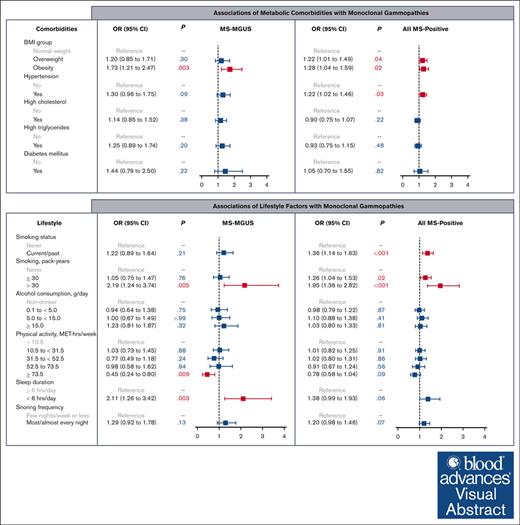
Among individuals at elevated risk of multiple myeloma, obesity is positively associated with mass spectrometry–detected MGUS.
High physical activity is inversely associated with MGUS, whereas heavy smoking and short sleep are positively associated with MGUS.
Visual Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant condition of multiple myeloma with few known risk factors. The emergence of mass spectrometry (MS) for the detection of MGUS has provided new opportunities to evaluate its risk factors. In total, 2628 individuals at elevated risk for multiple myeloma were enrolled in a screening study and completed an exposure survey (PROMISE trial). Participant samples were screened by MS, and monoclonal proteins (M-proteins) with concentrations of ≥0.2 g/L were categorized as MS-MGUS. Multivariable logistic models evaluated associations between exposures and MS outcomes. Compared with normal weight (body mass index [BMI] of 18.5 to <25 kg/m2), obesity (BMI of ≥30 kg/m2) was associated with MS-MGUS, adjusting for age, sex, Black race, education, and income (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.21-2.47; P = .003). High physical activity (≥73.5 metabolic equivalent of task (MET)-hours per week vs <10.5 MET-hours per week) had a decreased likelihood of MS-MGUS (OR, 0.45, 95% CI, 0.24-0.80; P = .009), whereas heavy smoking and short sleep had increased likelihood of MS-MGUS (>30 pack-years vs never smoker: OR, 2.19; 95% CI, 1.24-3.74; P = .005, and sleep <6 vs ≥6 hours per day: OR, 2.11; 95% CI, 1.26-3.42; P = .003). In the analysis of all MS-detected monoclonal gammopathies, which are inclusive of M-proteins with concentrations of <0.2 g/L, elevated BMI and smoking were associated with all MS-positive cases. Findings suggest MS-detected monoclonal gammopathies are associated with a broader range of modifiable risk factors than what has been previously identified. This trial was registered at www.clinicaltrials.gov as #NCT03689595.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant clonal proliferation of plasma cells that consistently precedes the development of multiple myeloma (MM).1 MGUS is not universally screened for in routine clinical practice, as it is unclear what groups would benefit clinically from enhanced surveillance.2,3
Indeed, only a few risk factors have been implicated in MGUS development, including older age, male sex, Black or African American race, and family history.4 Although obesity is a well-established, potentially modifiable risk factor for MM,5 its association with MGUS has been less consistent.6-8 One of the earliest studies to report a positive association observed a twofold higher risk of MGUS among obese than nonobese women.6 Although a subsequent study based on a large primary care database also found a positive association between obesity and MGUS, the association was no longer statistically significant after adjusting for the number of laboratory tests before MGUS diagnosis, possibly indicating that the association may be influenced by the higher frequency of medical evaluations among obese individuals, which increases their chance of being incidentally diagnosed with MGUS.8 Additionally, studies evaluating other potentially modifiable risk factors, such as obesity-related comorbidities and lifestyle factors, for MGUS are limited.4,6,8
Furthermore, to date, nearly all studies have used serum protein electrophoresis supplemented by immunofixation (SPEP/IFX), which have been the primary methods for identifying monoclonal proteins (M-proteins) for >40 years. However, mass spectrometry (MS)-based approaches have allowed for the detection and quantification of M-proteins with superior sensitivity,9-11 revealing that the prevalence of MGUS may be 2 to 3 times higher than previously estimated with SPEP/IFX.9 Recent MS-based screening studies have observed that traditional myeloma risk factors, such as older age, male sex, Black race, and family history of hematological malignancies, are associated with MGUS.9,11 We aimed to build upon these findings by investigating novel, potentially modifiable risk factors for this condition.
