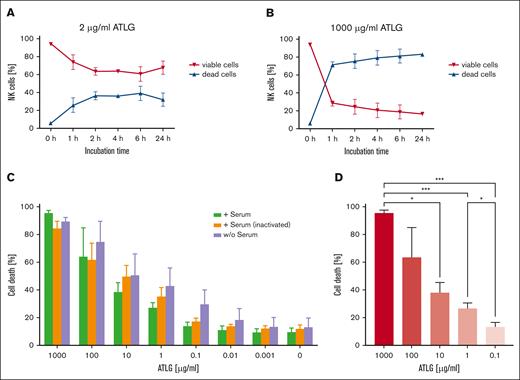
To test whether the negative effect of ATLG on NK cell viability is time-dependent, apoptosis assays were performed after incubating NK cells with “low” (2 μg/mL) or “high dose” (1000 μg/mL) ATLG for 1, 2, 4, 6, and 24 hours (Figure 2A-B). After a 1-hour incubation time, 74% of NK cells were viable in the presence of “low dose” ATLG, whereas only 28% of NK cells remained viable after “high dose” ATLG treatment. Importantly, low dose ATLG treatment beyond 6-hour incubation did not result in further NK cell apoptosis and/or necrosis. Moreover, the rate of viable NK cells started plateauing at ∼60% upon 2-hour incubation (Figure 2A). This indicates that in the presence of “low dose” ATLG a significant portion of NK cells can be preserved. Otherwise, “high dose” ATLG led to a vast and fast decrease of NK cell viability within the first hour (72% of cell death) not plateauing after a 1-day incubation (Figure 2B).
Time-dependent effect of ATLG and role of complement in the presence of ATLG on NK cell viability. (A-B) Isolated NK cells were either incubated with 2 μg/mL ATLG (A) or 1000 μg/mL ATLG (B) for 1, 2, 4, 6, and 24 hours, respectively. Induction of cell death was determined by FACS after annexin V/PI staining at these time points. A minimum of 50 000 events was analyzed per sample. Dead cells include early and late apoptotic (annexin V+/annexin V+ and PI+) and necrotic (PI+) cells. Shown are means and standard deviation of duplicates of 2 different donors. (C) Isolated NK cells were incubated with various ATLG concentrations, as indicated, for 24 hours in the presence (i) of native human serum in a ratio of 1:4; (ii) of heat-inactivated human serum; or (iii) without human serum. Induction of cell death was determined by FACS after annexin V/PI staining. A minimum of 50 000 events was analyzed per sample. No significant differences within 1 ATLG dose (0-100 μg/mL) could be shown independent of the presence/absence of (active) complement (Kruskal-Wallis test; Dunn multiple comparisons). Only at 1000 μg/mL ATLG, cell death rates differed significantly between samples with functional and with inactivated sera (P = .03). Shown are means of dead cells of 3 independent experiments, and bars indicate standard deviation. (D) Cell death rates of isolated NK cells (sum of annexin V+/annexin V+ and PI+/PI+ stained CD56+ NK cells) preincubated with native human serum (1:4) and ATLG, concentrations as indicated, for 24 hours. Same assay as in panel C, analyzed by Dunnett T3 test.
Time-dependent effect of ATLG and role of complement in the presence of ATLG on NK cell viability. (A-B) Isolated NK cells were either incubated with 2 μg/mL ATLG (A) or 1000 μg/mL ATLG (B) for 1, 2, 4, 6, and 24 hours, respectively. Induction of cell death was determined by FACS after annexin V/PI staining at these time points. A minimum of 50 000 events was analyzed per sample. Dead cells include early and late apoptotic (annexin V+/annexin V+ and PI+) and necrotic (PI+) cells. Shown are means and standard deviation of duplicates of 2 different donors. (C) Isolated NK cells were incubated with various ATLG concentrations, as indicated, for 24 hours in the presence (i) of native human serum in a ratio of 1:4; (ii) of heat-inactivated human serum; or (iii) without human serum. Induction of cell death was determined by FACS after annexin V/PI staining. A minimum of 50 000 events was analyzed per sample. No significant differences within 1 ATLG dose (0-100 μg/mL) could be shown independent of the presence/absence of (active) complement (Kruskal-Wallis test; Dunn multiple comparisons). Only at 1000 μg/mL ATLG, cell death rates differed significantly between samples with functional and with inactivated sera (P = .03). Shown are means of dead cells of 3 independent experiments, and bars indicate standard deviation. (D) Cell death rates of isolated NK cells (sum of annexin V+/annexin V+ and PI+/PI+ stained CD56+ NK cells) preincubated with native human serum (1:4) and ATLG, concentrations as indicated, for 24 hours. Same assay as in panel C, analyzed by Dunnett T3 test.
