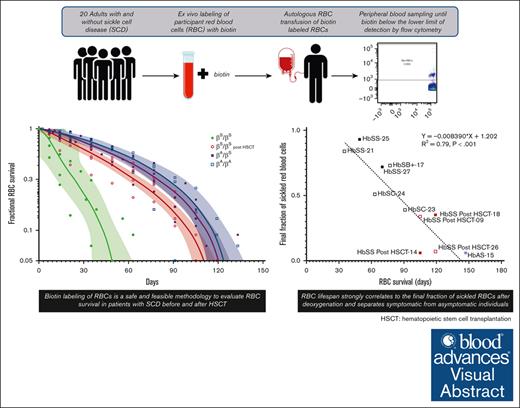
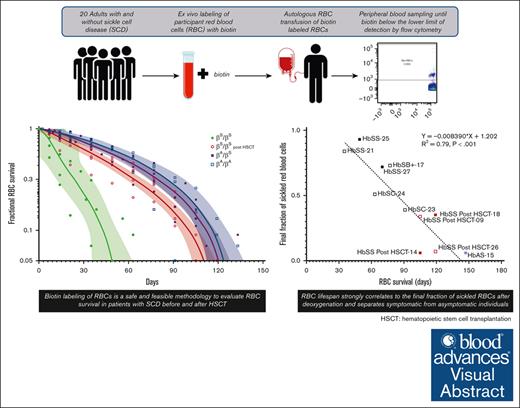
Biotin labeling of RBCs is a safe and feasible methodology to evaluate RBC survival in patients with SCD before and after HSCT.
Understanding differences in RBC survival may inform hemoglobin composition thresholds required to reverse the phenotype after gene therapy.
Visual Abstract
Stable, mixed-donor–recipient chimerism after allogeneic hematopoietic stem cell transplantation (HSCT) for patients with sickle cell disease (SCD) is sufficient for phenotypic disease reversal, and results from differences in donor/recipient–red blood cell (RBC) survival. Understanding variability and predictors of RBC survival among patients with SCD before and after HSCT is critical for gene therapy research which seeks to generate sufficient corrected hemoglobin to reduce polymerization thereby overcoming the red cell pathology of SCD. This study used biotin labeling of RBCs to determine the lifespan of RBCs in patients with SCD compared with patients who have successfully undergone curative HSCT, participants with sickle cell trait (HbAS), and healthy (HbAA) donors. Twenty participants were included in the analysis (SCD pre-HSCT: N = 6, SCD post-HSCT: N = 5, HbAS: N = 6, and HbAA: N = 3). The average RBC lifespan was significantly shorter for participants with SCD pre-HSCT (64.1 days; range, 35-91) compared with those with SCD post-HSCT (113.4 days; range, 105-119), HbAS (126.0 days; range, 119-147), and HbAA (123.7 days; range, 91-147) (P<.001). RBC lifespan correlated with various hematologic parameters and strongly correlated with the average final fraction of sickled RBCs after deoxygenation (P<.001). No adverse events were attributable to the use of biotin and related procedures. Biotin labeling of RBCs is a safe and feasible methodology to evaluate RBC survival in patients with SCD before and after HSCT. Understanding differences in RBC survival may ultimately guide gene therapy protocols to determine hemoglobin composition required to reverse the SCD phenotype as it relates directly to RBC survival. This trial was registered at www.clinicaltrials.gov as #NCT04476277.
Introduction
Sickle cell disease (SCD) is a severe form of hemolytic anemia in which an inherited mutation in the β globin gene results in sickle hemoglobin (HbS) polymerization in red blood cells (RBCs). HbS polymers distort and damage RBCs, leading to an overall shortened RBC survival compared with the lifespan of RBCs from healthy donors and those with sickle cell trait (SCT).1-5 The only current curative treatment for SCD is allogeneic hematopoietic stem cell transplantation (HSCT) which aims to replace the hematopoietic stem cells (HSCs) containing the mutated β globin gene with donor HSCs from either SCT or unaffected individuals. Approximately 20% stable, mixed-donor–recipient chimerism after HSCT is sufficient to reverse the sickle cell phenotype by virtue of improved donor red cell survival compared with that of SCD red cells.6,7
Certain molecular factors are thought to affect the severity of anemia in patients with SCD, likely reflected by different rates of hemolysis and therefore different Hb levels at steady state. Several biochemical markers of hemolysis correlate with RBC survival in patients with SCD including absolute reticulocyte count (ARC), α globin gene number, and percentage of fetal hemoglobin (HbF).2,3,5 Mathematical models to determine the minimally sufficient donor myeloid chimerism (DMC) to predict alleviation of disease pathology are reliant on differences between donor and recipient RBC half-lives.6,8 Because stable mixed chimerism is intrinsically linked to the differences in RBC survival in healthy and the SCD genotype, understanding the variability and predictors of red cell survival among patients with SCD vs individuals with SCT and healthy donors is critical for gene therapy approaches, which seek to generate sufficient corrected Hb to overcome the pathology of SCD.
Biotin labeling of RBCs is a safe and effective means of in vivo measurement of RBC lifespan and has been used in healthy donors and in patients with homozygous HbSS disease.2,5,9-13 Biotin labeling of RBCs tags a representative population of circulating RBCs irrespective of RBC age and confers advantages over previously used radiolabeled methods. Biotin can be easily tracked via flow cytometry; the analysis can be complexed with additional characterization parameters, such as fraction sickled after deoxygenation; and lifespan can be determined by the temporal decline in labeled RBCs. Here, we describe a pilot study to determine and compare RBC survival in participants with SCD, participants with SCD who have undergone HSCT, individuals with SCT (HbAS), and healthy donors (HbAA), the latter 2 groups who serve as donors for patients with SCD undergoing HSCT. We included several novel groups in which biotin labeling has not been done; in patients with SCD with genotypes other than homozygous HbSS, in patients with SCD after HSCT, and in participants with SCT. We demonstrate the safety and feasibility of this approach in patients with SCD and confirm that RBC lifespan strongly correlates with the final fraction of sickled RBCs after deoxygenation, separating symptomatic from asymptomatic individuals. This work may inform future allogeneic HSCT and gene therapy protocols by determining a target chimerism or gene modification level that sufficiently reduces polymerization and, therefore, sickling needed to reverse the SCD phenotype as it relates directly to RBC survival.
