Parsaclisib, a highly selective, potent PI3Kδ inhibitor, demonstrated durable responses and an overall manageable safety profile in R/R MZL.
Investigation is needed to determine patients who may benefit from PI3K pathway inhibition as an alternative to standard-care agents used for second-line and beyond.
Visual Abstract
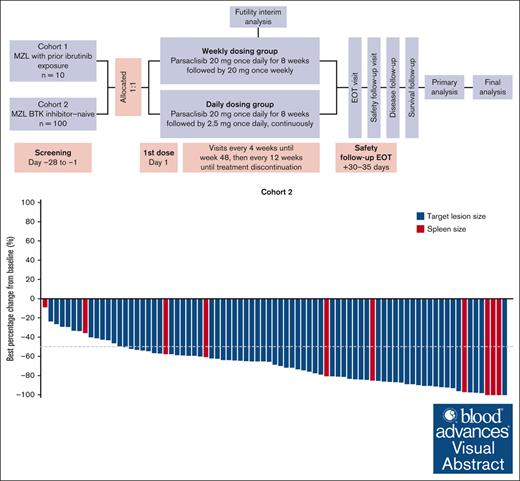
Parsaclisib, a potent and highly selective PI3Kδ inhibitor, has shown clinical benefit in patients with relapsed or refractory (R/R) B-cell lymphomas. The phase 2 CITADEL-204 study (NCT03144674, EudraCT 2017-000970-12) assessed efficacy and safety of parsaclisib in Bruton tyrosine kinase (BTK) inhibitor–experienced (cohort 1) or BTK inhibitor–naive (cohort 2) patients with R/R marginal zone lymphoma (MZL). Patients aged ≥18 years with histologically confirmed R/R MZL, treated with ≥1 prior systemic therapy (including ≥1 anti-CD20 antibody) received parsaclisib 20 mg once daily for 8 weeks then 20 mg once weekly (weekly dosing group [WG]) or parsaclisib 20 mg once daily for 8 weeks then 2.5 mg once daily (daily dosing group [DG]); DG was selected for further assessment. Primary end point of the study was objective response rate (ORR). Owing to slower than expected recruitment, cohort 1 was closed with 10 patients (WG, n = 4; DG, n = 6) enrolled. Based on a planned interim analysis in cohort 2, the futility boundary was not crossed, and enrollment continued to study completion. At data cutoff (15 January 2021), 100 patients were enrolled and treated in cohort 2 (WG, n = 28; DG, n = 72). In the DG, the ORR was 58.3% (95% confidence interval [CI], 46.1-69.8), with a complete response rate of 4.2% (95% CI, 0.9-11.7); the lower bound of the ORR 95% CI exceeded the protocol-defined threshold of 40%. The median duration of response was 12.2 months (95% CI, 8.1-17.5) and progression-free survival was 16.5 months (95% CI, 11.5-20.6); median overall survival was not reached. The most common treatment-emergent adverse events (TEAEs) among all patients were diarrhea (47.0%), cough (23.0%), and rash (18.0%); the most common grade ≥3 TEAEs included diarrhea (12.0%), neutropenia, and pneumonia (9.0% each). TEAEs led to dose interruptions, reductions, and discontinuations in 56.0%, 16.0%, and 29.0% of all patients, respectively. Durable responses and an overall manageable safety profile were demonstrated in patients with R/R MZL treated with parsaclisib monotherapy.
Introduction
Marginal zone lymphoma (MZL), an indolent non-Hodgkin lymphoma (NHL), accounts for 5% to 15% of all NHLs in Western countries.3,4 Treatment options for symptomatic patients include rituximab with or without chemotherapy5; however, patients tend to experience serial relapses and require multiple lines of therapy.6 Second- and subsequent-line options for patients with relapsed or refractory (R/R) MZL include lenalidomide in combination with anti-CD20 therapies,5,7,8 and zanubrutinib (Bruton tyrosine kinase [BTK] inhibitor) after at least 1 prior anti-CD20–based therapy9,10 (accelerated approval of another BTK inhibitor ibrutinib was recently withdrawn by the sponsor for this indication).11
In B-cell malignancies, upregulation of the phosphatidylinositol-3 kinase (PI3K) pathway, particularly the PI3Kδ isoform, is a critical driver of tumor growth and survival.12,13 PI3K inhibitors have demonstrated clinically meaningful efficacy in the treatment of various R/R NHLs, with reported objective response rates (ORRs) of 39% to 87% in MZL.14-17 However, challenges surrounding safety and tolerability from on-target and off-target effects related to the selectivity and potency of current therapies restrict the ability to optimize efficacy outcomes.13
Parsaclisib is a potent, highly selective, next-generation PI3Kδ inhibitor designed to improve tolerability while maintaining strong inhibition.18 Parsaclisib inhibited PI3Kδ activity (half-maximal concentration = 1 nM) and was at least 10 000-fold more selective for PI3Kδ compared with PI3Kα, PI3Kβ, and PI3Kγ in biochemical assays.18,19 In the phase 1/2 CITADEL-101 study (NCT02018861),20 parsaclisib demonstrated differentiated tolerability, with near absence of grade ≥2 transaminitis, and encouraging clinical outcomes in patients with R/R NHL including MZL. Here, we report primary results from the CITADEL-204 study conducted to further evaluate the efficacy and safety of parsaclisib in patients with R/R MZL.