Role of complement on the viability of ATLG–treated NK cells in vitro
Beside the induction of cell death, ATLG is known to act by depleting T cells via complement dependent cytotoxicity. Therefore, we investigated whether the presence of complement (native human serum) also functions in modulating NK cells in respect to apoptosis and/or necrosis during ATLG treatment for 24 hours (Figure 2C). Interestingly, addition of functional human serum did not increase the overall ATLG-induced (0-100 μg/mL) death rate of NK cells, compared with samples without or with heat-inactivated serum. Decreasing ATLG concentrations from 1000 to 10 (1; 0.1) μg/mL resulted in significantly lower death rates of NK cells (P = .02; P < .001; P < .001), as exemplarily shown in the presence of native human serum (Figure 2D).
Determination of ATLG serum concentration in patients treated with 3 different ATLG doses
In our retrospective analysis, 51 patients were treated with either 3 × 5 mg/kg (n = 8), 3 × 10 mg/kg (n = 34), or 3 × 20 mg/kg (n = 9) ATLG. ATLG was uniformly applied IV on days −12 until −9, preceded by an ATLG test dose (1 mg/kg) on day −12. ATLG serum concentrations were serially determined in 18 of 51 patients by measuring T-cell–specific rabbit IgG by FACS (Figure 3; Table 2; supplemental Table 1). T-cell–specific rabbit IgG represents the biologically active fraction of the total amount of administered ATLG, therefore seen as the important factor impairing effector immune cells of the graft.
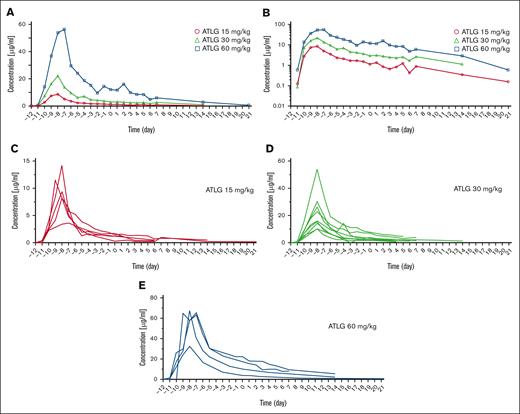
Serum concentration profiles of ATLG IV applied from day –12 to day –9 before haplo-HSCT. Day 0 is defined as the day of transplant. (A) Mean serum concentrations of T-cell–specific rabbit ATLG over time according to dosing (circle, 15 mg/kg; triangle, 30 mg/kg; square, 60 mg/kg). (B) Mean serum concentration curves of T-cell–specific rabbit ATLG over time, shown on semilogarithmic scale. ATLG concentrations declined according to a monoexponential log-linear function. (C-E) ATLG serum concentrations, shown as spaghetti plots, in 18 patients after IV administration of (C) 15 mg/kg (n = 5), (D) 30 mg/kg (n = 9), or (E) 60 mg/kg (n = 4) ATLG.
Serum concentration profiles of ATLG IV applied from day –12 to day –9 before haplo-HSCT. Day 0 is defined as the day of transplant. (A) Mean serum concentrations of T-cell–specific rabbit ATLG over time according to dosing (circle, 15 mg/kg; triangle, 30 mg/kg; square, 60 mg/kg). (B) Mean serum concentration curves of T-cell–specific rabbit ATLG over time, shown on semilogarithmic scale. ATLG concentrations declined according to a monoexponential log-linear function. (C-E) ATLG serum concentrations, shown as spaghetti plots, in 18 patients after IV administration of (C) 15 mg/kg (n = 5), (D) 30 mg/kg (n = 9), or (E) 60 mg/kg (n = 4) ATLG.