Methods
Participant selection
We conducted a single-site pilot study to determine and compare RBC survival in patients with SCD, patients with SCD who have undergone HSCT, individuals with SCT, and healthy donors. The study was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute and was monitored by an independent data and safety monitoring board (#NCT04476277). All participants provided informed consent before enrollment. Patients with SCD who received an allogeneic HSCT were previously enrolled to separate institutional review board–approved studies (National Institutes of Health: #NCT00061568, #NCT02105766, and #NCT03077542). An investigational new drug application for the use of CC-TAG Biotin in humans was approved by the Food and Drug Administration (IND19550). Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for AEs (CTAE), version 5.0.
Participants aged ≥18 years were eligible if there was a confirmed diagnosis of SCD (HbSS, HbSC, HbSβ+, and HbSβ0), SCT (HbAS), or ethnically–matched healthy volunteer (HbAA) with normal renal function (creatinine <1.5 mg/dL), negative direct antiglobulin test, and ability to provide informed consent. Participants were excluded if there was any uncontrolled chronic illness other than SCD, active infection, consumption of biotin supplements or raw eggs within 30 days, blood loss >540 mL within the previous 8 weeks, pregnancy, or preexisting, naturally-occurring antibodies against biotin.
All participants were evaluated with a medical history, physical examination, screening labs, and venous assessment. Assessment parameters used at screening and throughout the study are listed in supplemental Table 1.
Biotinylation of RBCs
Approximately 100 mL of venous blood was collected via venipuncture in acid-citrate-dextrose anticoagulant (Baxter Healthcare Corp, Deerfield, IL) to obtain >15 mL of packed RBCs for biotinylation of RBCs (∼80 ± 5 mL), routine labs, and for α-globin genotyping (supplemental Table 1). Whole blood was centrifuged at 1000g (2142 r.p.m.), deceleration (DEC) 4 at room temperature (17-25°C) for 10 minutes. The plasma layer was aspirated and saved for use in reconstituting RBCs after biotin labeling. RBCs were washed 3 times at 4 to 1 ratio in PlasmaLyte A (Baxter Healthcare Corp) and resuspended to a 25% suspension of RBCs. CC-TAG-Biotin (ChemConnection BV, Oss, The Netherlands) was reconstituted to a 1 mg/mL solution and the appropriate volume of biotin solution was added to the 25% RBC suspension to give the desired final concentration of biotin of 18 μg/mL. Cells were incubated at room temperature for 30 minutes, washed, and resuspended. The washed biotinylated RBCs (BioRBCs) were passed through an 18 μm filter (Hemo-Nate Syringe Filter, Utah Medical Products, Inc, Midvale, UT) and resuspended in autologous plasma at a hematocrit (Hct) of 50% (range, 40%-60%). Samples were removed for complete blood count, biotin labeling efficiency determination via flow cytometry, and safety assays (sterility and endotoxin) before release of the cellular product.
Infusion of product and sample collection
BioRBCs were reinfused by gravity per nursing standard operating procedure for cellular therapy product administration via an 18- or 20-gauge peripheral IV when central access was not available. The bag was back flushed with normal saline at completion of the infusion. The time at 20 minutes after the end of the infusion was designated time 0.
Approximately 5 mL of venous blood was collected at 20 minutes after infusion (time 0) in all participants. Participants with SCD who did not undergo transplantation had samples collected bi-weekly for the first 2 weeks, then weekly until the percentage of BioRBCs decreased to the lower limit of detection (less than ∼0.06%). All other participants (SCD post-HSCT, SCT, and healthy donors) had samples drawn weekly for the first 4 weeks then every other week until the percentage of BioRBCs decreased to the lower limit of detection. All participants had antibiotin antibody testing done at screening, at 12 weeks after infusion, and at 6 months after infusion. At every encounter, participants were questioned about any illness, including emergency room visits, hospitalizations, pain, or transfusions since their last visit. A complete list of assessment parameters, including additional hematologic measurements used throughout the study, are listed in supplemental Table 1.
Determination of BioRBC survival, F-cells, and Hb content
Samples were analyzed in real time for percent survival of BioRBCs by flow cytometry (streptavidin-phycoerythrin; S-866; Molecular Probes, Eugene, OR) and F-cell percentage using anti-HbF allophycocyanin–conjugated antibody (Clone HBF-1, Thermo Fisher, Grand Island, NY). The flow cytometry plots and gating strategy from an example participant throughout his or her RBC lifespan is shown in supplemental Figure 1. Hb composition was determined using high performance liquid chromatography on Hb from whole-blood samples, on the biotin-positive fraction after isolation by flow cytometry, and on reticulocytes. Reticulocytes were isolated at least once on every participant using MACS CD71 magnetic bead separation (130-046-201; Miltenyi Biotech, Auburn, CA).
