BT does not have a significant influence on response rates, progression-free survival, and overall survival for patients who receive axi-cel.
BT with radiation results in similar outcomes as nonradiation-based BT.
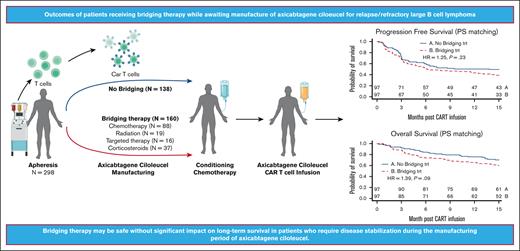
Visual Abstract
During the manufacturing period of autologous chimeric antigen receptor (CAR) T-cell therapy, patients may experience a decline in their condition due to cancer progression. In this study, we investigated the impact of bridging therapy (BT) on the outcome of patients with relapsed/refractory large B-cell lymphoma who received antilymphoma treatment between leukapheresis and axicabtagene ciloleucel (axi-cel) infusion. We conducted our analysis using data from the multicenter US Lymphoma CAR-T Consortium, with a median follow-up of 33 months (range, 4.3-42.1). Out of the 298 patients who underwent leukapheresis, 275 patients received axi-cel. A total 52% of patients (n = 143) who received BT had a higher baseline risk profile than patients who did not receive BT, and these patients, as a group, had inferior outcomes compared with those who did not receive BT. However, after propensity score matching between the 2 groups, there were no statistically significant differences in overall response rate (77% vs 87%; P = .13), complete response rate (58% vs 70%; P = .1), progression-free survival (hazard ratio [HR], 1.25; P = .23), and overall survival (HR, 1.39; P=.09) between the BT group and the no-BT group, respectively. Analyzing the effects of BT in the whole cohort that underwent leukapheresis regardless of receiving axi-cel (intention-to-treat analysis) showed similar results. Radiation BT resulted in outcomes similar to those observed with nonradiation BT. Our findings suggest that BT may be safe without a significant impact on long-term survival for patients who require disease stabilization during the manufacturing period. Moreover, our results suggest that there is no clear advantage to using radiation-based BT over nonradiation-based BT.
Introduction
Three autologous anti-CD19 chimeric antigen receptor (CAR) T-cell products, including axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel), are approved for treatment of relapsed/refractory large B-cell lymphoma (R/R LBCL) on the basis of the pivotal ZUMA-1, JULIET, and TRANSCEND clinical trials.1-3 For all 3 products, patients first undergo leukapheresis to obtain autologous T cells for manufacturing and then need to wait as their T cells are expanded and genetically engineered to express the anti-CD19 CAR construct. The dration of the manufacturing period varies depending on the manufacturer’s capacity and the processes used. For example, in the ZUMA-1 clinical trial, the time from leukapheresis to the delivery of axi-cel to the receiving institution was 17 days.1 In the JULIET trial, the time from enrollment to tisa-cel infusion was 54 days,2 and in the TRANSCEND trial,3 the time from leukapheresis to liso-cel infusion was 24 days. Similarly, bridging therapy (BT), defined as antilymphoma therapy delivered after leukapheresis and before the start of lymphodepleting chemotherapy, was not allowed in ZUMA-1 but was administered to 92% of patients in JULIET and 59% of patients in TRANSCEND. It remains unclear whether BT affects the subsequent outcomes of CAR T-cell treatment, particularly for axi-cel. Possible outcomes of BT could be a reduction in tumor volume and/or an improvement in patient status, but BT may also be toxic or ineffective. We previously reported the outcomes of 298 patients who underwent leukapheresis with the intention to receive standard-of-care axi-cel in a multicenter consortium.4 In this study, we conducted an analysis of our large multicenter cohort to gain a better understanding of the outcomes observed in patients who received BT compared with outcomes in those who did not receive BT during axi-cel manufacturing.
Methods
Patients
Seventeen academic centers located in the United States participated in this study. Each of the participating centers obtained independent institutional review board approval for observational retrospective analysis of their patients, and the study was conducted in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonization. All authors contributed to the conduct of the study, data analyses, and writing of the manuscript. We conducted both intention-to-treat (ITT) and modified ITT (mITT) analyses. The mITT analysis included all patients who received axi-cel, whereas the ITT analysis included all patients who underwent leukapheresis, regardless of whether they ultimately received an axi-cel infusion. We present the results of the mITT analysis in the main manuscript and the results of the ITT analysis in the supplemental Data. The cohort of 298 patients who underwent leukapheresis with the intention of standard-of-care axi-cel, as of 30 September 30 2018, was previously described.4 All axi-cel infusions occurred between November 2017 and November 2018.