In the current study, therefore, we investigated obesity, obesity-related comorbidities, and lifestyle factors and their associations with the prevalence of MGUS in a US nationwide study of individuals at high risk for MM, screened by MS.
Methods
PROMISE study cohort
The Predicting Progression of Developing Myeloma in a High-Risk Screened Population (PROMISE, NCT03689595) is the first US nationwide screening study for individuals at high risk for MM. As described previously,9 individuals were eligible to participate if they self-identified as: (1) Black or of African descent; or (2) non-Black but have a family history of hematological malignancy, or MM precursor condition. Enrollment began at age 30 years, but for individuals with ≥2 first-degree family members with hematological malignancy or MM precursor condition, enrollment eligibility started at age 18 years. Individuals with a prior diagnosis of MGUS, smoldering multiple myeloma, MM, Waldenström macroglobulinemia, and/or other malignancies requiring active therapy were excluded.
Enrollment occurred either online or at participating study sites. Written informed consent was obtained from participants upon recruitment. Participants received a screening kit at their home addresses, and serum samples were drawn at local phlebotomy sites. All participants were invited to complete an exposure assessment survey querying sociodemographic, comorbidity, and lifestyle factors.
This study was conducted upon approval of protocol 18-370 by the institutional review board of the Dana-Farber Cancer Institute. Individual participant data, including data dictionaries, are not available for sharing, in accordance with the PROMISE study protocol (PROMISE, NCT03689595).
Measurement of monoclonal proteins
Serum samples underwent SPEP/IFX and serum free light chain testing in a Clinical Laboratory Improvement Amendments–approved environment. Remaining serum (500 μL) were used for MS testing with the EXENT system (The Binding Site Group Ltd, Birmingham, UK). The system uses matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) MS for the identification of M-proteins and the Optilite free light for polyclonal immunoglobulin G (IgG), IgA, IgM, and serum free light chains. The EXENT-iQ software was used for quantitative analysis of M-proteins. The lower limit of measurable interval for M-proteins by MALDI-TOF MS was 0.015 g/L.9
MS-detected MGUS (MS-MGUS) was defined by the presence of M-protein with a concentration of ≥0.2 g/L, as quantified by MALDI-TOF MS. This definition is based on work (previously described by El-Khoury et al.9) that determined the lower limit of M-protein detection for SPEP/IFX by comparing SPEP/IFX and MALDI-TOF results from PROMISE study samples and separate serial dilution sensitivity experiments. The aforementioned study demonstrated that >99% of M-proteins concomitantly detected by both SPEP/IFX and MALDI-TOF MS had concentrations of ≥0.2 g/L. It also demonstrated the superior sensitivity of MALDI-TOF MS, compared with SPEP/IFX, in being able to detect a larger pool of M-proteins, even at, or above, the 0.2 g/L concentration threshold.
All MS-detected monoclonal gammopathies (MS-positive cases) were defined as the presence of M-proteins detected by MS at any concentration level, including those <0.2 g/L.