Antibiotin antibody detection
Plasma samples were tested for antibiotin antibodies using gel card antibody detection (MTS anti-IgG Card, MTS084024, Ortho Clinical Diagnostics, Rochester, NY). Random group O donor RBCs were biotinylated (Pierce Premium Grade Sulfo-NHS-SS-Biotin, PG82077, Thermo Scientific, Rockford, IL) and were prepared at 0.8% Hct using diluent 2 (MTS9230, Ortho Clinical Diagnostics, Rochester, NY). Fifty microliter of diluted BioRBCs were layered atop the buffer layer in the gel card microtube. Twenty-five microliters of participant plasma or positive control diluted in ratio 1:100 (generously provided by Guohua An, University of Iowa) was added into the target BioRBC layer. Cells were incubated at 37°C for 30 minute (ID-MTS Incubator MTS9680, Ortho Clinical Diagnostics) and centrifuged at 895 r.p.m. (80-90 r.c.f.) for 10 minutes (ID-MTS Centrifuge MT515060; Ortho Clinical Diagnostics).
Metabolite and sickling measurements
Samples were prepared for metabolite measurement (2,3 diphosphoglyceric acid [2,3-DPG] μg/mL, adenosine triphosphate [ATP] μg/mL, pyruvate kinase protein [ECLU/Hb] gm/dL) at Agios Pharmaceuticals (Cambridge, MA) and for sickling analysis. Whole-blood levels of ATP and 2,3-DPG were measured using liquid chromatography tandem mass spectrometry. For measurement of fractions sickled at the end of deoxygenation, whole-blood samples were collected at specific intervals, diluted 1500- (for HbSS blood) or 3000-fold (for HbAS blood) into a phosphate buffered saline solution; 10 μL of the cell suspension was added to each well of a polypropylene 384-well plate (part no. 3770, Corning, Corning, NY). The plate was inserted into the 37°C humidified chamber of a BioTek FX “Lionheart” automated microscope (Agilent Technologies, Santa Clara, CA) and deoxygenated using the Agilent/BioTek O2 gas controller (part number 1210013) with 100% nitrogen to 5% oxygen to induce sickling. Images of cells were collected every 15 minutes for 9 hours. Sickling times for each cell were determined at 15 minute intervals with an in-house developed, robust image analysis software, which uses machine learning to compare several metrics of the cell image to determine at each 15-minute interval whether or not the cell is sickled.14-17 The output of an experiment is a plot of the fraction of sickled cells vs time.
Single cell Western
To determine the proportion of RBCs that contained HbA, HbS, or both in whole-blood or biotin enriched fractions, ∼50 000 RBCs were loaded onto a small single-cell Western chip (Bio-Techne, Devens, MA) and allowed to settle for 10 minutes. After washing, sodium dodecyl sulfate–polyacrylamide gel electrophoresis separation is performed on-chip using the ProteinSimple Milo Instrument (Bio-Techne). After electrophoresis and protein fixing, the chip is probed with an anti-HbA unconjugated antibody (clone 14G2.G11.F11; Rockland Immunochemicals Inc, Pottstown, PA), a donkey anti-mouse secondary antibody conjugated with Alexa FluorTM 555 (Invitrogen, Waltham, mA) and a custom anti-HbS allophycocyanin–conjugated antibody (Green Mountain Antibodies, Inc Burlington, VT) and analyzed using the Scout software to determine the proportion of occupied wells that were positive for HbA, HbS, or both.
Data and statistical analysis
Fractional RBC survival was determined by plotting the percentage of BioRBCs in circulation vs time after transfusion. Median RBC survival and RBC half-life were calculated from these curves using linear regression. Average lifespan among the groups studied was compared using means and standard deviations. The mean of each participant’s clinical laboratory values during the study period was used for correlation with RBC survival. Correlation of mean RBC survival with various markers of red-cell survival was performed using Pearson Correlation. A P value of <.05 was considered statistically significant. The differences between groups were used to examine the feasibility and guide the design of a larger study; in this small pilot study, statistically significant differences within groups were not anticipated because the size was not powered to observe an effect.
Results
Twenty-seven participants (healthy volunteers, patients with SCD pre- or postallogeneic HSCT, and individuals with SCT) were screened on protocol #NCT04476277 of which 20 participants met eligibility criteria, were enrolled, and completed the study between April 2021 and April 2023. One participant with SCD before transplantation with a history of stroke(s) had a stroke 4 weeks after infusion of BioRBCs which required exchange transfusion. His final RBC survival curve was therefore extrapolated based on the weekly percent biotin decline and clearance up until 4 weeks.
The 20 participants included participants with SCD pre-HSCT (N = 6), participants with SCD post-HSCT (N = 5), participants with SCT (HbAS) (N = 6), and healthy volunteers (HbAA) (N = 3) (Table 1). Median age was 44 years (range, 19-62 years). Most participants were female (N = 13; 65%). Of the participants with SCD pre-HSCT (N = 6), genotypes included HbSS (N = 3; 50%), HbSC (N = 2; 33%), and HbSβ+ (N = 1; 17%). Four participants with SCD before HSCT were administered hydroxyurea (HU) (N = 4; 67%) (Table 2). All participants with SCD after HSCT had HbSS (N = 5; 100%) and received nonmyeloablative conditioning per National Institutes of Health protocols from either a matched sibling (N = 3; 60%) or a haploidentical (N = 2; 40%) donor (Table 2). Donor genotypes were HbAS (N = 4; 80%) or HbAA (N = 1; 20%). Median time from transplantation was 29 months (range, 6-62 months). At the time of enrollment, 3 participants with SCD after HSCT (N = 3; 60%) had full (100%) DMC and 2 participants (N = 2; 40%) had mixed myeloid chimerism (17% and 28%, respectively).
Biotin labeling of RBCs is safe and feasible in participants with SCD before and after transplantation
There were no AEs attributable to the use of biotin or the infusion of BioRBCs during this study. One participant with SCD before HSCT, who had a prior history of stroke(s), required admission and exchange RBC transfusion for acute ischemic stroke 4 weeks after infusion of BioRBCs that was attributable to his underlying SCD. There were no other SCD-related hospitalizations, emergency room visits, pain crises, or health-related events during the observation period among the remainder of the cohort. No antibodies to biotin were detected throughout the study.