Methods
Trial oversight
CITADEL-204 was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable local regulations. The protocol and all amendments were reviewed and approved by institutional review boards or independent ethics committees before study initiation, and written informed consent was obtained from each patient before study enrollment.
Study design
This phase 2, multicenter, open-label study evaluated efficacy and safety of parsaclisib in patients with R/R MZL, with (cohort 1) or without (cohort 2) prior treatment with the BTK inhibitor ibrutinib (the only BTK inhibitor approved when the study was designed and initiated; supplemental Figure 1). This report focuses on cohort 2; owing to slower than expected recruitment, cohort 1 was closed early.
The first 60 patients enrolled in cohort 2 and all patients in cohort 1 were assigned originally in a 1 to 1 ratio into either the weekly dosing group (WG) or the daily dosing group (DG); before opening of the expansion cohort, it was decided to enroll all patients into the DG based on collective preliminary data across the CITADEL-203, -204, and -205 trials. At the end of the enrollment in cohort 2, only 28 patients were enrolled in the WG and 72 in the DG. Patients in the WG received oral parsaclisib 20 mg once daily for 8 weeks followed by 20 mg once weekly (QW); patients in the DG received oral parsaclisib 20 mg once daily for 8 weeks followed by 2.5 mg once daily. After the initial 20-mg once-daily dosing, the subsequent lower dosing of 20 mg QW or 2.5 mg once daily was proposed to maintain response while reducing the intensity and frequency of adverse events (AEs). An additional 30 patients in cohort 2 were planned for enrollment for better understanding of safety and efficacy. All patients were required to receive a standard Pneumocystis jirovecii pneumonia (PJP) prophylaxis regimen while receiving parsaclisib and for 2 to 6 months after the last dose of parsaclisib. Cytomegalovirus infection was monitored by polymerase chain reaction.
After an initial evaluation of safety and efficacy from this study and other monotherapy studies in NHL, the DG was selected as the study treatment regimen; all patients were enrolled in the DG thereafter. Patients in the WG could cross over to the DG or remain on their current regimen. Treatment for all patients continued until disease progression, death, unacceptable toxicity, or consent withdrawal.
Patients
Key inclusion criteria included patients aged at least 18 years with histologically confirmed R/R MZL (extranodal, nodal, and splenic subtypes); prior treatment with at least 1 systemic therapy, including at least 1 anti-CD20 antibody; radiographically measurable (computed tomography [CT] or magnetic resonance imaging [MRI]) lymphadenopathy or extranodal lymphoid malignancy (defined as having at least 1 lesion that measures >1.5 cm in the longest transverse diameter and ≥1.0 cm in the longest perpendicular diameter; patients with splenic MZL who did not meet radiographically measurable disease criteria were eligible if bone marrow [BM] infiltration was histologically confirmed); willingness to undergo an incisional or excisional lymph node or tissue biopsy or provide a lymph node or tissue biopsy from the most recent available archival tissue; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2, and adequate hematologic, hepatic, and renal function.
Key exclusion criteria were known histologic transformation from indolent NHL to diffuse large B-cell lymphoma; history of primary or secondary central nervous system lymphoma; prior treatment with PI3K inhibitors; receiving allogeneic or autologous stem cell transplantation, immunosuppressive therapy, anticancer, potent CYP34A inducers or inhibitors, or investigational drugs within protocol-defined intervals before the study; active graft-versus-host disease; history of stroke or intracranial hemorrhage within 6 months of the study; chronic or active infection requiring treatment (including human immunodeficiency virus, hepatitis B virus, and hepatitis C virus); or exposure to a live vaccine within 30 days of dosing.
Study end points and assessments
The primary end point was independent review committee (IRC)–determined ORR assessed by CT or MRI according to Lugano classification response criteria for lymphomas.21 Secondary end points included complete response (CR) rate, best percentage change in disease burden (measured as the best percentage change in the sum of product of diameters of all target lesion sizes for patients with measurable disease at baseline, or the best percentage change in the enlarged portion of the spleen for patients with splenomegaly only at baseline), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety and tolerability. Measurable disease by CT or MRI and BM examinations were performed at screening to determine disease status and followed until disease progression. If BM disease was present at baseline, a BM biopsy was required to confirm CR.