Assessment of height, weight, and body mass index (BMI)
Participants reported their height and weight. BMI was calculated as weight (kilograms) divided by height (meters) squared (kg/m2). BMI was categorized into underweight (<18.5 kg/m2), normal weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2).5
Assessment of smoking, alcohol, physical activity, and sleep factors
Survey questions assessing lifestyle factors were adapted from validated questionnaires used in other large prospective cohorts.12-14 Specifically, smoking history was assessed by querying whether participants ever smoked ≥20 packs of cigarettes in their lifetime. If ever smokers, participants reported the average number of cigarettes smoked per day across age categories. Smoking pack-years, defined as smoking 20 cigarettes per day for 1 year, were calculated by multiplying the number of cigarette packs smoked per day by the number of years of smoking. Among ever smokers, ≤30 pack-years was categorized as light-to-moderate smoking, and >30 pack-years, as heavy smoking.
Alcohol consumption was assessed by asking participants how often, on average, they consumed regular and light beer (1 glass, bottle, or can), red and white wine (5 oz glass), and liquor (1 drink or shot) over the previous year.13 Average alcohol consumption (grams per day) was calculated as the sum of daily drinks multiplied by average alcohol content in the type of drink (beer, 12.8 g per 12-oz serving; light beer, 11.3 g per 12-oz serving; wine, 13.75 g per 5-oz serving; and liquor, 14.0 g per serving).13 Participants were grouped into 4 categories of average alcohol consumption: nondrinker, light (0.1 to <5.0 g per day), moderate (5.0 to <15.0 g per day), and high-to-heavy (≥15.0 g per day).
For physical activity, participants reported average weekly time spent engaging in specified recreational activities: walking, running/jogging, bicycling (low, medium, or high intensity), lap swimming, tennis/racket sports, aerobics/calisthenics, lower intensity exercise (e.g., yoga and stretching), vigorous exercise (e.g., lawn mowing), and weight training/resistance exercise.14 Type, intensity, and duration of reported activities were used to calculate the total metabolic equivalent of task (MET)-hours per week.15 MET-hours per week were categorized into <10.5, 10.5 to <31.5, 31.5 to <52.5, 52.5 to <73.5, and ≥73.5 intervals, which correspond to walking <30 minutes per day, 30 minutes per day to <1.5 hour per day, 1.5 to <2.5 hours per day, 2.5 to <3.5 hours per day, and ≥3.5 hours per day (or running/jogging 45-60 minutes per day or more), respectively.
Other covariates
Participants reported sociodemographic information, including age, sex, race, highest education level attained, and annual family income. For comorbidities, participants reported whether a clinician ever diagnosed them with obesity-related comorbidities, specifically hypertension, high cholesterol, high triglycerides, and diabetes mellitus.
Statistical analyses
Multivariable logistic regression models evaluated the associations of obesity, obesity-related comorbidities, and lifestyle factors with the odds of MS-MGUS. In likeness to our prior study,9 the referent population was defined as individuals who screened negative by MS. Models adjusted for sociodemographic covariates of age (continuous variable), sex (male vs female), Black race (Black vs other race), annual family income (≥$50 000, <$50 000, and “don’t know”/declined), and education (college graduate vs non–college graduate). Participants with missing value(s) for any variable included in a model (i.e., no response was provided) were excluded from that respective model.
Given close associations between obesity and other comorbidities (hypertension, high cholesterol, high triglycerides, and diabetes), an extended model additionally adjusted for all metabolic comorbidities to evaluate the independent association of obesity with MS-MGUS. Additional models evaluating physical activity and sleep as the exposures further adjusted for BMI group, given the association between these exposures and BMI.5,17,18
Based on a priori hypotheses that the association of obesity and MGUS may vary by sex,19,20 we assessed the interaction between BMI group and sex in a model that evaluated the main effects of the 2 variables and their cross-product term, adjusting for sociodemographic covariates. Similarly, we assessed the interaction between BMI group and physical activity.