Definitions
BT is defined as any lymphoma-directed treatment administered after leukapheresis and before the initiation of lymphodepleting chemotherapy and a CAR T-cell infusion. The treating physician determined whether BT was admistered as well as the type of BT provided. The type of BT was categorized as (1) chemotherapy, (2) corticosteroids, (3) radiation, or (4) targeted therapies. When more than 1 category was used in combination, the patient was assigned to the highest category based on the following hierarchy: chemotherapy, targeted therapy, radiation, and corticosteroids. Use of rituximab was not considered in the categorization (ie, corticosteroids plus rituximab was categorized as corticosteroids). Details of the individual regimens given and their categorization are listed in supplemental Table 1.
Statistical analysis
Descriptive statistics include mean, standard deviation, median, and range for continuous variables, such as age, frequency counts, and percentages, and for categorical variables, such as stage and response status. The distributions of baseline demographic and clinical characteristics between patients in bridging vs no-BT groups were compared using Fisher exact test, χ2 square test, analysis of variance, or Kruskal-Wallis rank sum test, as appropriate. Univariate and multivariable logistic regression models were fitted to assess the effect of important covariates on response status (complete response [CR] or overall response rate [ORR]). The variables that had a P value < .2 from the univariate analysis were included in the initial multivariable model. A backward selection method was used, and a significant level of .2 was set as the criterion for a variable to stay in the multivariate model. Collinearity diagnostics were performed on the final models and indicated no collinearity problem. Kaplan-Meier product limit method was used to estimate progression-free survival (PFS) and overall survival (OS), and log-rank test was used to evaluate the difference in PFS or OS between the BT and no-BT groups. Multivariable Cox proportional hazards models were also performed for PFS and OS, with the backward selection procedure as described above to retain important and significant covariates. The Schoenfeld residual was used to check the proportional hazards assumption. Collinearity diagnostics were performed for the final models and indicated no collinearity problem. To validate the results of the multivariate analysis of the Cox model, we performed a matched pair comparison of a subset of closely matched patients selected based on propensity score (PS) matching. Key factors included in PS matching were 7 variables significant in multivariate analysis of OS or PFS (sex, Eastern Cooperative Oncology Group [ECOG] status, stage, number of lines of therapy, refractory, prior autologous stem cell transplant, and lactate dehydrogenase [LDH] at conditioning) and 3 variables that were significant in univariate OS/PFS and significantly imbalanced between groups (ZUMA-1 eligibility, Lymphoma International Prognostic Index score [IPI] score, and bulky disease). For each treated case, a matched control was identified as the one with the closest distance (within 0.25 × standard deviation of the PS in the treatment group). The aforementioned analyses were performed on both mITT and ITT cohorts. PS matching was also performed to compare outcomes in patients who received radiation-based BT vs in those who received nonradiation-based BT in both the mITT and ITT cohorts. All data analyses were performed using SAS 9.4 (SAS Institutes, Cary, NC), and statistical significance was defined as a two-tailed P value < .05 for all analyses.
Results
Patient characteristics
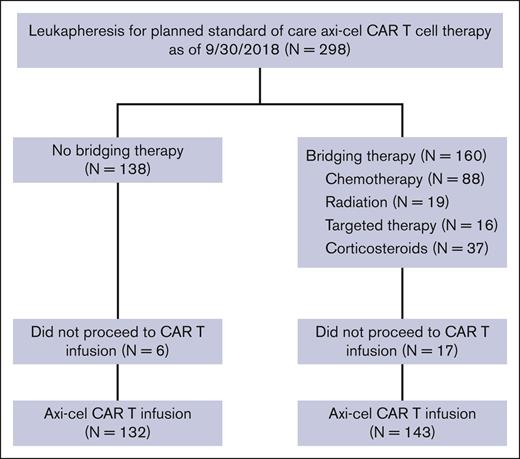
Of the 298 patients who underwent leukapheresis, 23 patients did not receive axi-cel, 20 declined in performance status or died from disease progression before CAR T cells could be administered, 1 was deferred because of a serious infection, 1 was deferred because of renal failure, and 1 had nonmeasurable disease after BT with steroids. Of the 275 patients who received axi-cel (infused or mITT cohort), 143 patients (52%) had received BT. The median time from leukapheresis to initiation of lymphodepleting chemotherapy was 26 days in the whole cohort. Of the 298 who underwent leukapheresis or the ITT cohort, 160 patients (54%) received BT. Although 89% of patients (143 of 160) who underwent leukapheresis followed by BT ended up receiving axi-cel, 96% of patients (132 of 138) of patients with no BT after leukapheresis ended up receiving axi-cel infusion (Figure 1).