Safety was assessed by monitoring AE frequency, duration, and severity (National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03), as well as physical examinations, vital signs, 12-lead electrocardiogram, ECOG PS, and clinical laboratory blood and urine samples. The AEs of special interest, including colitis, diarrhea, exfoliative dermatitis, febrile neutropenia, rash, intestinal perforation, pneumonitis, pneumonia and PJP, cytomegalovirus, herpes simplex virus, and varicella zoster virus infections, were monitored. Laboratory events of special interest included decreased neutrophils, increased alanine transferase (ALT), and increased aspartate aminotransferase (AST). Colonoscopies with biopsies were recommended but not mandated as part of the evaluation of an event, such as colitis, and no formal data were collected.
Exploratory end points included profiles of blood biomarkers at baseline and on treatment associated with response, resistance, and safety of parsaclisib. Whole blood was collected for pharmacokinetic analysis and translational biomarker analysis of serum proteins (ie, changes in protein analytes associated with B-cell lymphoma and immune cell function). Study assessments were performed at protocol-defined time points (supplemental Table 1), and serum samples were analyzed for relative expression levels of ∼1000 proteins by proximity extension assays (Olink Proteomics, Boston, MA).
Statistical analyses
A sample size of ∼90 patients was selected so that if the true ORR is 60% for patients in both treatment groups, there would be ∼96% probability of observing the lower bound of the 95% confidence interval (CI) of ORR ≥40%. An interim futility analysis was planned once 30 patients in cohort 2 were treated and evaluated for response or had permanently discontinued study treatment owing to disease progression, withdrawal of consent, or death. The study was to be terminated if the futility boundary was crossed (≤10/30 patients responded [ie, CR or partial response (PR)] based on IRC assessment).
The full analysis set (used for summary of demographics, baseline characteristics, patient disposition, and all efficacy analyses) and the safety population (used for all safety analyses) consisted of patients who received at least 1 dose of parsaclisib. The pharmacokinetic or pharmacodynamic-evaluable population included patients who received at least 1 dose of parsaclisib and provided at least 1 postdose plasma sample.
No statistical comparisons were planned for this study. Patients initially assigned to the WG who switched to the DG before starting the 20-mg QW period were included in the DG for analyses, and those who switched after starting the 20-mg QW period were included in the WG for analyses. Unless otherwise stated, all efficacy data presented were determined by the IRC.
Results
Patient demographics and disposition
Between 18 December 2017 and the 15 January 2021 primary analysis data cutoff date, 100 patients were enrolled in cohort 2 at 44 international study sites and treated with parsaclisib (28 patients in the WG; 72 patients in the DG). Three patients switched from weekly to daily dosing after starting the 20-mg QW period. In the total population, median age was 71.0 (range, 35-95), 53.0% were male, and 83.0% were White. At baseline, 95% of patients had an ECOG PS score of 0 to 1 and 82.0% had advanced disease (Ann Arbor Stage III-IV); 31%, 34%, and 35% of patients had nodal, extranodal, and splenic MZL subtypes, respectively. The median number of prior therapies was 2 (range, 1-8), and 49.0% of patients had lymphoma refractory to their most recent prior therapy (Table 1). Ten patients (4 in the WG; 6 in the DG) had been enrolled in cohort 1 of the study before it was closed; baseline characteristics of these patients are presented in supplemental Table 2. Results reported hereon are primarily for cohort 2 and the DG unless otherwise specified, and data for cohort 1 are presented in supplemental Data.
In the DG, 50 patients (69.4%) discontinued treatment, and 22 patients (30.6%) remained on treatment at the primary analysis cut-off; the main reasons for treatment discontinuation were AEs (37.5%) and progressive disease (25.0%) (supplemental Table 3). The median duration of parsaclisib treatment in the DG was 11.6 months (range, 0.4-30.9), and median follow-up time was 21.0 months (range, 11.9-37.0) from the first dose to data cutoff date (supplemental Table 3). Data for the WG and overall population are presented in supplemental Table 3.
Efficacy
In an interim analysis of the first 30 treated patients in cohort 2, the ORR was 46.7% (95% CI, 28.3-65.7), and all 14 responders were PR. The futility boundary (≤10 of the 30 patients responded) was not crossed, and enrollment continued until 100 patients were treated. The efficacy data presented below refer to the DG in cohort 2. At the data cutoff for primary analysis, the ORR based on IRC assessment was 58.3% (95% CI, 46.1-69.8), exceeding the protocol-defined ORR 95% CI lower bound of 40%. The CR rate was 4.2% (95% CI, 0.9-11.7) and stable disease was observed in 30.6% of patients (Table 2). The ORR was 56.5% in extranodal, 48.0% in nodal, and 70.8% in splenic MZL subgroups. In a subgroup analysis of ORR based on patient baseline characteristics, response rates were generally consistent with the primary analysis in the total population and for the DG (supplemental Figure 2).