In exploratory analysis, models evaluating metabolic comorbidities, physical activity, and sleep factors, the exposures were adjusted for smoking pack-years and alcohol consumption, in addition to sociodemographic covariates, given reported associations of smoking and alcohol with these exposures21,22 as well as various cancers.23
A prior study by Vachon et al.,11 evaluated the association of risk factors (Black race and family history) for all MS-positive cases, which included M-proteins at concentrations of <0.2 g/L. In this study, therefore, all models were replicated using all MS-positive cases, which are inclusive of low-level M-proteins (<0.2 g/L), as the outcome. Finally, in supplemental analysis, multivariable models evaluated associations of exposures with the prevalence of MGUS detected by SPEP/IFX (SPEP/IFX-MGUS), because SPEP/IFX is the most widely available test used in routine practice. The referent group for those models were individuals who screened negative by SPEP/IFX.
Results
Between February 2019 and March 2022, 3180 individuals were screened in the PROMISE study, and a total of 2628 participants completed the exposure assessment survey (83% response rate) and comprised our final analytic cohort. Participants were from all 50 US states and the District of Columbia. MS-MGUS was primarily IgG isotype (64%), followed by IgM (20%) and IgA (16%). The median M-protein concentration was 0.63 g/L (interquartile range, 0.30-1.76). The prevalence of MS-MGUS was 9% in the total cohort (Table 1).
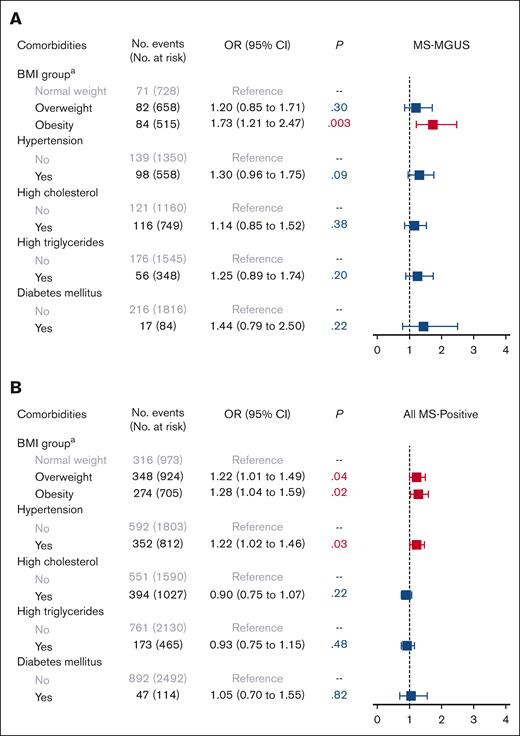
In multivariable logistic models adjusted for age, sex, Black race, education, and income, we observed an association of obesity with MS-MGUS, as compared with normal weight (Figure 1). Specifically, being obese was associated with a 73% higher odds of MS-MGUS, relative to individuals with normal weight (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.21-2.47; P = .003). In an extended model that further adjusted for all other metabolic comorbidities, the association between obesity and MS-MGUS remained statistically significant (OR, 1.57; 95% CI, 1.07-2.29; P = .02; model not shown). Adjustment of smoking pack-years and alcohol consumption in addition to sociodemographic variables did not meaningfully change the association of obesity with MS-MGUS (OR, 1.76; 95% CI, 1.23-2.53; P = .002). The association between obesity and MS-MGUS was not modified by sex or physical activity level (both Pinteraction > .05).
Association of metabolic comorbidities with monoclonal gammopathies. Multivariable logistic regression models for (A) MS-MGUS and (B) all MS-positive monoclonal gammopathies, adjusted for sociodemographic variables of age (continuous variable), sex, Black race, education, and income. The referent population for all models was defined as individuals who screened negative by MS. aBMI group: normal weight refers to BMI of 18.5 to <25 kg/m2; overweight, BMI of 25 to <30 kg/m2; and obesity, BMI of ≥30 kg/m2. Underweight (BMI < 18.5 kg/m2) is not provided in the figure because of the small number of individuals in this group leading to uninterpretable results. MS-MGUS, defined as M-protein concentration of ≥0.2 g/L); MS-positive defined as MS-detected monoclonal gammopathies across all M-protein concentration levels.