Hct was measured before and after biotin labeling, and packed RBC was calculated for each donor. RBC recoveries after labeling or washing were >95% for all tested groups regardless of genotype. No differences were observed between healthy volunteers (HbAA) and those with SCD or SCT, suggesting that RBC loss during processing was minimal.
Hematologic markers are improved, and RBC survival normalizes after HSCT for SCD
Compared with participants with SCD before HSCT, participants with SCD after HSCT had significantly elevated average Hb (12.4 vs 8.9 gm/dL before HSCT, P = .01), Hct (36.6% vs 25.2% before HSCT, P = .01), and HbA% (56.8% vs 0% before HSCT, P < .001), and significantly lower HbF% (1% vs 10.2% before HSCT, P = .04) and HbS% (39.1% vs 68.8% before HSCT, P < .005) (Table 2). There were no statistical differences in hemolytic markers, such as ARC, aspartate transaminase, total bilirubin, or lactate dehydrogenase (LDH) between participants with SCD before and after HSCT. Hematologic data for each individual participant with SCD are listed in supplemental Table 2.
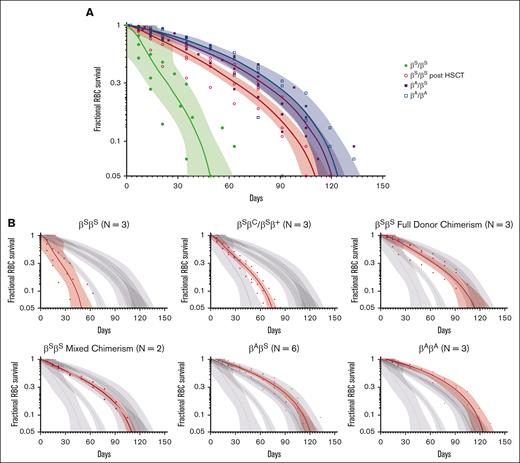
The average RBC lifespan was significantly shorter for participants with SCD before HSCT (64.1 days, range 35-91) compared with those with SCD after HSCT (113.4 days, range 105-119), HbAS (126.0 days, range 119-147), and HbAA (123.7 days, range 91-147) (P < .001). Specifically for participants with HbSS, those before HSCT had significantly shortened RBC lifespan (51.3 ± 17.6 days, range 35-70) compared with participants with HbSS after HSCT (113.4 ± 6.8 days, range 105-119 days), HbAS (126.0 ± 10.7 days, range 119-147 days), and HbAA ([123.7 ± 23.8 days, range 91-147 days] P < .001) (Figure 1A-B). There were no statistically significant differences in lifespan among the different genotypes of participants with SCD before HSCT (HbSS 51.3 ± 17.6 days, range 35-70 vs HbSC/HbSβ+ 77.0 ± 14.0 days, range 63-91, ns) or lifespan of participants with SCD after HSCT with full vs mixed donor chimerism (114.3 ± 8.1 days, range 105-119 vs 112.0 ± 9.9 days, range 105-119, ns) (Figure 1B). Median RBC half-life for participants with HbSS before HSCT was significantly shorter (14.3 ± 4.4 days, range 7.5-18.7) than those with HbSS after HSCT (45.8 ± 6.7 days, range 38.2-52.9) (P < .001).
Fractional RBC Survival Peripheral blood samples were collected at regular intervals in all participants. Samples were analyzed in real time for percent survival BioRBCs by flow cytometry using a streptavidin-conjugate fluorochrome (streptavidin-phycoerythrin; S-866; Molecular Probes) until the percentage of BioRBCs decreased to the lower limit of detection (approximately <0.06%). Values are represented as the RBC fractional survival given absolute differences in the starting percent biotin fraction among participants. Dots represent individual participant BioRBC fractional survival, shown with a nonlinear regression line and 99% confidence interval bands. (A) Fractional RBC survival curves for participants with HbSS pre- and posttransplant, HbAS, and HbAA. (B) Fractional RBC survival curves for each cohort as compared to the larger group are highlighted in red, including HbSS vs non-HbSS genotypes (HbSC and HbSβ+) in the pre-HSCT cohort, and full DMC vs mixed chimerism in the post-HSCT cohort.
Fractional RBC Survival Peripheral blood samples were collected at regular intervals in all participants. Samples were analyzed in real time for percent survival BioRBCs by flow cytometry using a streptavidin-conjugate fluorochrome (streptavidin-phycoerythrin; S-866; Molecular Probes) until the percentage of BioRBCs decreased to the lower limit of detection (approximately <0.06%). Values are represented as the RBC fractional survival given absolute differences in the starting percent biotin fraction among participants. Dots represent individual participant BioRBC fractional survival, shown with a nonlinear regression line and 99% confidence interval bands. (A) Fractional RBC survival curves for participants with HbSS pre- and posttransplant, HbAS, and HbAA. (B) Fractional RBC survival curves for each cohort as compared to the larger group are highlighted in red, including HbSS vs non-HbSS genotypes (HbSC and HbSβ+) in the pre-HSCT cohort, and full DMC vs mixed chimerism in the post-HSCT cohort.