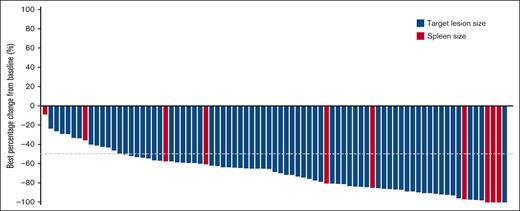
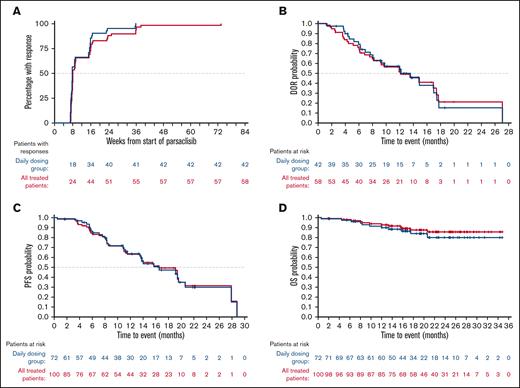
Among all 81 evaluable patients in cohort 2 with baseline and at least 1 valid postbaseline disease assessment (target lesion for patients with measurable disease at baseline or spleen for patients with splenomegaly only at baseline), all patients had tumor regression and among them, 82.7% (67/81) achieved a >50% reduction in disease burden from baseline (Figure 1). In the DG, the median time to response among 42 responders was 8.1 weeks, with 66.7% of responders demonstrating an objective response at the first 8-week assessment (Figure 2). The median DOR was 12.2 months (95% CI, 8.1-17.5), median PFS was 16.5 months (95% CI, 11.5-20.6) (Figure 2), and estimated 6- and 12-month PFS rates were 86.5% (95% CI, 74.7-93.0) and 63.6% (95% CI, 49.3-74.9) in the DG, respectively. The median OS was not reached in the DG (neither in the total population nor in the WG) (Figure 2); the estimated 6- and 12-month OS rates were 97.2% (95% CI, 89.2-99.3) and 89.7% (95% CI, 79.7-95.0), respectively. For the WG data, see supplemental Figure 3.
Waterfall plot of best percentage change from baseline in patients with R/R MZL receiving parsaclisib treatment. Best percentage change from baseline in either target lesions for patients with measurable disease at baseline and at least 1 valid postbaseline measurement (blue bars, n = 71) or enlarged portion of spleen for patients with splenomegaly only at baseline and at least 1 valid postbaseline measurement of spleen (red bars, n = 10) by IRC (cohort 2 WG and DG).
Waterfall plot of best percentage change from baseline in patients with R/R MZL receiving parsaclisib treatment. Best percentage change from baseline in either target lesions for patients with measurable disease at baseline and at least 1 valid postbaseline measurement (blue bars, n = 71) or enlarged portion of spleen for patients with splenomegaly only at baseline and at least 1 valid postbaseline measurement of spleen (red bars, n = 10) by IRC (cohort 2 WG and DG).
Response time, DOR, PFS, and OS in patients with R/R MZL receiving parsaclisib treatment. (A) Cumulative time to response curves, and (B) Kaplan-Meier estimates of DOR, (C) PFS, and (D) OS in the DG (blue) and all treated patients (red) (cohort 2). All response assessments were by IRC.
Response time, DOR, PFS, and OS in patients with R/R MZL receiving parsaclisib treatment. (A) Cumulative time to response curves, and (B) Kaplan-Meier estimates of DOR, (C) PFS, and (D) OS in the DG (blue) and all treated patients (red) (cohort 2). All response assessments were by IRC.
Among the 10 patients with prior ibrutinib treatment enrolled in cohort 1, ORR by IRC was 40.0% (95% CI, 12.2-73.8), with 4 PRs (supplemental Table 4) in the total population. The observed DOR for the 4 responders ranged from 1.9 to 8.8 months, and observed PFS for all 10 patients ranged from <1 to 10.6 months. Best percentage change from baseline in target lesion size for patients treated in cohort 1 is presented in supplemental Figure 4.