Figure 3 shows that higher ATLG dosing correlated with higher ATLG serum levels. Moving ATLG application to days −12 to −9 resulted in mean peak levels of T-cell–specific rabbit IgG of 9.39 (day −8), 22.1 (day −8), and 57.5 μg/mL (day −7), which declined to 1.14, 2.99, and 12.10 μg/mL (15, 30, and 60 mg/kg ATLG) on day 0, respectively. Within all 3 ATLG subgroups, absolute lymphocyte counts (ALCs) measured in patients before the start of ATLG application correlated neither with ATLG serum levels at the day of transplant (supplemental Figure 1A-C) nor with ATLG peak levels (supplemental Figure 1D-F).
PK parameters
ATLG PKs were compatible with linear first-order kinetics with a nearly parallel concentration decline after escalating doses (Figure 3B). As common for population kinetics, the geometric mean values are stated in addition to the arithmetic means (Table 2). With increasing ATLG doses, there was an overproportionate increase of the maximum concentration (Cmax) and the area under the concentration time curves (AUC). Geometric means of clearance (Cl) and volume of distribution (Vz) decreased with higher doses. Due to the simultaneous decrease of both parameters, values for half-life (T1/2) are almost congruent and only slightly increased from 5.4 to 6.6 and 7.6 days (Table 2) with the fourfold rise in ATLG dose (T1/2 = 0.693 × Vz/Cl).
ATLG decelerates posttransplant immune recovery
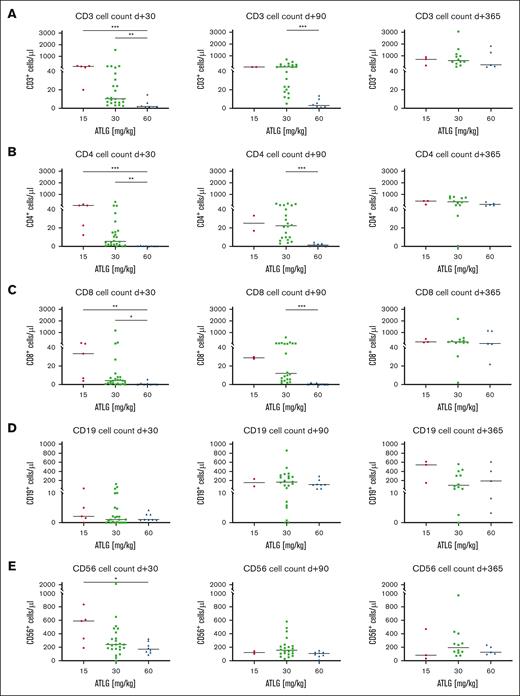
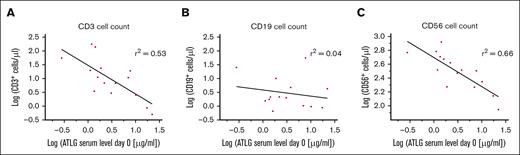
Besides ATLG’s negative impact on NK cell function and viability, we asked whether ATLG also influences the posttransplant recovery of T, B, and NK cells quantitatively in our patient cohort. CD3+ T-cell reconstitution was significantly compromised by ATLG in a dose dependent manner: at day +30, CD3+ T cells recovered significantly faster in the 15 mg/kg (P < .001) and the 30 mg/kg (P = .007) ATLG subgroup compared with the 60 mg/kg ATLG subgroup (Figure 4A). At day +90, median CD3+ T-cell counts still differed substantially between the 30 and the 60 mg/kg ATLG subgroups (Figure 4A; 55 vs 3 CD3+ T cells/μL; P < .001). Significant differences in T-cell recovery between ATLG subgroups were also observed for CD4+ and CD8+ T-cell subsets at days +30 and +90 (Figure 4B-C). Restoration of CD19+ B cells was slow in all subgroups without significant difference at any time (Figure 4D). Contrary to T and B cells, CD56+ NK cells were not depleted by graft manipulation and recovered fast and robust in all 3 subgroups, with significantly higher NK cell counts at day +30 in the 15 mg/kg subgroup than in the 60 mg/kg ATLG subgroup (Figure 4E; P = .03). Analyzing immune reconstitution (day +30) with respect to ATLG serum levels at day 0 in patients with serum analytics available (Figure 5) revealed regression coefficients (r2) of 0.53 (CD3+ T cells) and 0.66 (CD56+ NK cells).