Patients who received BT had a higher proportion of high-risk baseline characteristics than those who did not (Table 1). These included poor performance status (ECOG status ≥ 2), higher IPI scores, higher rates of bulky disease, a higher LDH level, and higher rates of patients not meeting ZUMA-1 eligibility criteria (Table 1).
Response and survival
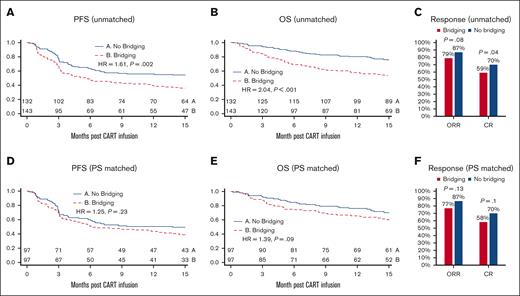
Unadjusted PFS (hazard ratio [HR], 1.61; 95% confidence interval [CI], 1.18-2.18; P = .002) and OS (HR, 2.04; 95% CI, 1.42-2.92; P < .001) periods were shorter in the BT group than in the no-BT group (Figure 2A-B). In multivariate analysis, after correcting for confounding factors (including sex, ECOG, stage, number of lines of therapy, refractory, prior autologous stem cell transplant, LDH at conditioning, bulky disease, and meeting all the eligibility criteria for ZUMA-1), there was no longer a difference in PFS (HR, 1.13; 95% CI, 0.8-1.6; P = .481) or OS (HR, 1.47; 95% CI, 0.99-2.18; P = .053) between the BT and no-BT groups. After axi-cel infusion, patients in the no-BT group had a significantly higher complete response (CR) rate (70% vs 59%; P = .04) and a numerically higher ORR that did not reach statistical significance (87% vs 79%; P = .08) (Figure 2C) than those in the BT group. PS matching analysis showed no statistically significant differences in PFS (HR, 1.25; P = .23), OS (HR, 1.39; P = .09), ORR, and CR rates between the BT and no-BT groups (Figure 2D- F).
PFS, OS, and response to axi-cel in the mITT cohort. Kaplan-Meier curves of PFS and OS. Curves start at the time of axi-cel infusion and are stratified according to BT. Unadjusted mITT analyses compare the n = 132 patients without BT with the n = 143 patients who received BT. After PS matching, n = 97 patients were matched and compared in each group. Unadjusted PFS and OS (A,B) and PFS and OS after adjustment for baseline characteristics with PS matching (D,E). The ORR and CR rates in the mITT cohort before matching (C) and after PS matching (F). CART, CAR T-cell therapy.
PFS, OS, and response to axi-cel in the mITT cohort. Kaplan-Meier curves of PFS and OS. Curves start at the time of axi-cel infusion and are stratified according to BT. Unadjusted mITT analyses compare the n = 132 patients without BT with the n = 143 patients who received BT. After PS matching, n = 97 patients were matched and compared in each group. Unadjusted PFS and OS (A,B) and PFS and OS after adjustment for baseline characteristics with PS matching (D,E). The ORR and CR rates in the mITT cohort before matching (C) and after PS matching (F). CART, CAR T-cell therapy.
Toxicities
The toxicities among patients who received BT vs no BT in the mITT group are listed in Table 2. After axi-cel infusion, patients in the BT group had similar rates of severe (grade 3 or higher) cytokine release syndrome (CRS) (8% vs 5%; P = .35) and severe immune effector cell–associated neurotoxicity syndrome (ICANS; 34%, vs 27%; P = .2). However, the rate of intensive care unit (ICU) admission was higher in the BT group (42% vs 23%; P = .001), as was the median duration of hospital stay (mean, 18.5 vs 15.7 days; P = .04). Although data on cytopenia were not collected, the use of granulocyte colony-stimulating factor (G-CSF) after CAR T-cell therapy was higher in the BT group (48% vs 33%; P = .01; Table 2). After PS matching, there were no statistically significant differences in rates of grade 3 or higher CRS and grade 3 or higher ICANS, use of G-CSF, rates of ICU admission, and duration of hospital stay between the BT vs no-BT groups (Table 2).