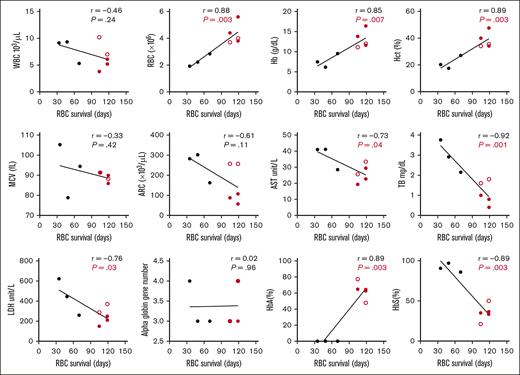
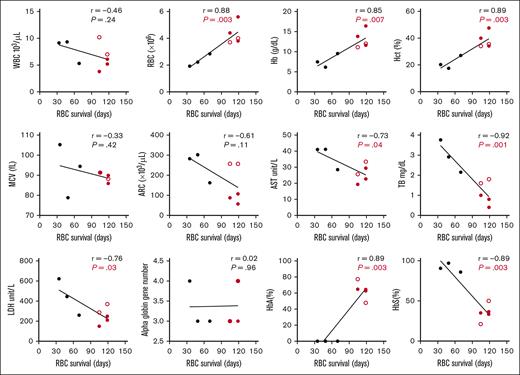
RBC survival in participants with HbSS before and after HSCT (N = 8) was positively correlated with RBC count (P < .01), Hb (P < .01), Hct (P < .01), and %HbA (P < .01), and negatively correlated with %HbS (P < .01), aspartate transaminase (P = .04), total bilirubin (P < .01), and LDH (P = .03) (Figure 2). ARC was negatively correlated with RBC survival in the full cohort (N = 20) (supplemental Figure 2), but lost significance when comparing participants with HbSS before and after HSCT (Figure 2). Correlation of RBC survival to measurable hematologic parameters in all participants with SCD before HSCT (N = 6) is shown in supplemental Figure 3. There was no significant correlation of hematologic parameters with RBC survival in the participants with SCD after HSCT (N = 5).
Correlation of RBC survival to measurable hematologic parameters in participants with HbSS pre- vs posttransplant. Hematologic parameters were measured with each sample throughout the study and correlated to the final RBC survival (days). AST, aspartate transaminase; Hb, hemoglobin; Hct, hematocrit; HbA, adult hemoglobin; HbS, sickle hemoglobin; MCV, mean corpuscular volume; TB, total bilirubin; WBC, white blood cell count. Black, HbSS pretransplant; Red, HbSS posttransplant ( mixed chimerism;
mixed chimerism;  full-donor chimerism).
full-donor chimerism).
Correlation of RBC survival to measurable hematologic parameters in participants with HbSS pre- vs posttransplant. Hematologic parameters were measured with each sample throughout the study and correlated to the final RBC survival (days). AST, aspartate transaminase; Hb, hemoglobin; Hct, hematocrit; HbA, adult hemoglobin; HbS, sickle hemoglobin; MCV, mean corpuscular volume; TB, total bilirubin; WBC, white blood cell count. Black, HbSS pretransplant; Red, HbSS posttransplant ( mixed chimerism;
mixed chimerism;  full-donor chimerism).
full-donor chimerism).
Enrichment in a non-HbS Hb prolongs RBC survival
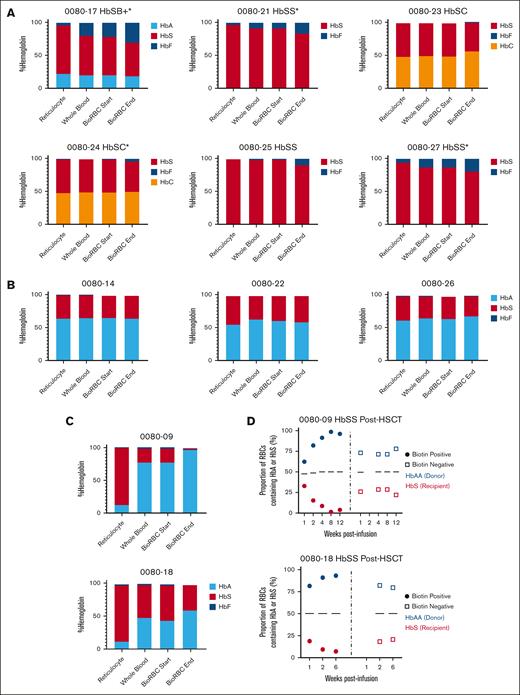
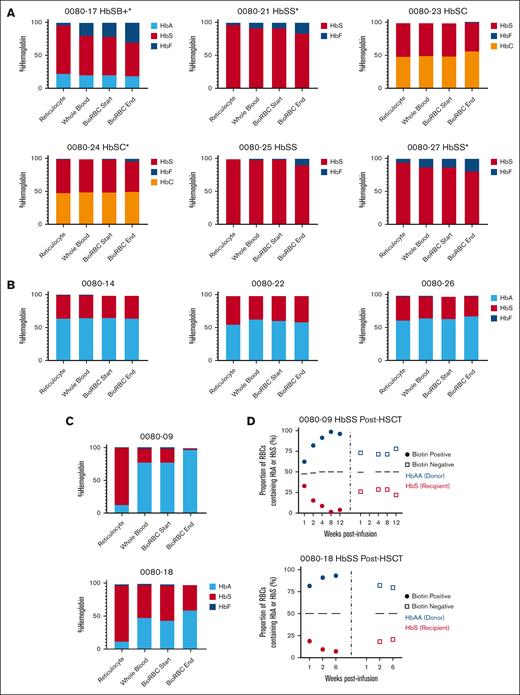
Participants with SCD before HSCT demonstrated an enrichment in %HbF (participants with HbSS or HbSβ+) or %HbC (participants with HbSC), and a decline in %HbS in surviving BioRBCs as compared with whole-blood high performance liquid chromatography (Figure 3A). There was no difference in %HbA or %HbS in BioRBCs vs whole blood in participants with SCD after HSCT who had full DMC (Figure 3B); however, those with mixed chimerism demonstrated a decline in %HbS and an enrichment in %HbA in surviving BioRBCs (Figure 3C) which was corroborated by single-cell Western (Figure 3D). F-cells were enriched over time in all participants with SCD before transplantation (supplemental Figure 4). The difference in F-cell percentage in the surviving BioRBCs compared with whole blood in participants with SCD before HSCT was significantly higher in participants who were on HU (P < .001).