Influence of ATLG dosing on posttransplant immune reconstitution in recipients of CD3/CD19–depleted stem cell grafts. FACS analysis of (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) CD19+ B cells, and (E) CD56+ NK cells at days +30, +90, and +365 after transplant in patients treated with 15 mg/kg, 30 mg/kg, or 60 mg/kg ATLG before HSCT. Shown are scatterplots with median cell numbers per μL of all patients without graft rejection. Statistical analysis: Kruskal-Wallis test (A-E).
Influence of ATLG dosing on posttransplant immune reconstitution in recipients of CD3/CD19–depleted stem cell grafts. FACS analysis of (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) CD19+ B cells, and (E) CD56+ NK cells at days +30, +90, and +365 after transplant in patients treated with 15 mg/kg, 30 mg/kg, or 60 mg/kg ATLG before HSCT. Shown are scatterplots with median cell numbers per μL of all patients without graft rejection. Statistical analysis: Kruskal-Wallis test (A-E).
Influence of ATLG serum concentration on the day of transplant on posttransplant immune recovery. Immune reconstitution of (A) CD3+ T cells, (B) CD19+ B cells, and (C) CD56+ NK cells on day +30 after CD3/CD19-depleted HSCT, shown as a function of respective ATLG serum levels on day 0. Regression lines are shown in black, r2 indicates regression coefficients (A-C). Equations of regression lines are given by (A) y = −1.03x + 1.48, (B) y = −0.22x + 0.58, (C) y = −0.42x + 2.70.
Influence of ATLG serum concentration on the day of transplant on posttransplant immune recovery. Immune reconstitution of (A) CD3+ T cells, (B) CD19+ B cells, and (C) CD56+ NK cells on day +30 after CD3/CD19-depleted HSCT, shown as a function of respective ATLG serum levels on day 0. Regression lines are shown in black, r2 indicates regression coefficients (A-C). Equations of regression lines are given by (A) y = −1.03x + 1.48, (B) y = −0.22x + 0.58, (C) y = −0.42x + 2.70.
ATLG reduces graft rejection dose-dependently
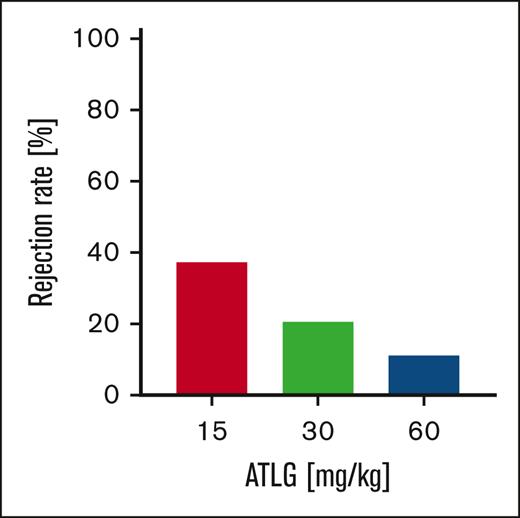
Graft rejection occurred in 3 of 8 patients (37.5%) treated with 15 mg/kg ATLG, in 7 of 34 patients (20.6%) with 30 mg/kg ATLG, and in 1 of 9 patients (11.1%) with 60 mg/kg ATLG (Figure 6). However, this trend in reducing graft rejection by higher ATLG doses was not significant (P = .459). Final engraftment was achieved in all patients by reconditioning and stem cell infusion from an alternative donor or after transfusion of an autologous stem cell back up.
ATLG dosing influences graft rejection in CD3/CD19-depleted haplo-HSCT. Graft rejection occurred in 37.5% (15 mg/kg ATLG) vs 20.6% (30 mg/kg ATLG) vs 11.1% (60 mg/kg ATLG) of patients (n = 51). Associations between ATLG doses and rejection were explored using the Freeman-Halton extension of Fisher exact test (2-sided); 2-tailed probability: P = .459.
ATLG dosing influences graft rejection in CD3/CD19-depleted haplo-HSCT. Graft rejection occurred in 37.5% (15 mg/kg ATLG) vs 20.6% (30 mg/kg ATLG) vs 11.1% (60 mg/kg ATLG) of patients (n = 51). Associations between ATLG doses and rejection were explored using the Freeman-Halton extension of Fisher exact test (2-sided); 2-tailed probability: P = .459.