As of the data cutoff on 11 July 2021, a total of 129 patients in the mITT cohort had died, and of those, 82 patients (64%) had received BT. Nine patients (7%) in the BT group and 3 (2%) in the no-BT group died of nonrelapse causes.
Effects of BT subtypes on outcomes
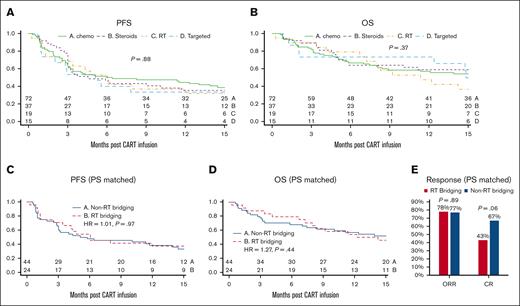
Bridging therapies were provided at the discretion of the treating physician, resulting in variation in dosing, modalities, and regimens used (supplemental Table 1). To facilitate analysis, we categorized the BT regimens (mITT N = 143) used into chemotherapy (n = 72), targeted therapy (n = 15; ie, lenalidomide, ibrutinib, etc), radiation therapy only (n = 19), and corticosteroids (n = 37). No significant difference was observed in the PFS (median PFS, 6.2, 5.1, 6.0, and 4.5 months, respectively, with univariate P = .88 and multivariate analysis P = .75) and OS (median OS, 19.6, 14.9, 11.4 months for the first 3 groups and not reached for corticosteroids, with univariate P = .37 and multivariate analysis P = .64) among patients who received bridging chemotherapy, targeted therapy, radiation therapy, and corticosteroids, respectively (Figure 3A-B). In addition, we compared BT regimens that included radiation therapy (RT bridging) (N = 26) with all other BT regimens combined (non-RT bridging) (N = 117). RT bridging group in this analysis included 19 patients who received only bridging radiation therapy and 7 patients who received bridging radiation plus another systemic therapy and were then infused with axi-cel (mITT). Given the potential confounding variables associated with the use or nonuse of radiation as BT, we used PS matching to identify a group of 44 subjects from the non-RT bridging group who had matched baseline characteristics to 24 subjects who received RT bridging. After PS matching, no statistically significant differences were observed in PFS, OS, ORR, or CR rates between the RT bridging and non-RT bridging groups (Figure 3C-E).
Outcome after axi-cel stratified based on the category of BT. Kaplan-Meier curves starting from the time of axi-cel infusion, showing unadjusted PFS (A) and OS (B) comparing patients who received chemotherapy (chemo; n = 72), corticosteroids only (steroids; n = 37), radiation therapy alone (RT; n = 19), and targeted therapies (targeted; n = 15). Kaplan-Meier curves depicting PFS (C) and OS (D) after PS matching stratified based on any RT (n = 24) bridging vs non-RT bridging (n = 44). The ORR and CR rates in RT bridging vs non-RT bridging after PS matching (E).
Outcome after axi-cel stratified based on the category of BT. Kaplan-Meier curves starting from the time of axi-cel infusion, showing unadjusted PFS (A) and OS (B) comparing patients who received chemotherapy (chemo; n = 72), corticosteroids only (steroids; n = 37), radiation therapy alone (RT; n = 19), and targeted therapies (targeted; n = 15). Kaplan-Meier curves depicting PFS (C) and OS (D) after PS matching stratified based on any RT (n = 24) bridging vs non-RT bridging (n = 44). The ORR and CR rates in RT bridging vs non-RT bridging after PS matching (E).