Change in hemoglobin content in surviving BioRBCs compared with whole blood. (A) Change in % sickle, fetal, or C hemoglobin as performed by high performance liquid chromatography in the participants with SCD pretransplant in reticulocytes, whole blood, and the biotin-positive fraction sorted by flow cytometry at the time of product infusion and near the end of the RBC lifespan. (B-C) Change in % sickle or adult hemoglobin in the participants with SCD who underwent HSCT and had full DMC (B) or mixed chimerism (C). (D) Single cell Western analysis demonstrating single cell proportion of HbA to HbS of the biotin-positive fraction after sorting compared to the biotin negative fraction on the 2 participants with HbSS post-HSCT with mixed donor chimerism. ∗Participants on HU.
Change in hemoglobin content in surviving BioRBCs compared with whole blood. (A) Change in % sickle, fetal, or C hemoglobin as performed by high performance liquid chromatography in the participants with SCD pretransplant in reticulocytes, whole blood, and the biotin-positive fraction sorted by flow cytometry at the time of product infusion and near the end of the RBC lifespan. (B-C) Change in % sickle or adult hemoglobin in the participants with SCD who underwent HSCT and had full DMC (B) or mixed chimerism (C). (D) Single cell Western analysis demonstrating single cell proportion of HbA to HbS of the biotin-positive fraction after sorting compared to the biotin negative fraction on the 2 participants with HbSS post-HSCT with mixed donor chimerism. ∗Participants on HU.
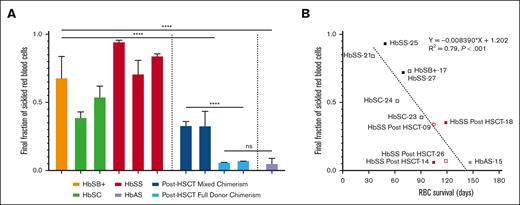
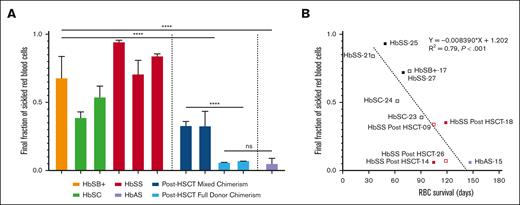
The final fraction of sickled RBCs after deoxygenation strongly correlates with RBC lifespan
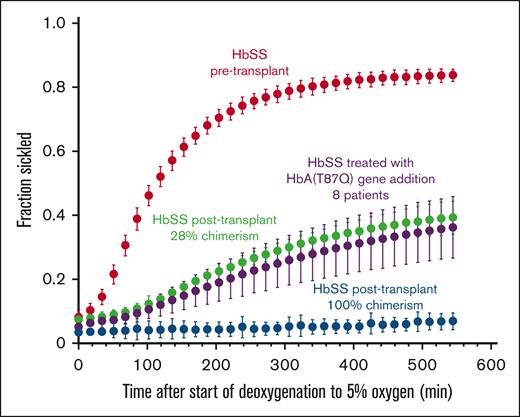
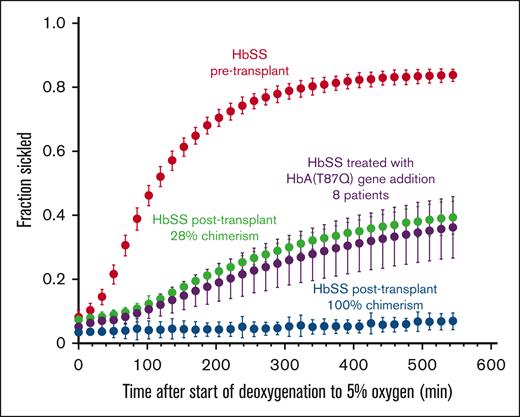
Figure 4 shows plots of the fraction sickled vs time after the start of deoxygenation to 5% oxygen for a participant with HbSS before transplant, a participant with HbSS before transplantation with 28% DMC, and a participant with HbSS after transplantation with 100% DMC, in addition to previously reported average for 8 participants treated with HbAT87Q globin addition.17 The sickling curve for the patient after transplantation with 100% chimerism is the same as sickling curves for individuals with HbAS trait, who also show essentially no sickling in the assay. We describe the sickling curves with a single parameter—the fraction sickled at the end of the 9 hours of deoxygenation to a final oxygen percentage of 5% (38 torr). The average final fraction of sickled RBCs after deoxygenation was much higher in participants with SCD before HSCT (0.66 ± 0.19) compared with those with SCD after HSCT (0.24 ± 0.14, P < .001), and HbAS (0.06 ± 0.02, P < .001) (Figure 5A) and was strongly negatively correlated with RBC survival (Figure 5B). The fraction of sickled RBCs from whole blood were much higher in participants with SCD after HSCT with mixed chimerism compared with those with full-donor chimerism (0.33 ± 0.08 vs 0.06 ± 0.02, P < .001) owing to the continued presence of recipient RBCs. There was no difference in fraction of sickled RBCs in those with full DMC and those with HbAS (0.06 ± 0.02 vs 0.06 ± 0.02, ns). There was no correlation to levels of 2,3-DPG, ATP, or pyruvate kinase protein to RBC survival in the full cohort or in the cohort with SCD (supplemental Figure 5).