Effect of ATLG dosage on incidence of acute and chronic GVHD
Among all patients, acute GVHD (aGVHD; grade 2-4) occurred in 4 of 51 patients (7.8%) and chronic GVHD (cGVHD; extensive form) in 4 of 47 patients (8.5%). Differences of GVHD rates among ATLG subgroups were not significant; 12.5% (8.8%) of patients of the 15 (30) mg/kg ATLG subgroup developed aGVHD (grade 2-4), and extensive cGVHD was observed in 14.3% (9.4%) in the corresponding subgroups. Neither aGVHD (grade 2-4) nor cGVHD (limited/extensive forms) manifested in patients who received 60 mg/kg ATLG. Whether or not day 0 and/or peak ATLG serum concentrations influenced the incidence of aGVHD (grade 2-4) and/or cGVHD (extensive form) could not be evaluated due to low patient numbers (supplemental Figure 2).
Discussion
In this analysis, we investigated whether a different dosing schedule of ATLG can improve posttransplant immune reconstitution without increasing the rate of rejection in patients who received T- and B-cell–depleted haploidentical stem cell grafts. Additionally, we evaluated the time course of ATLG serum levels.
Improving posttransplant immune recovery is clinically essential to reduce the incidence of infectious complications and relapse.11 Early posttransplant recovery is dominated by cells of the innate immune system, among which NK cells play an essential role with regard to antiviral and antileukemic effects.11 Given that, in the haploidentical TCD setting, production of NK cells originating from donor hematopoietic stem cells takes ∼2 months, allo-reactive NK cells transfused within the graft are needed not to delay the graft-versus-leukemia effect.11-13 Here, our data showed that using lower ATLG doses (15 mg/kg) significantly increased posttransplant NK cell counts at day +30 compared with higher ATLG doses (60 mg/kg). Additionally, in contrast to T-cell–replete haplo-HSCT, most of our patients received only a short-term and single-agent immunosuppressive prophylaxis, thus only shortly compromising posttransplant NK cell activity in vivo. Recovery of the adaptive immune system generally occurs after NK cell reconstitution.14 De Koning et al15 and others16 showed that, across different transplant platforms, CD4+ T-cell counts >50/μL until day +100 accurately predict survival and nonrelapse mortality. In this regard, we could demonstrate that higher ATLG dosing, correlating with higher serum levels of T-cell–specific ATLG, significantly prolonged immune reconstitution: patients treated with “low” (15 mg/kg) and “medium dose” (30 mg/kg) ATLG recovered significantly faster regarding CD3+ T-cell counts at day +30 than did patients who received “high dose” (60 mg/kg) ATLG. Most importantly, also at day +90, T cells in patients with “medium dose” ATLG recovered markedly faster than in patients with “high dose” ATLG. We would assume a significant difference in T-cell counts at day +90 also between the “low” and “high dose” ATLG groups. However, due to low patient numbers evaluable at day +90 in the 15 mg/kg subgroup, we could not show statistical significance in T-cell recovery between these 2 subgroups.
In turn, there was a clear trend toward reduced rejection using higher ATLG doses. However, possibly due to the size of our subgroups, differences in rejection rates were not significant (Figure 6; P = .459). The rate of rejection we observed is consistent to the results of a prospective phase 2 study by Merli et al,17 who evaluated the outcome of haplo-HSCT using TCRαß/CD19-depleted grafts in 70 children with nonmalignant disorders; after ATLG application (12 mg/kg) over 3 days (day −5 to −3), graft failure occurred in 30.4% of patients, resembling a rejection rate of 37.5% in our “low dose” subgroup.
Evaluating T-cell and NK-cell reconstitution (day +30) as a function of ATLG serum levels on day 0 revealed regression coefficients of 0.53 and 0.66, respectively (Figure 5), indicating that ATLG levels (day 0), in parallel to ATLG doses, markedly determine both immune cell counts (day +30). Because, however, ATLG levels were determined only in 18 of 51 patients and only 2 of these patients rejected their graft, we could analyze the incidence of rejection only by ATLG doses and not by ATLG serum levels.