ITT analysis
We conducted an ITT analysis comparing BT (n = 160) vs no BT (n = 138) for all patients who underwent leukapheresis, regardless of whether they ultimately received axi-cel infusion (Figure 1). Baseline characteristics of the leukapheresis/ITT cohort are listed in supplemental Table 2. After PS matching, the ITT analysis yielded results similar to those of the mITT analysis. No statistically significant differences were observed between the BT and no-BT groups in terms of ORR, rate of CR, PFS, OS, rates of grade 3 and higher CRS and grade 3 and higher ICANS, need for G-CSF, rates of ICU admission, and duration of hospital stay (supplemental Table 3; supplemental Figure 1). On analyzing the effects of different subtypes of BT, there was no difference in PFS and OS among patients who received bridging chemotherapy, targeted therapy, radiation therapy, and corticosteroids (supplemental Figure 2A-B). Additionally, we compared RT bridging (N = 27) with non-RT bridging (N = 133). To address the potential confounding variables associated with the use or nonuse of radiation as BT, we used PS matching to identify a cohort of 43 subjects from the non-RT bridging group with similar baseline characteristics to 25 subjects who received RT bridging. After PS matching, there were no statistically significant differences in the PFS, OS, CR rate, and ORR in RT bridging vs non-RT bridging group on ITT analysis (supplemental Figure 2C-E).
Discussion
In this multicenter, retrospective study, we analyzed the characteristics and outcomes of patients who received BT before axi-cel infusion for R/R LBCL. We found that patients chosen to receive BT had a higher proportion of high-risk features for axi-cel failure, including poor performance status, high IPI score, bulky disease, and elevated LDH. In unadjusted analysis patients who received BT and axi-cel in mITT had significantly worse PFS and OS and lower CR rates. However, after multivariate analysis and PS matching to adjust for differences in baseline characteristics between the 2 groups there was no significant difference between PFS, OS, ORR, and CR rates. Although BT did not increase the risks of CAR T-cell therapy, the types of BT used were also unsuccessful at improving the outcomes of patients with high-risk features.
We attempted to determine whether any 1 type of BT was superior to others. In our study, patients received bridging with 1 or more of chemotherapy, corticosteroids, radiation, and targeted therapies. Our analysis was limited by a relatively small sample size for each category. Nevertheless, we found no statistically significant differences in the PFS or OS between the different types of BTs.
Previous preclinical studies have suggested that radiation therapy could be a superior BT for patients receiving CAR T-cell therapy, because of the abscopal effect it can create in radiated tissue and tumor sites outside of the radiation field.5-7 However, in our study, we found no statistically significant differences in treatment outcomes between patients who received RT bridging and those who received non-RT bridging, after performing PS matching to correct for baseline confounding factors. Interestingly, patients who received bridging radiation therapy had a numerically lower CR rate than those who received other systemic BTs, even after PS matching, but this difference did not reach statistical significance (43% vs 67%, respectively; P = .06). It is important to note that our study as limited by its retrospective nature, in that, no single dose or schedule of radiation was used. In addition, we did not examine differences between focal or comprehensive radiation, the latter of which was recently shown to be associated with improved CAR T-cell therapy efficacy in a single-center study.8
In our previous analysis of this cohort, we performed multivariable modeling and found that bridging was a covariate associated with worse OS but not PFS at an average follow-up of 12.9 months after axi-cel infusion.9,10 We now find that, at longer follow-up, there are no statistically significant differences in the PFS and OS between BT and no-BT groups after multivariate analysis and PS matching.
A limitation of retrospective data is that it cannot account for unknown covariates; in this case, that may affect the propensity to receive BT. To account for these covariates, in addition to performing multivariate analysis, we conducted PS matching analysis, comparing patients who received BT vs those who did not. Our PS matching analysis showed no statistically significant differences in treatment outcomes after axi-cel infusion between patients who received BT and those who did not.
Given that the primary goal of BT was to keep the patient alive until the CAR T cells could be manufactured, one could argue that to fully assess the impact of BT, we should analyze its effects on patients who underwent leukapheresis, regardless of whether they ultimately received an axi-cel infusion. This type of analysis, known as ITT analysis, would allow us to better evaluate the true impact of BT. Our ITT analysis, which included all patients who underwent leukapheresis, produced results similar to those of the mITT analysis, which included patients who ultimately received an axi-cel infusion. Specifically, we found no statistically significant differences in treatment outcomes between the BT and no-BTgroups in the ITT analysis. Furthermore, our ITT analysis also showed no significant differences in outcomes between the different subgroups of BT, including the comparison of radiation therapy vs other BTs combined.