Representative sickling curves. The fraction sickled vs time after the start of deoxygenation with nitrogen to 5% oxygen is shown for blood samples from: 1 participant with HbSS pretransplant (red points) (0080-27), 1 participant with HbSS posttransplant with 28% donor chimerism (green points) (0080-18), 1 participant with HbSS posttransplant with 100% donor chimerism (blue points) (0080-26), and the average for 8 participants treated with HbAT87Q globin addition (purple points) as previously published.17 The error bars represent 1 standard deviation from the average value at each time point for the 8 patients and are primarily due to differences in chimerism among the patients and are not the result of error in the experimental measurements or variation of the sickling curves for the individual patients. Only curves for the patients following 6 months or more after transplant were included, because it requires 6 months for the hemoglobin composition of the whole blood to stabilize.
Representative sickling curves. The fraction sickled vs time after the start of deoxygenation with nitrogen to 5% oxygen is shown for blood samples from: 1 participant with HbSS pretransplant (red points) (0080-27), 1 participant with HbSS posttransplant with 28% donor chimerism (green points) (0080-18), 1 participant with HbSS posttransplant with 100% donor chimerism (blue points) (0080-26), and the average for 8 participants treated with HbAT87Q globin addition (purple points) as previously published.17 The error bars represent 1 standard deviation from the average value at each time point for the 8 patients and are primarily due to differences in chimerism among the patients and are not the result of error in the experimental measurements or variation of the sickling curves for the individual patients. Only curves for the patients following 6 months or more after transplant were included, because it requires 6 months for the hemoglobin composition of the whole blood to stabilize.
Fraction of sickle RBCs at the end of deoxygenation. (A) The average final fraction of sickled RBCs based on genotype is shown. Whole-blood samples from participants with SCD pre- and posttransplantation were deoxygenated with nitrogen to 5% oxygen. (B) The average final fraction of sickled RBCs from whole blood is compared with the measured RBC survival in days. Samples from 1 posttransplant participant with full DMC only underwent deoxygenation to 0% oxygen and are not included. Samples from 1 participant with HbAS that underwent deoxygenation with 5% oxygen is included. Open symbols represent participants with single alpha globin gene deletion.
Fraction of sickle RBCs at the end of deoxygenation. (A) The average final fraction of sickled RBCs based on genotype is shown. Whole-blood samples from participants with SCD pre- and posttransplantation were deoxygenated with nitrogen to 5% oxygen. (B) The average final fraction of sickled RBCs from whole blood is compared with the measured RBC survival in days. Samples from 1 posttransplant participant with full DMC only underwent deoxygenation to 0% oxygen and are not included. Samples from 1 participant with HbAS that underwent deoxygenation with 5% oxygen is included. Open symbols represent participants with single alpha globin gene deletion.
Discussion
Biotin labeling of RBCs is a safe and feasible methodology to evaluate RBC survival in patients with SCD before and after HSCT. Here, we demonstrate the safety and feasibility of this approach in patients with SCD and confirm that RBC lifespan strongly correlates to the final fraction of sickled RBCs after deoxygenation and separates symptomatic from asymptomatic individuals. We included several novel groups in which biotin labeling has not been done; in patients with SCD after HSCT, and in participants with SCT who often serve as donors for allogeneic transplantation in whom only assays with radiolabeled 51Cr has been done,4 and in patients with SCD with genotypes other than homozygous HbSS. Our results confirm a shorter RBC half-life in participants with HbSS and was consistent with previous reports using cohort or population-based methodologies.5,11,12
Studies have shown 20% DMC is sufficient to reverse the sickle phenotype after allogenic HSCT because of longer donor red-cell survival; however, not all patients experience full benefit after allogeneic HSCT with this DMC. The clinic variability at 20% DMC suggests differences in recipient RBC lifespan, and as shown here, variable enrichment in non-HbS, antisickling Hb (HbF, HbC, or HbA). Biotin labeling of RBCs after allogeneic HSCT for SCD is a safe and feasible methodology to directly measure RBC survival in order to characterize how RBC populations and Hb fractions may differ after transplantation in which DMC may differ. Additionally, because one-time autologous gene therapies continue to be developed for SCD, these characterization data could inform minimally sufficient gene modification rates necessary to reduce sickling and achieve a clinical benefit, akin to DMC.
RBC lifespan can be correlated to hematologic parameters as well as complexed with additional characterization parameters to better understand factors that influence or predict RBC lifespan. Here, we were able to show a clear negative correlation of RBC lifespan with the percentage of RBC sickling in our assay, an important variable in determining the severity of the various sickle syndromes.18 Two participants with mixed chimerism after HSCT (DMC 17% and 28%) demonstrated RBC lifespans that were similar to participants after HSCT with full DMC despite having a statistically higher final fraction sickled RBCs after deoxygenation. Based on this study and the results for patients who are asymptomatic after treatment with globin addition of HbAT87Q,19 there may be a narrow range of final fraction sickled RBCs that divides asymptomatic and symptomatic individuals, and may be a critical target requirement for current and future therapeutic interventions. Of note, participant 0080-23 is a participant with HbSC before HSCT who has had no SCD-related complications and had a similarly low fraction of sickled RBCs after deoxygenation in this study as those who are asymptomatic with mixed chimerism, again suggesting a potential target requirement of a gene therapy product to best predict a clinically meaningful outcome if within or below the target final fraction of sickled RBCs after deoxygenation.