Altogether, our data show that combining high pretransplant with low posttransplant ATLG exposure would be ideal. This observation is in line with others: in a large study by Admiraal et al,18 including 251 patients receiving thymoglobulin (first dose starting 5 days [range, 1-19 days] before transplant), graft failure was significantly reduced by high thymoglobulin exposure before transplant, whereas low posttransplant exposure was essential for immune reconstitution. Thus, scheduling of thymoglobulin application was relevant to decrease posttransplant levels of this TCD agent. In our study, ATLG, formerly applied on days −4 until −1, was uniformly administered on days −12 to −9. Thereby, it was feasible to obtain mean T-cell–specific ATLG levels (15 and 30 mg/kg subgroups) of only 2.07 μg/mL at day 0. Using low ATLG concentrations in our in vitro assays, we could demonstrate that viability and cytotoxicity of NK cells were notably enhanced compared with higher ATLG doses, pointing out the benefit of our ATLG scheduling: NK cell–mediated lysis against K562 cells was only reduced by 18% to 22% compared with lysis in the absence of ATLG. Preserving viable and functionally active NK cells by achieving ATLG serum levels as low as possible on day 0 appears to be clinically relevant. Maggs et al showed that higher NK-cell counts in the grafts significantly reduced relapse rates from 40% to 6% at 2 years after transplant in 107 patients.19 In another series of 47 pediatric patients with malignant diseases, NK cell–mediated antileukemic capacity was repetitively determined after T-cell–depleted HSCT.20 Relapse probability at 2 years after transplant was significantly lower in patients with favorable than with low NK-cell activity.20
Another graft manipulation method, TCRαβ/CD19 depletion, preserves γδ T cells in addition to NK cells. We also could show that low ATLG concentrations did not affect the expression of activation markers on γδ T cells in vitro (supplemental Figure 3), suggesting that starting ATLG application as early as on day -12 would also be beneficial in the case of HSCT using TCRαβ/CD19-depleted grafts.
Taking into account the lower rejection rate in the 60 mg/kg subgroup, advantages and disadvantages of our 3 ATLG subgroups are best balanced in the 30 mg/kg subgroup. What could be done to further decrease ATLG serum concentrations on day 0 to accelerate immune recovery? Three options are conceivable in principle:
Thus, for example, in the 60 mg/kg subgroup (mean C−5 = 24.2 μg/mL; T1/2 = 7.58 days), the concentration of 2 μg/mL was not reached until day +23. Consequently, ATLG application ought to be completed already on day −32. To calculate this time period, only one measurement 4 days after the last ATLG application is necessary.
Secondly, posttransplant ATLG concentrations could be lowered by plasmapheresis. Zhang et al21 used this method to decrease posttransplant thymoglobulin levels before an adoptive T-cell transfer. Although thymoglobulin concentrations were rapidly lowered by ∼75% by 1-day plasmapheresis, levels persisted above the therapeutic threshold. Moreover, plasmapheresis usually performed for several days is a stressful procedure, hard to establish during conditioning.
Thirdly, by inactivating ATLG enzymatically straight before transfusing the graft. Imlifidase (Idefirix; Hansa Biopharma, Lund, Sweden), an enzyme of Streptococcus pyogenes, cleaves all human IgG subclasses.22 In a randomized dose-escalation phase 1 study, human IgG was quickly hydrolyzed in F(ab’)2 and Fc fragments with the maximum effect already at 2 to 6 hours after application of imlifidase.23 Because imlifidase is also capable to cleave rabbit ATLG, using this enzyme straight before transplant would be an optimal procedure to inactivate rabbit ATLG abruptly.
However, it is important to note that decreasing ATLG levels at day 0 is not feasible in the context of unmanipulated allogeneic HSCT, because sufficient ATLG levels are needed for GVHD prevention. In our cohort, GVHD prophylaxis was primarily maintained by CD3/CD19 ex vivo depletion. In this setting, the main function of ATLG is to prevent graft rejection. We observed an additional but nonsignificant effect of ATLG on GVHD prevention by higher doses, which is most likely due to residual allo-reactive donor T cells after TCD in the graft itself. Nevertheless, even patients treated with “low dose” in our cohort developed aGVHD (chronic extensive) in only 12.5% (14.3%) of cases. With an average incidence of 7.8% (aGVHD) and 8.5% (cGVHD) in all subgroups, GVHD rates in our haploidentical cohort were low: Peters et al24 reported a rate of 14% (6%) of extensive cGVHD in children with ALL who received unmanipulated grafts from matched sibling (matched unrelated) donors and 60 mg/kg of ATLG.