Association of metabolic comorbidities with monoclonal gammopathies. Multivariable logistic regression models for (A) MS-MGUS and (B) all MS-positive monoclonal gammopathies, adjusted for sociodemographic variables of age (continuous variable), sex, Black race, education, and income. The referent population for all models was defined as individuals who screened negative by MS. aBMI group: normal weight refers to BMI of 18.5 to <25 kg/m2; overweight, BMI of 25 to <30 kg/m2; and obesity, BMI of ≥30 kg/m2. Underweight (BMI < 18.5 kg/m2) is not provided in the figure because of the small number of individuals in this group leading to uninterpretable results. MS-MGUS, defined as M-protein concentration of ≥0.2 g/L); MS-positive defined as MS-detected monoclonal gammopathies across all M-protein concentration levels.
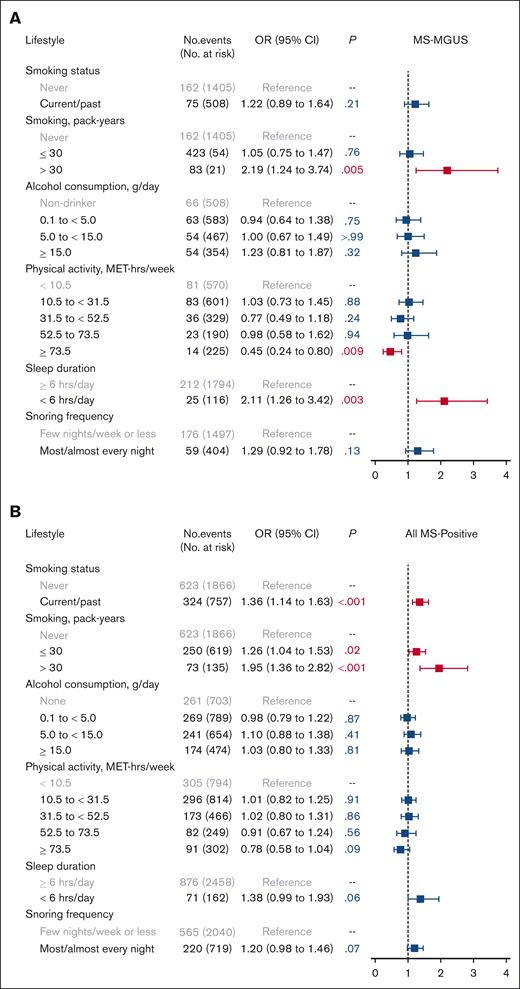
In evaluating lifestyle factors (Figure 2), heavy smoking (>30 pack-years), compared with never smoking, was associated with MS-MGUS (OR, 2.19; 95% CI, 1.24-3.74; P = .005). High levels of physical activity at ≥73.5 MET-hours per week (equivalent to running/jogging 45-60 minutes per day or more), compared with the lowest level of <10.5 MET-hours per week (equivalent to walking <30 minutes per day), was inversely associated with MS-MGUS (OR, 0.45; 95% CI, 0.24-0.80; P = .009). This association was not meaningfully different after adjustment for BMI group (OR, 0.51; 95% CI, 0.27-0.91; P = .03) or adjustment for smoking pack-years and alcohol consumption (OR, 0.46; 95% CI, 0.24-0.82; P = .01; models not shown). Furthermore, short sleep (<6 hours per day), compared with sleeping ≥6 hours per day, was associated with MS-MGUS (OR, 2.11; 95% CI, 1.26-3.42; P = .003). This association remained statistically significant after additionally adjusting for BMI group (OR, 2.06; 95% CI, 1.23-3.33; P = .004) or adjusting for smoking pack-years and alcohol consumption (OR, 2.10; 95% CI, 1.25-3.42; P = .004; models not shown).