Safety
The safety population in cohort 2 included all patients who received at least 1 dose of parsaclisib (N = 100). Data from the overall population and the DG are presented in the text and Table 3; data from the WG are provided in Table 3 only. Treatment-emergent AEs (TEAEs) occurred in 96 patients (96.0%) overall and in 70 patients (97.2%) in the DG (Table 3). The most common TEAEs were diarrhea (overall, 47.0%; DG, 52.8%), cough (overall, 23.0%; DG, 26.4%), and rash (overall, 18.0%; DG, 18.1%). Among patients who experienced any-grade diarrhea, the median time to onset was 2.7 (range, 0.03-23.85) and 2.6 (range, 0.03-23.85) months overall and in the DG, respectively; the median time to resolution of any-grade diarrhea was 1.6 (95% CI, 1.0-2.6) and 1.5 (95% CI, 0.9-2.6) months overall and in the DG, respectively, and the median time to improvement to grade ≤2 from grade ≥3 diarrhea was 0.4 (95% CI, 0.1-0.8) months, both overall and in the DG. TEAEs led to parsaclisib discontinuation in 29 (29.0%) and 27 (37.5%) patients overall and in the DG, respectively; the most common TEAEs leading to parsaclisib discontinuation were diarrhea (overall, 9.0%; DG, 12.5%) and colitis (overall, 5.0%; DG, 6.9%). Treatment interruptions because of TEAEs were required by 56 (56.0%) and 43 (59.7%) and dose reductions by 16 (16.0%) and 12 (16.7%) patients overall and in the DG, respectively. Treatment-related AEs were reported in 83 (83.0%) and 60 (83.3%) patients overall and in the DG group, respectively. The most common treatment-related AEs were diarrhea (overall, 42.0%; DG, 48.6%) and pruritus (overall, 11.0%; DG, 11.1%). Treatment-related hematological events included neutropenia (overall, 10.0%; DG, 9.7%), anemia (overall, 7.0%; DG, 8.3%), eosinophilia (overall, 3.0%; DG, 2.8%), and thrombocytopenia (overall, 3.0%; DG, 1.4%).
Grade ≥3 TEAEs occurred in 63 patients (63.0%) in the total population and in 52 patients (72.2%) in the DG in cohort 2. Grade ≥3 TEAEs occurring in ≥5% of patients in the total population were diarrhea (overall, 12.0%; DG, 15.3%), neutropenia (overall, 9.0%; DG, 11.1%), pneumonia (overall, 9.0%; DG, 9.7%), colitis (overall, 7.0%; DG, 9.7%), anemia (overall, 6.0%; DG, 8.3%), and febrile neutropenia (overall, 5.0%; DG, 5.6%). Serious TEAEs occurred in 47 patients (47.0%) in the total population and 40 patients (55.6%) in the DG; serious TEAEs occurring in ≥5% of patients in the total population were pneumonia (overall, 9.0%; DG, 9.7%), colitis (overall, 6.0%; DG, 8.3%), and febrile neutropenia (overall, 5.0%; DG, 5.6%). Six patients experienced fatal TEAEs during the study; worsened general condition (in the WG), and COVID-19 pneumonia, Enterobacter sepsis, febrile neutropenia, mental status change, and sepsis (each in the DG).
For AEs of special interest, in addition to diarrhea, colitis, rash, and pneumonia (presented above), febrile neutropenia occurred in 5 patients (5.0%; 4 patients in the DG), cytomegaloviral infection and pneumonitis in 2 patients each (2.0%; both in the DG), and herpes simplex viral infection in 1 patient (1.0%; DG) (Table 4; data for the WG are presented in Table 4). No AEs of PJP were reported as of data cutoff.
Select new or worsening hematological and chemistry laboratory abnormalities are presented in supplemental Table 5. The most common new or worsening hematology laboratory parameters for the total population included decreases in neutrophil count (53.0%), hemoglobin level (32.0%), and platelet count (20.0%). Any-grade or grade 3 or 4 increases in ALT occurred among 27.0% and 5.0% of patients, respectively, and AST occurred among 22.0% and 3.0% of patients, respectively.
Nine of the 10 patients (90%) in cohort 1 had at least 1 TEAE (supplemental Table 6), and 6 (60%) had TEAEs that were considered related to parsaclisib by the investigator. Grade ≥3 TEAEs occurred in 6 patients (60%) and serious TEAEs in 4 patients (40%). One patient experienced a fatal TEAE (sepsis); no TEAEs were reported as leading to discontinuation of parsaclisib in cohort 1.
Biomarker analysis
Baseline and on-treatment serum samples were available for 75 patients for exploratory translational analyses. Serum proteomic analysis from samples at week 4 demonstrated that parsaclisib reduced the expression of several cytokines, chemokines, and transmembrane receptors from baseline to week 4, including CXCL13/BCA1, FCER2, IL10, LTA/TNFB, TNFRSF4, TNFRSF9, and TNFRSF13B (supplemental Table 7 for all significantly [P < .05] changed proteins and supplemental Figure 5 for representative CXCL13 and TNFRSF9 results).