There is clearly a subset of patients who will require BTs, without which they would die or not be able to receive CAR T cells; however, identification of these patients at the time of leukapheresis is difficult and frequently relies on clinical judgment. One of the potential advantages of allogeneic CAR T cells is to provide an off-the-shelf product that eliminates the bridging period. However, allogeneic CAR T cells continue to have barriers in terms of persistence, which is likely driven by host responses against the donor CAR T cells, and it remains to be seen whether they can match the efficacy of autologous CAR T cells.11
Although our study included only patients treated with axi-cel in the standard-of-care setting, it is possible that bridging has a similar effect for other CAR T-cell products for LBCL, including tisa-cel or liso-cel. In the JULIET trial of tisa-cel, 92% of patients received bridging, whereas in the TRANSCEND trial of liso-cel, 59% of patients received BT.2,3 This is in contrast to ZUMA-1, in which BT was not allowed before axi-cel infusion.1 However, for JULIET and TRANSCEND, the manufacturing time periods were longer than for ZUMA-1, and the decision to provide BT in JULIET and TRANSCEND might have been different from that in our standard-of-care axi-cel cohort.
Shahid et al recently reported that BT in a group of patients with mainly LBCL and follicular lymphoma receiving 5 different types of CAR T-cell therapies was not associated with differences in ORR, CR rate, or PFS. However, they found that BT was associated with inferior OS.12 Our study exclusively analyzed patients with LBCL who all received axi-cel. Furthermore, in addition to multivariate analysis, we performed PS matching to better assess the potential impact of confounding variables on our findings.
Pinnix et al reported effects of BT in patients with large cell lymphoma receiving axi-cel at a single center.13 They reported no differences in ORR, CR rate, PFS, and OS between BT vs no-BT groups. On analyzing the different subtypes of BT, they reported that radiation therapy was associated with improved PFS compared with systemic BT. However, our analysis, which had a larger cohort, a longer follow-up period ,and included PS matching analysis, did not show any significant differences in treatment outcomes between patients who received radiation BT and those who received systemic BT.
Notably, this study did not include any patients who received BT with polatuzumab vedotin, which, at the time of this study, was not approved by the Food and Drug Administration. This antibody-drug conjugate is reported to have an ORR of 70% when combined with bendamustine among patients with R/R DLCBL.14 Our study is limited to BTs available before 2019; therefore, none of our patients received bridging with polatuzumab vedotin or other more recently approved therapies. Further investigation is needed to determine the impact of novel bridging strategies on the outcomes of patients undergoing CAR T-cell therapy. Indeed, it has been shown that response to BT is a predictor of post–CAR T-cell therapy outcomes.15,16 In our standard-of-care study, patients were inconsistently restaged after BT, and we were, therefore, not able to assess response rates to the various bridging strategies.
Another limitation is that events that are less frequent (specifically ICU admission and deaths) may be underpowered to observe differences. This is exacerbated by PS matching because patients without matches are excluded. In unmatched analyses, ICU admissions and nonrelapse mortality were higher in the bridging group. After PS matching, the differences between groups were smaller and did not meet the threshold for significance. Further study of larger data sets are needed to determine if bridging contributes to ICU admissions or deaths from nonrelapse mortality.
In summary, this study suggests that at long-term follow-up, current bridging therapies do not significantly affect treatment outcomes after CAR T-cell therapy, and bridging radiation therapy is not superior to nonradiation BT. However, decisions to provide BT should be carefully considered on an individual basis. Additional studies are needed to determine whether any type of BT can improve the outcomes of patients receiving autologous CAR T-cell products.
Acknowledgments
The research reported in this publication was supported by grants from the National Institutes of Health National Cancer Institute K12CA167540 (M.T.J.), the ASCO Young Investigator Award via Conquer Cancer Foundation (M.T.J.), and Dean’s Scholars award from the Washington University Division of Physician-Scientists, which is funded by a Burroughs Wellcome Fund Physician-Scientist Institutional award (M.T.J.). F.L.L. is funded by the Leukemia and Lymphoma Society as a Clinical Scholar, and by the National Institutes of Health National Cancer Institute (R01CA244328-01; principal investigators, F.L.L. and Jim; P30 CA076292; principal investigator, Cleveland)
Authorship
Contribution: M.T.J., M.D.J., A.G., and F.L.L. conceptualized the study; M.T.J., M.D.J., F.G., A.G., and F.L.L. designed the study; and all authors provided study materials or patients, collected and assembled data, performed data analysis and interpretation, wrote the manuscript, and approved it.