Association of lifestyle factors with monoclonal gammopathies. Multivariable logistic regression models for (A) MS-MGUS and (B) all MS-positive monoclonal gammopathies, adjusted for sociodemographic variables of age (continuous variable), sex, Black race, education, and income. The referent population for all models was defined as individuals who screened negative by MS. MS-MGUS, defined as M-protein concentration of ≥0.2 g/L); MS-positive defined as MS-detected monoclonal gammopathies across all M-protein concentration levels.
Association of lifestyle factors with monoclonal gammopathies. Multivariable logistic regression models for (A) MS-MGUS and (B) all MS-positive monoclonal gammopathies, adjusted for sociodemographic variables of age (continuous variable), sex, Black race, education, and income. The referent population for all models was defined as individuals who screened negative by MS. MS-MGUS, defined as M-protein concentration of ≥0.2 g/L); MS-positive defined as MS-detected monoclonal gammopathies across all M-protein concentration levels.
In the analysis of all MS-positive monoclonal gammopathies at any M-protein concentration level, the prevalence of having any detectable monoclonal protein was 36% (Table 1). The isotype was predominantly IgM (58%), followed by IgG (25%) and IgA (16%). In multivariable models evaluating obesity and obesity-related comorbidities as the exposures (Figure 1), elevated BMI (both overweight and obese) and hypertension were associated with MS positivity. We did not observe any evidence of effect modification by sex or physical activity in the association between BMI group and MS positivity (both Pinteraction > .05). As for lifestyle factors (Figure 2), being a current/past smoker, light-to-moderate smoking, and heavy smoking were associated with MS positivity.
In our supplemental analysis of SPEP/IFX-detected MGUS, the prevalence of MGUS was 4% (supplemental Table 2). In models of SPEP/IFX-MGUS (supplemental Figure 3), obesity and obesity-related comorbidities were not associated with SPEP/IFX-MGUS, nor did we observe any evidence of effect modification by sex or physical activity in the association between BMI group and SPEP/IFX-MGUS (both Pinteraction > .05). Furthermore, associations of lifestyle factors with SPEP/IFX-MGUS did not reach statistical significance after adjustment for sociodemographic covariates (supplemental Figure 4).
Discussion
In this study, we observed that obesity, heavy smoking, and short sleep were associated with an increased likelihood of screening positive for MS-MGUS, whereas high physical activity levels were associated with a decreased likelihood. In the analysis of all MS-detected monoclonal gammopathies, elevated BMI and smoking were also associated with all monoclonal gammopathies detected by MS, including at wider ranges of exposure levels (i.e., overweight and obesity for BMI group; light-to-moderate and heavy levels for smoking pack-years). These results are the first to be reported for MS-detected monoclonal gammopathies, providing important preliminary insights into their risk factors.
Prior studies on the association between obesity and MGUS have yielded inconsistent results. The population-based Age, Gene/Environment-Susceptibility-Reykjavik Study in Iceland found no associations between MGUS and various surrogate measures of obesity, including BMI, abdominal circumference, and percent body fat.7 However, a smaller study of Black and White women in a population-based Southern Community Cohort Study in the United States showed a positive association between BMI of >30 and excess MGUS risk.6 Differences among study populations may, in part, account for these varying results, including the fact that the Age, Gene/Environment-Susceptibility-Reykjavik Study recruited the surviving members of the original Reykjavik Study who had a mean age of 77 years, which the authors point out may imply selection for a healthier population.7 Our study is comprised of a geographically diverse cohort that includes only individuals at high risk for MM, based on self-reported Black race and/or family history of hematological malignancy. Thus, it is possible that the association of obesity and MGUS depend on baseline predispositions to MGUS. For example, obesity may increase the risk of MGUS in individuals who already may be more susceptible based on family history or African ancestry,24 as have been observed for some cancers.25,26 Further studies are needed to evaluate the interaction of biological and environmental factors in MM and its precursor conditions.