Optimal dosing and timing of ATLG is an ongoing matter of debate, also in matched allogeneic HSCT.25 Although EBMT guidelines generally recommend the use of 30 mg/kg for HLA-identical siblings and 60 mg/kg ATLG in unrelated HSCT in myeloablative conditioning regimens,6,26 Admiraal et al27 propose a more sophisticated dosing: they recommend to adjust the thymoglobulin dose on ALCs and not, as usual, on body weight. They argue that patients with high ALCs performed better with higher thymoglobulin doses, probably because lymphocytes display targets for thymoglobulin, with higher ALCs resulting in less active serum levels and faster thymoglobulin clearance.27,28 The same result was observed in children.29 In our haploidentical cohort, however, ALCs before the start of ATLG application did not negatively correlate with peak or day 0 levels of ATLG in all ATLG subgroups (supplemental Figure 1), possibly because ATLG serum analytics was performed only in 18 of 51 patients.
In the aforementioned pediatric study of the Admiraal group,18 successful CD4+ T-cell reconstitution by day +100 after thymoglobulin treatment was significantly associated with better overall survival. The authors proposed an AUC-based dosing model and concluded that the AUC gives a better prediction of drug effects than the concentration at discrete time points.18 AUC estimates, however, need 3 to 5 concentration measurements at least. Analysis of our clinical data showed that a dosing scheme reaching mean levels of about 2 μg/mL of T-cell–specific ATLG on day 0 predicts significant faster reconstitution of NK and T cells. Transferring our PK results into clinics, we propose to measure C−5 in patients. With respect to our PK analysis, ttarget and, thus, the day of transplant on which a desired ATLG concentration below the lympholytic level will be reached, can easily be calculated. However, this procedure necessitates the use of frozen grafts to flexibly change the day of transplant.
In conclusion, dosing and timing of ATLG administration markedly influence the clinical outcome. Prospective trials are necessary to evaluate options further reducing ATLG serum levels upon transplant to preserve best immune cell subsets with known antitumor activity.
Acknowledgments
This work was supported by grants from the Deutschen Konsortium für Translationale Krebsforschung, the excellence cluster “Image-Guided and Functionally Instructed Tumor Therapies (iFIT)” (EXC 2180) gefördert durch die Deutsche Forschungsgemeinschaft im Rahmen der Exzellenzstrategie des Bundes und der Länder—WXC 2180—390900677, the Reinhold-Beitlich Stiftung, the Wilhelm-Sander Stiftung, the Schickedanz Kinderkrebs Stiftung, the Förderverein und Stiftung für krebskranke Kinder Tuebingen e.V. (all to P.L.), and by research funding from Neovii Biotech (Graefelfing, Germany; P.L.).
Authorship
Contribution: P.L., R.H., and C.K. designed the study and supervised the project; C.-P.M. wrote the manuscript; C.K., H.M., and R.T. performed experiments; C.-P.M., C.K., and P.L. performed statistical analysis and interpreted data; F.K. was involved in pharmacokinetic analysis and data interpretation; C.M.S., M.D., R.M., F.S., B.G., T.E., T.F., R.H., and P.L. provided serum samples from patients; C.-P.M., C.K., F.H., F.K., A.R., A.-M.A., G.A., T.E., H.M., S.N., J.H.S., C.L., and P.L. critically discussed the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: P.L. received research funding from Neovii Biotech (Graefelfing, Germany). H.M. is an employee of Neovii Biotech (Graefelfing, Germany). The remaining authors declare no competing financial interests.
Correspondence: Claus-Philipp Maier, University Hospital Tuebingen, Hoppe-Seyler-Str 1, Tuebingen 72076, Germany; email: claus-philipp.maier@med.uni-tuebingen.de.
References
Author notes
Data will be available upon reasonable request from the corresponding author, Claus-Philipp Maier (claus-philipp.maier@med.uni-tuebingen.de).
The full-text version of this article contains a data supplement.