Conflict-of-interest disclosure: M.D.J. reports consultancy/advisory for Kite/Gilead and Myeloid Therapeutics and received research funding from Kite/Gilead, Incyte, and Loxo@Lilly. L.J.N. has received honorarium for participation in advisory boards/consulting from AbbVie, ADC Therapeutics, Atara Biotherapeutics, Bristol Myers Squibb (BMS)/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, Janssen, Incyte, Merck, Novartis, and Takeda and received research support from BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. Y.L. reports consulting or advisory role at Kite/Gilead, Novartis, bluebird bio, Celgene, Juno Therapeutics, BMS, Gamida Cell, Legend Biotech, Sorrento Therapeutics, Vineti, Janssen Oncology, and Pfizer (all to institution); and received research funding from Janssen Oncology, Janssen Oncology, Merck, Takeda, Boston Scientific, Kite/Gilead, BMS, and bluebird bio (all to institution). S.D. reports consulting or advisory role at Kite/Gilead. M.L. reports consultancy/advisory for AbbVie, Acrotech, ADC Therapeutics, AstraZeneca, Astellas, BMS, Caribou, CRISPR, Daiichi Sankyo, EUSA, Fate Therapeutics, Genentech, Genmab, Instil Bio, Ipsen, Janssen, Kite, Loxo, Miltenyi, MorphoSys, Novartis, Nurix, Pharmacyclics, Regeneron, Sanofi, Seagen, and Takeda, TG Therapeutics and research Funding for BMS and Curis. P.R. received research support from Seagen and Seattle Genetics and consulting for Kite Pharma-Gilead and Caribou Biosciences. O.O. reports consulting or advisory role at Kite/Gilead, Legend Biotech, Curio Science, Novartis, ADC Therapeutics, Syncopation Life Sciences, Nektar, Gilead Sciences, and Epizyme and received research Funding from Kite, a Gilead company (to the institution). J.M. reports consulting/advisory for AlloVir, Novartis, Kite, and BMS. A.D. reports consulting or advisory role at Novartis, Kite/Gilead, Agios, Juno/Celgene, Janssen, and Adicet Bio. A.R.S. received research funding from Kite Pharma–Gilead and BMS–Juno Therapeutics. A.G. received grant/research support as a principal investigator (research to institution) for Acerta, AstraZeneca, BMS, Celgene, Genentech, Infinity Pharmaceuticals, Janssen, Janssen Global Services, Karyopharm, Kite Pharma, Pharmacyclics, Seattle Genetics, and Verastem; is a stock/shareholder of COTA, Alloplex, Resilience, and Genomic Testing Cooperative; is a consultant at Pharmacyclics LLC, an AbbVie Company, and Janseen Global Services LLC, AbbVie, Clinical Advances in Hematology & Oncology, Novartis, Michael J. Hennessey Associates, Novartis, and BMS; is on the Advisory board of Kite, Janseen Biotech, and Pharmacyclics LLC; is a speaker at Physicians Education Resource, LLC and third GCC Hematology Expert Forum; is on the board of directors at COTA, Resilience, Genomic Testing Cooperative; is a consulting faculty at Michael J Hennessey Associates, , and Physcians Education Resource, LLC; is on the scientific advisory board at Alloplex, BMS, and Vincerx; is on the steering committee at Astrazenca, Pharmacyclics LLC, an AbbVie Company, and Janssen Biotech. C.A. reports consulting or advisory role at Gilead Sciences, Kite, a Gilead company, Karyopharm Therapeutics, Atara Biotherapeutics, Incyte, TG therapeutics, and Epizyme and received research funding from Novartis, Merck, BMS, and Genmab. J.M. received honoraria from Kyowa Hakko Kirin, Seattle Genetics, Targeted Oncology, Onc view, Curio Science, and Physicians' Education Resource; reports a consulting or advisory role at Kite, a Gilead company, Pfizer, Pharmacyclics, Bayer, Alexion Pharmaceuticals, BMS, Janssen, Seattle Genetics, Gilead Sciences, Kyowa Hakko Kirin, Juno Therapeutics, Genentech, Celgene, BeiGene, Fosun Kite, Innovent Biologics, Debiopharm Group, Karyopharm Therapeutics, Genmab, ADC Therapeutics, Epizyme, Servier, Novartis, MorphoSys, Aurobindo, Lilly, and Secura Bio; is on the speakers' bureau at Kite, a Gilead