Discussion
Approved treatment options for patients with R/R MZL in the United States include anti-CD20 plus lenalidomide7 and therapy targeting the BTK pathway (zanubrutinib).9 Patients with R/R MZL who do not respond to or who experience excessive toxicity on approved treatments have few alternatives, underscoring the need for new therapies. Parsaclisib, a potent and highly selective PI3Kδ inhibitor, was investigated in this phase 2 study to evaluate efficacy and safety in patients with R/R MZL. In the overall population in cohort 2, treatment with parsaclisib achieved an IRC-assessed ORR of 58.0%, and a >50% reduction in disease burden from baseline (ie, best percentage change in target lesions for patients with measurable target lesions at baseline or enlarged spleen for patients with only splenomegaly as measurable disease at baseline, as defined in the study end points and assessment section above) was observed in 82.7% of patients. In the DG, the IRC-assessed ORR was 58.3%, including 3 patients with a CR, 39 patients with a PR, and 22 patients who achieved stable disease. Although the CR rate was relatively low (4.2%), parsaclisib produced a rapid and durable response, with a median time to onset of response of 8.1 weeks and a clinically relevant DOR of 12.2 months. Treatment with parsaclisib also demonstrated efficacy in the WG, with an ORR of 57.1% and observed DOR range of 1.9 to 8.8 months among responders. The overall efficacy findings from this study are consistent with those observed in the phase 1 CITADEL-101 study in patients with NHL,20 and the CITADEL-203 study in R/R follicular lymphoma.22
Several AEs associated with PI3K inhibition appear to be a class effect; inhibitors of the δ isoform (such as idelalisib,23 umbralisib,16 and zandelisib24) are associated with AEs including transaminitis, diarrhea, colitis, pneumonitis, neutropenia, and rash. Inhibition of the ubiquitously expressed α isoform (such as with copanlisib and a dual α and δ inhibitor15) is associated with AEs including hyperglycemia and hypertension, whereas infections and autoimmune toxicities have been observed upon inhibition of δ and γ isoforms (such as with duvelisib14). Consistent with the known safety profile of PI3Kδ inhibitors, no novel safety events were reported with parsaclisib monotherapy in either weekly or daily treatment regimens; more events were reported with continuous dosing and observed toxicities were likely attributable to on-target PI3Kδ-associated effects.13 TEAEs were experienced by 96.0% of patients overall, with the most common being diarrhea, cough, and rash. Diarrhea occurred more frequently and had a shorter time to onset (2.6 vs 3.6 months) in patients from the DG compared with the WG; discontinuation of treatment towing to toxicity was also more common in the DG (37.5% vs 7.1%). Although AEs were managed by dose reductions in 56% to 60% or interruptions in 16% to 17% of patients, TEAEs led to parsaclisib discontinuation in 29% to 37.5% of patients. Most patients treated with parsaclisib maintained normal ALT and AST values throughout the study, likely owing to the high selectivity and structural design of parsaclisib to reduce PI3Kδ inhibitor–associated hepatotoxicity.18,19 No clinically meaningful trends were observed on health-related quality-of-life outcomes, assessed using Functional Assessment of Cancer Therapy-Lymphoma total score, while patients were on the study (data not shown).
Parsaclisib demonstrated a durable response with a safety profile consistent with PI3K inhibitor treatment in patients with R/R MZL. It is important to note that this is the only study of a PI3K inhibitor in patients with R/R MZL that was designed with a predetermined induction and maintenance phase dosing schedule. This design was implemented to evaluate the ability of a maintenance phase with lower dosing intensity to address tolerability while maintaining efficacy. Two different maintenance doses and dosing schedules were investigated in the current study. Pharmacokinetic modeling data from the phase 1/2 CITADEL-101 study showed that weekly dosing of parsaclisib (20 mg QW) achieved a plasma concentration in excess of the 90% inhibitory concentration (IC90) for maximal inhibition of the protein kinase B pathway for 36 hours, but resulted in a plasma concentration below the half-maximal inhibitory concentration (IC50) for approximately half of the dosing interval based on parsaclisib having a half-life between 8.6 and 11.5 hours.20 Comparatively, similar modeling data showed that a daily dosing of parsaclisib (2.5 mg once daily) achieved a plasma concentration in excess of IC50 for ∼90% of a weekly dosing interval, indicating less maximal, but more consistent inhibition of protein kinase B, which was hypothesized to offer better clinical efficacy while reducing the severity and frequency of late-onset AEs during the maintenance phase. Based on the pharmacokinetic modeling data, safety and efficacy data from multiple studies investigating parsaclisib monotherapy in the treatment of B-cell lymphoma,20,22,25 and early safety and efficacy data from this study, continuous 2.5 mg once daily dosing was selected as the preferred maintenance dosing regimen for the later part of the study—continuous daily dosing at a lower dose was expected to potentially allow patients to experience durable responses while minimizing side effects.