An expert panel convened by the International Agency for Research on Cancer reported compelling evidence for the causal effect of obesity on MM risk.5 Obesity is associated with a chronic state of low-grade inflammation with dysregulated levels of proinflammatory cytokines (e.g., tumor necrosis factor α and interleukin-6) and adipokines (e.g., adiponectin and leptin) that have been linked with myelomagenesis.27,28 These disturbances (e.g., elevated tumor necrosis factor α and decreased adiponectin) may also play a role in the development of obesity-related insulin resistance with altered levels of insulin and insulin-like growth factor 1 signaling pathways that have also been linked to the proliferation and survival of clonal plasma cells.27,28 With some studies, including ours, observing an association between obesity and MGUS, as well as the growing evidence that obesity may play a role in the transformation of MGUS to MM,19,20 it is plausible that biological links between obesity and myelomagenesis apply across the MGUS-to-MM disease continuum.
Physical activity influences the same cytokine and metabolic pathways dysregulated in obesity and posited to affect myelomagenesis.17 Although most but not all prior studies investigating physical activity and MM risk have found nonsignificant but suggestive inverse associations,14,29 we found that high levels of physical activity were inversely associated with MS-MGUS. The inverse association was observed for only the highest levels of physical activity (≥73.5 MET-hours per week, equivalent to running/jogging 45-60 minutes per day or more), which is greater than definitions of high physical activity used in prior investigations of MM and, thus, may partially account for the discrepant results across studies.
The finding that short sleep was associated with an increased likelihood of MS-MGUS was a novel finding and may be explained by the effect of short sleep on reduced melatonin levels and, therefore, their possible antitumorigenic effects, disturbances to the immune system, and dysregulation of metabolic systems related to obesity.18,22 Although there is currently no consensus regarding the influence of sleep duration on cancer risk, evidence formulated by the International Agency for Research on Cancer of the possible link between sleep disorders and onset of cancers has stimulated a growing interest in understanding possible associations and mechanisms.22
Heavy smoking was strongly associated with MS-MGUS and with all MS-detected monoclonal gammopathies. Although smoking has been hypothesized to increase the risk of MGUS via chronic antigenic stimulation, most of the evidence for its association for overt MM has been null.30 There are only a few studies evaluating smoking as a risk factor specifically for MGUS (including those reviewed in Castaneda-Avila et al.),4,31 including 3 studies that observed that ever-smoking status was associated with MGUS4,8,31 and another that showed only heavy smoking (defined in that study as >20 cigarettes per day for >5 years) was associated with MGUS,4 a dose-dependent finding that is largely consistent with our study results.
Our observation that certain risk factors were associated with MS-MGUS but did not reach statistical significance with SPEP/IFX-MGUS in supplemental analysis is hypothesized to be primarily because of the low numbers of MGUS cases identified by SPEP/IFX. MS also allowed for a greater refinement of the referent population by excluding those with M-proteins of low concentrations (<0.2 g/L), thereby offering a finer comparison of participants with MS-MGUS and those who were MS negative. In a recent MS-based screening study using the same definitions of MS-MGUS and referent group, we had observed that traditional myeloma risk factors (older age, male sex, and Black race) were associated with MS-MGUS.9 This study builds upon these findings by identifying novel, potentially modifiable risk factors (obesity, heavy smoking, and short sleep) for MS-MGUS.
Strengths of our study include the use of MS for screening M-proteins, the geographic diversity of our cohort, use of validated exposure measurements,12-14 and ability to adjust for socioeconomic status in models. Limitations include the cross-sectional design of our study, allowing for the possibility of reverse causation between the main exposures and outcomes, as well as the relatively low percentage of Black individuals (6%) in our cohort. Furthermore, the high-risk demographic of our cohort and self-selection of participants into the PROMISE study may affect the generalizability of findings. We also acknowledge that, at the present, the impact of the identified risk factors and MS-MGUS may also be limited, because MS is currently not a test used as standard-of-care in routine medical practice. Larger longitudinal studies are needed to evaluate how exposures influence the development of monoclonal gammopathies and their potential progression to overt MM.