company, Bayer, Pharmacyclics/Janssen, AstraZeneca, Gilead Sciences, Seattle Genetics, Kyowa Hakko Kirin, Acrotech Biopharma, BeiGene, Verastem, Celgene, and AbbVie/Genentech; and received research funding from Kite, a Gilead company, Celgene, Portola Pharmaceuticals, Incyte, Genentech/AbbVie, Pharmacyclics/Janssen, Seattle Genetics, and Millennium. J.C.C. reports consultancy/advisory/honoraria for Kite/Gilead, Novartis, Karyopharm, MorphoSys, BeiGene, AbbVie, ADC Therapeutics, BMS, Epizyme, Genentech, and Bayer and research funding from AstraZeneca, Merck, and Adaptive. N.N.B. received research funding from Kite Pharma-Gilead and Affimed and is an advisory board member for Daiichi Sankyo, Verastem, and Purdue Pharma. J.M.V. received honoraria from Acerta Pharma/AstraZeneca, MorphoSys, Johnson and Johnson, MEI Pharma, Lilly, AbbVie, and Merck; received research funding from Celgene, Incyte, Acerta Pharma, Kite, a Gilead company, Seattle Genetics, Novartis, BMS, AstraZeneca (all to institution), Loxo, and Epizyme. D.B.M. received honoraria from Janssen and Fosun Kite Biotechnology; reports consulting or advisory Role at Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics, and Janssen; received research funding from Pharmacyclics, Novartis, Roche/Genentech, Kite, a Gilead company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, and Adicet Bio; reports patent held with Pharmacyclics supporting ibrutinib for chronic graft-versus-host disease (no royalty claim). S.S.N. holds stock and other ownership interests in Longbow Immunotherapy Inc; received honoraria from Bio Ascend, Medscape, Aptitude Health, and MJH Life Sciences; reports consulting or advisory role at Merck Sharp & Dohme, Kite, a Gilead company, Novartis, Incyte, Gilead Sciences, Alimera Sciences, BMS, Adicet Bio, Calibr, Athenex, Sellas Life Sciences, bluebird bio, and Sana Biotechnology; received research funding from BMS, Kite, a Gilead company, Cellectis, Poseida Therapeutics, Unum Therapeutics, Gilead Sciences, Alimera Sciences, Precision BioSciences, and Adicet Bio; and reports patents related to cellular therapy and royalty income from Takeda Pharmaceuticals. F.L.L. reports consulting or advisory Role at Novartis, Celgene, Calibr, Alimera Sciences, Gerson Lehrman Group, EcoR1 Capital, Amgen, bluebird bio, BMS, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, BMS/Celgene, Janssen, A2 Biotherapeutics, Miltenyi Biotec, Caribou Biosciences, Takeda, and Umoja Biopharma; received research funding from Kite, a Gilead company, Alimera Sciences, Novartis, bluebird bio, BMS/Celgene (all to the institution); reports patents, royalties, and other intellectual property (all to the institution): Double Mutant Survivin Vaccine, US010414810B2; CAR T cells with enhanced metabolic fitness, serial no.: 62/939 727; methods of enhancing CAR T-cell therapies, serial no.: 62/892 292; evolutionary dynamics of non-Hodgkin lymphoma CAR T-Cell therapy, serial no.: 62/879 534; and received travel, accommodation and expenses from Kite, a Gilead company, and A2 Biotherapeutics. A.G. received research support from Kite Pharma-Gilead and Amgen and is an advisory board member/consultant for Kite Pharma-Gilead, Amgen, Celgene, EUSA, Atara, CRISPR Therapeutics, and Wugen. The remaining authors declare no competing financial interests.
Correspondence: Armin Ghobadi, Division of Medical Oncology, Section of Stem Cell Transplant and Leukemia, Clinical Director, Center for Gene and Cellular Immunotherapy (CGCI), Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007-29, St Louis, MO 63110; email: arminghobadi@wustl.edu.
References
Author notes
M.D.J. and M.T.J. contributed equally to this work.
F.L.L. and A.G. contributed equally to this work.
Deidentified patient data are available on a case-by-case basis on request from the corresponding author, Armin Ghobadi (arminghobadi@wustl.edu).
The full-text version of this article contains a data supplement.