Currently there are multiple ongoing clinical trials investigating various methodologies for gene therapy in patients with SCD.20 Early results from the first group of patients with SCD treated with gene therapy (NCT02140554 using LentiGlobin BB305 [bb1111, lovotibeglogene autotemcel])(group A, n = 7) demonstrated low peripheral blood vector copy numbers and HbAT87Q production with minimal clinical benefit given sustained stress erythropoiesis.21,22 Subsequent improvements to the trial design were made, many of which have been implemented across clinical trials for gene therapy in SCD.23 Such improvements for those who have received LentiGlobin BB305 (bb1111, lovotibeglogene autotemcel)(group C; N = 35) now demonstrate high and sustained near-pancellular expression of HbAT87Q that was >40% of total Hb by 6 months after treatment.19 Clinically there was a reduction in HbS, reduction in the propensity of RBCs to sickle,17 and resolution of severe vaso-occlusive events. Although total bilirubin, LDH, and haptoglobin levels normalized, ARCs improved but did not normalize suggesting ongoing host-derived hemolysis from uncorrected or under corrected RBCs. Despite this, previous data suggest fraction sickled RBCs in patients with HbAT87Q are near those observed in this study in participants with SCD after HSCT with mixed-donor chimerism and similarly remain clinically asymptomatic,17 suggesting the feasibility of identifying a target chimerism or gene modification level that results in decreased sickling, improved RBC lifespan, and a lack of clinical symptoms after HSCT. Our sickling assay, or similar, may therefore have the sensitivity to predict clinically meaningful resolution of symptoms after autologous gene therapy.
Improved but continued hemolysis is described in other clinical gene therapy studies,24,25 and may vary in trials that target HbF induction based on F cell and HbF percentage.25,26 HbF induction is the primary target in gene editing trials to date given the protective antisickling property of HbF27 and the ability to easily target genes that regulate HbF expression. The blood concentration of HbF, or the number of cells with detectable HbF (F-cells), is not representative, however, of the amount of HbF per cell. The distribution of HbF can vary such that a proportion of F-cells have insufficient concentrations of HbF to inhibit HbS polymerization.18 F-cells are measured by flow cytometry and can detect cells with around 6 pg of HbF, but deoxyHbS polymerization is prevented at physiologic venous and capillary O2 saturations of 40% to 70% when HbF/F cell is 9 to 12 pg.28 When HbF levels are ∼30%, as is commonly seen in patients with HbS-hereditary persistence of HbF, the number of protected cells approaches 70% and a longer delay time is observed.17,29 However, when HbF levels fall <30%, the distribution of HbF per F cell can vary greatly even if the mean HbF is constant. Unlike the heterocellular distribution and variable clinical response observed with HU,30 HbF induction after gene editing is more likely to be pancellular when editing efficiencies are high and engraftment is achieved after myeloablation. If the distribution is heterocellular and patients continue to have hemolysis and/or vaso-occlusive events after HbF gene editing, combination treatment with drugs that might broaden the distribution of F cells or increase the amount of HbF in unprotected F cells with HbF <10 pg per cell, such as with HU, might be the most appropriate therapy to allow sustained, protective HbF induction. The goal, however, should be to maximize HbF per F cell after editing rather than relying on mean HbF or F-cell percentage as a target outcome measure because it erroneously assumes that all F cells contain the same amount of HbF. Biotin labeling after gene therapy is a feasible method to answer questions surrounding RBC lifespan after genetic modification of HSCs, including if the therapy results in pancellular or heterocellular distribution of therapeutic protein and, if heterocellular, what threshold will have an adequate reduction in sickling after deoxygenation to reverse clinical phenotype.
Enrichment in a non-HbS, antisickling Hb (HbF, HbC, or HbA) prolongs RBC survival as demonstrated in this pilot study of patients with SCD before and after HSCT. Understanding the variability and predictors of RBC survival using this method may inform future allogeneic HSCT and gene therapy protocols by determining a target chimerism or gene modification level that sufficiently reduces polymerization and therefore sickling needed to reverse the SCD phenotype because it relates directly to RBC survival. Such understanding can better inform patients and providers after allogeneic HSCT with falling donor chimerism and set important standards for current and future gene therapy protocols, in order to offer patients an optimized therapy with a maximized chance of being one-time, sustained, and curative.
Acknowledgments
The authors thank John Widness and Jose Cancelas-Perez for their support in guiding this trial, given their expertise in biotinylation of RBCs. The authors thank Jose Cancelas-Perez for allowing the team to cross reference his previously submitted investigational new drug for the use of biotin in humans. The authors thank Guohua An and Demet Nalbant for providing the positive anti-biotin antibody control plasma. The authors thank Paige Hadley for analyzing samples. This trial was funded in a collaborative agreement between the National Institutes of Health and bluebird bio, Inc.
Authorship
Contribution: A.L., D.F., M.M.H., Z.I., C.L., and J.F.T. conducted the clinical trial; A.L., M.B., F.J.P., E.R.M., and J.F.T. designed the research; A.L., D.F., Z.I., R.C., K.E., S.D., B.G., M.H., J.D., Q.L., W.A.E., T.C., X.W., and S.L.T. analyzed samples; A.L., Z.I., T.C., M.B., F.J.P., E.R.M., and S.V. analyzed results; A.L., Z.I., Q.L., W.E., M.B., F.J.P., and S.V. made the figures; A.L. wrote the manuscript; D.F., M.M.H., Z.I., R.C., K.E., S.D., Q.L., W.E., X.W., S.L.T, E.R.M., S.V.N., M.B., F.J.P., and J.F.T. contributed to writing the manuscript.
Conflict-of-interest disclosure: S.V., M.B., and F.J.P. are employees and shareholders of bluebird bio, Inc. E.R.M. was an employee and shareholder of bluebird bio, Inc, at the time this work was completed. The remaining authors declare no competing financial interests.
Correspondence: John F. Tisdale, Cellular and Molecular Therapeutics Branch, National Insitutes of Health, 10 Center Drive, Bethesda, MD 20814; email: johntis@mail.nih.gov.
References
Author notes
Deidentified participant data are available upon reasonable request from the corresponding author, John F. Tisdale (johntis@mail.nih.gov).
The full-text version of this article contains a data supplement.



 mixed chimerism;
mixed chimerism;  full-donor chimerism).
full-donor chimerism).