Building on prior investigations, which established a high prevalence of MS-detected MGUS in the US population,9,11 our study of a US-based cohort of individuals at high risk shows associations with novel, potentially modifiable risk factors (obesity, physical activity, heavy smoking, and short sleep duration). These preliminary results will require validation and further investigation toward the effort of identifying groups that may benefit from targeted screening strategies, enhanced surveillance, and precision prevention strategies.
Acknowledgments
This study was funded, in part, by the National Institutes of Health (F32 CA220859, K22 CA251648, R25 AI147393, and R38 HL150212). This research was also supported by a Stand Up To Cancer Dream Team Research grant (grant number: SU2C-AACR-DT-28-18). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of Stand Up To Cancer. Editorial review of this manuscript was provided by Anna Justis, Department of Medical Oncology, Dana-Farber Cancer Institute, as part of her salaried position.
Authorship
Contribution: D.J.L., H.E-K., I.M.G., and C.R.M. designed the study; D.J.L., I.M.G., and C.R.M. oversaw conceptualization; I.M.G. and C.R.M. acquired the funding; H.E-K. processed samples; H.E-K., J-B.A., D.S., D.B., M.C.P., and S.H. acquired and analyzed the MS data; J.P., M.I.D., E.H., I.M.G., and C.R.M. managed the project, recruited participants, and acquired data for the PROMISE study cohort; D.J.L., H.E-K., I.M.G., and C.R.M. interpreted the data; D.J.L., A.C.T., and R.R. did formal data analysis, statistical modeling, and figure visualization; D.J.L., H.E-K., A.C.T., J.P., I.M.G., and C.R.M. had access to and verified the raw data; D.J.L., H.E-K., I.M.G., and C.R.M. were responsible for the decision to submit for publication; D.J.L., H.E-K., and C.R.M. drafted the manuscript; and all authors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: D.S., D.B., and M.C.P. are current employees of The Binding Site. S.H. is a current employee, member of the Board of Directors, and holds patents related to The Binding Site. I.M.G. has served as a consultant for AbbVie, Adaptive, Aptitude Health, BMS, Cellectar, CurioScience, Genentech, Janssen, Janssen Central American and Caribbean, Karyopharm, Medscape, Oncopeptides, Sanofi, Takeda, The Binding Site, Gene Network Sciences Healthcare, and GlaxoSmithKline. Spouse of I.M.G., William Savage, is chief medical officer and equity holder at Disc Medicine. C.R.M. serves as an adviser to Exact Sciences; and is a steering committee member for Natera. The remaining authors declare no competing financial interests.
The current affiliation for H.E.-K. is Department of Internal Medicine, University of Chicago, Chicago, IL.
Correspondence: David J. Lee, Department of Medicine, Massachusetts General Hospital, 55 Fruit Str, Bigelow 740, Boston, MA 02114; email: djlee@mgh.harvard.edu.
References
Author notes
D.J.L and H.E-K. contributed equally to this study.
Presented as a poster at the American Association for Cancer Research Annual Meeting 2022, New Orleans, LA, 13 April 2022; and as an oral presentation at the InterLymph Annual Meeting 2022 (Virtual), 14 June 2022. Data/text/tables/figures in this manuscript are posted in a preprint server (available at SSRN: https://dx.doi.org/10.2139/ssrn.4313707).
Individual participant data, including data dictionaries, are not available for sharing, in accordance with the PROMISE study protocol (PROMISE, NCT03689595). For other forms of data sharing, contact the corresponding author, David J. Lee (djlee@mgh.harvard.edu).
The full-text version of this article contains a data supplement.