Overall results in our study revealed that the rate of some TEAEs, especially diarrhea and colitis, were numerically less frequent in the WG compared with the DG. However, fewer patients were dosed in the WG, and the study was not designed to compare the 2 maintenance doses. With regard to efficacy, the ORR were similar between the DG (58.3%) and WG (57.1%) in our study of parsaclisib monotherapy in R/R MZL. These results may suggest that intermittent or weekly dosing providing regular pauses to PI3K pathway inhibition, compared with continuous daily dosing at similar dosing intensity, may improve certain safety events without reducing efficacy in patients treated with parsaclisib. However, further clinical investigation is required to make firm conclusions owing to study design and limited data from the WG in this study. Recently, continuous or intermittent dosing was evaluated with another PI3K inhibitor, zandelisib, in a phase 1b study of patients with R/R B-cell malignancies.24
The manageable benefit/risk profile of parsaclisib suggests that it may be an effective therapeutic option for patients with R/R MZL. The efficacy results with parsaclisib are generally consistent with other PI3K inhibitors that have been investigated for R/R MZL (duvelisib [ORR, 39%],14 idelalisib [ORR, 57%],23 and umbralisib [ORR, 49%]26). However, several PI3K inhibitors previously approved by the US Food and Drug Administration for follicular lymphoma (duvelisib, idelalisib, umbralisib), MZL (umbralisib), and chronic lymphocytic leukemia/small lymphocytic leukemia (idelalisib) have had indications or marketing authorization withdrawn owing to unfavorable feasibility of confirmatory studies or emerging data suggesting increased toxicity and reduced OS in patients with indolent NHL or chronic lymphocytic leukemia.27-31 In addition, the treatment landscape for R/R MZL has also evolved since the conduct of the CITADEL-204 study, with newer-generation BTK inhibitors increasingly used for second-line treatment of advanced MZL.5 Zanubrutinib was approved under accelerated approval for the treatment of adult patients with R/R MZL who have received at least 1 anti-CD20–based regimen. In phase 2 studies, acalabrutinib and zanubrutinib achieved ORRs of 52.5% and 68.2%, and 12-month DOR rates of 75.8% and 93.0%, respectively, in patients with R/R MZL.10,32
Given that data indicate AEs attributed to PI3K inhibitors are strongly correlated with chronic exposure, modification and optimization of dosing schedules are required to improve the safety and long-term survival benefit observed with PI3K inhibitors, including parsaclisib, in patients with NHL. Although parsaclisib demonstrated meaningful clinical benefit and an overall manageable safety profile in patients with R/R MZL in this study, a new study will be required to further test the hypothesis that reduction of certain side effects, such as diarrhea and colitis, could be achieved with a QW maintenance dosing schedule, with minimal impact on efficacy. Owing to feasibility of conducting confirmatory phase 3 trials and the evolving treatment landscape, a business decision was made by the sponsor, and no additional studies are planned with parsaclisib monotherapy in MZL.
Acknowledgments
The authors thank the patients, their families, and the site personnel who participated in this study.
This study was sponsored by Incyte Corporation (Wilmington, DE). Medical writing assistance was provided by Rachel Shparberg (Envision Pharma Group, Philadelphia, PA), and funded by Incyte Corporation.
Authorship
Contribution: All authors contributed to the acquisition, analysis, and interpretation of data, drafting and critical review of the manuscript, and provided approval of the final version to be published; T.J.P., P. Johnson, F.Z., E.R., W.Z., and P. Jiang directly accessed and verified the underlying data reported in the manuscript.
Conflict-of-interest disclosure: T.J.P. is a Scholar in Clinical Research of The Leukemia & Lymphoma Society, and reports consultancy for AbbVie, Bayer, BeiGene, Bristol Myers Squibb, Cardinal Health, Incyte Corporation, Karyopharm, and Seattle Genetics; travel, accommodations, or expenses (paid by any for-profit health care company) were provided by Incyte Corporation; and research funding was provided by AstraZeneca and Pharmacyclics. A.A. reports consultancy for Gilead Sciences, Pfizer, and Takeda; honoraria from Janssen; and research funding from Bristol Myers Squibb. R.G. reports consultancy for Gilead Sciences, Medison, Novartis, and Takeda; and honoraria from JC Health CARE and Roche. P.C. reports consultancy for AbbVie, Amgen, Celgene, Daiichi Sankyo, F. Hoffman-La Roche Ltd, Gilead Sciences, Incyte Corporation, Janssen, Kyowa Kirin, Kite, Novartis, Sanofi, Servier, and Takeda; honoraria from AbbVie, Amgen, Celgene, Daiichi Sankyo, F. Hoffman-La Roche Ltd, Gilead Sciences, Janssen, Kyowa Kirin, Kite, Novartis, Sanofi, Servier, and Takeda; travel, accommodations, and expenses (paid by any for-profit health care company) were provided by AbbVie, Amgen, Bristol Myers Squibb, Celgene, Gilead Sciences, Novartis, and Takeda. A.M. reports consultancy for AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead, Incyte Corporation, Kyowa Kirin, MorphoSys/Incyte Corporation, Novartis, Pharmacyclics, Seattle Genetics, and TG Therapeutics; research funding from Affimed, Celgene/Bristol Myers Squibb, Forty Seven, Inc./Gilead Sciences, I-MAB, Incyte Corporation, Innate Pharmaceuticals, Juno Pharmaceuticals/Bristol Myers Squibb, Kite Pharma/Gilead Sciences, Merck, Roche-Genentech, Seattle Genetics, Takeda, and TG Therapeutics; speakers’ bureau for AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead Sciences, Kyowa Kirin, Incyte Corporation, MorphoSys/Incyte, Pharmacyclics, and Seattle Genetics. I.S.L. reports consultancy for Janssen Scientific, Seattle Genetics, and Verastem Oncology; honoraria from Janssen Biotech and Verastem Oncology; travel, accommodations, and expenses (paid by any for-profit health care company) were provided by Janssen and Seattle Genetics; patents and royalties were provided by Stanford University; research funding was received from National Cancer Institute. P.L.Z. reports consultancy for Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead Sciences, Immune Design, Janssen, Merck Sharp & Dohme (MSD), Portola, Roche, Sandoz, Sanofi, and Verastem Oncology; honoraria from AbbVie, ADC Therapeutics, Bristol Myers Squibb, EUSA Pharma, Gilead Sciences, Incyte Corporation, Janssen, Kyowa Kirin, Merck, Roche, Takeda, TG Therapeutics, and Verastem Oncology; membership on an entity's board of directors or advisory committees at AbbVie, ADC Therapeutics, Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead Sciences, Immune Design, Incyte Corporation, Janssen, Kyowa Kirin, Merck, MSD, Portola, Roche, Sandoz, Sanofi, Takeda, and Verastem Oncology; research funding from Portola; speakers’ bureau for AbbVie, ADC Therapeutics, Bristol Myers Squibb, Celgene, Celltrion, EUSA Pharma, Gilead Sciences, Immune Design, Incyte Corporation, Janssen, Kyowa Kirin, Merck, MSD, Portola, Roche, Sandoz, Takeda, TG Therapeutics, and Verastem Oncology. C.T. reports consultancy for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Cellectis, Kite Pharma/Gilead Sciences, Novartis, and Roche; honoraria from AbbVie, Amgen, Bayer, Celgene, Cellectis, Incyte Corporation, Kite Pharma/Gilead Sciences, Novartis, and Roche; membership on an entity’s board of directors or advisory committees at AbbVie, Amgen, Celgene, Cellectis, Kite Pharma/Gilead Sciences, Novartis, and Roche; travel, accommodations, and expenses (paid by any for-profit health care company) were provided by AbbVie, Amgen, Bristol Myers Squibb, Celgene, Cellectis, Kite Pharma/Gilead Sciences, Novartis, and Roche; research funding from Hospira and Roche; and speakers’ bureau for Cellectis. W.J. reports consultancy for Acerta, AstraZeneca, BeiGene, Epizyme, European Medicines Agency, Janssen China R&D, and Sandoz-Novartis; research funding was provided by Acerta, Affimed, Bayer, BeiGene, Celgene, Gilead Sciences, MEI Pharma, Nordic Nanovector, Janssen China R&D, Merck, MorphyoSys, Pharmacyclics, Roche, Servier, Takeda, and TG Therapeutics; and former employment at Jagiellonian University, Kraków, Poland. F.Z. reports employment by and stock ownership in Incyte Corporation. E.R. reports employment by and stock ownership in Incyte Corporation. W.Z. reports former employment by and stock ownership in Incyte Corporation. P. Jiang reports former employment by and stock ownership in Incyte Corporation. P. Johnson reports consultancy for Epizyme, Janssen, MorphoSys, OncoImmune, and Takeda; honoraria from Bristol Myers Squibb, Celgene, Genmab, Incyte Corporation, Kite Pharma, Kymera, Novartis, and OncoImmune; and research funding from Epizyme. The remaining authors declare no competing financial interests.
The current affiliation of T.J.P. is City of Hope Comprehensive Cancer Center, Duarte, CA.
Correspondence: Tycel J. Phillips, City of Hope Comprehensive Cancer Center, 1500 E Duarte Rd, Duarte, CA 91010; email: tphillips@coh.org.
References
Author notes
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte Corporation for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least 1 major market (eg, United States, European Union, and Japan). Data will be available upon request after the primary publication or 2 years after the study has ended. Information on Incyte Corporation’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trialdata-sharing.pdf?ver=2020-05-21-132838-960.
The full-text version of this article contains a data supplement.