This study identified 59 new signals of DIIHA in Vigibase.
An association was found for some antibiotics, antifungal drugs, ibuprofen, acetaminophen, furosemide, azathioprine, and iomeprol.
Visual Abstract
More than 130 drugs have been suspected to induce immune hemolytic anemia. Comparative studies measuring the risk of drug-induced immune hemolytic anemia (DIIHA) are lacking. We aimed (1) to detect new signals of DIIHA, excluding vaccines, and (2) to assess the association between all suspected drugs and the occurrence of immune hemolytic anemia in a nationwide comparative study. The new signals were identified using a disproportionality study (case/noncase design) in the World Pharmacovigilance Database, Vigibase, among the cases of adverse drug reactions reported up to February 2020 (>20 million). We then conducted a comparative study in the French National health database that links sociodemographic, out-of-hospital, and hospital data for the entire population (67 million individuals). Associations between exposure to drugs (those already reported as DIIHA, plus new signals identified in Vigibase) and incident cases of immune hemolytic anemia (D59.0 and D59.1 diagnosis codes of the International Classification of Diseases, version 10) from 2012 to 2018 were assessed with case-control and case-crossover designs. In Vigibase, 3371 cases of DIIHA were recorded. Fifty-nine new signals were identified resulting in a final list of 112 drugs marketed in France and measurable in the nationwide cohort (n = 4746 patients with incident immune hemolytic anemia included in the case-control analysis matched with 22 447 controls from the general population). We identified an association between immune hemolytic anemia occurrence and some antibiotics, antifungal drugs, ibuprofen, acetaminophen, furosemide, azathioprine, and iomeprol.
Introduction
Autoimmune hemolytic anemia (AIHA) is a rare disease characterized by the destruction of red blood cells due to autoantibodies.1 The incidence rate of AIHA has been recently estimated in France between 2012 and 2017 at 2.44 per 100 000 person-years (95% confidence interval [95% CI], 2.39-2.48).2
Primary AIHA is defined by the absence of underlying disorder.1 Secondary AIHA, representing half of the cases, corresponds to the association of AIHA with hematologic malignancy, systemic autoimmune disease, infection, or inherited immunodeficiency.1,3 The international consensus on AIHA diagnosis and treatment also lists drug-induced immune hemolytic anemia (DIIHA) as a secondary cause of AIHA that should be systematically searched.1 The challenge is to rapidly diagnose DIIHA in order to withdraw the drug as soon as possible. Various mechanisms by which drugs stimulate autoantibodies formation have been suggested: drug-dependent autoantibodies due to an immuno-allergic mechanism, drug-independent autoantibodies due to molecular mimicry, or nonspecific stimulation of the immune system.4 For some drugs, nonimmunological mechanisms such as protein adsorption can be rarely involved.4 They are, nevertheless, listed by the international consensus as causes of DIIHA.1 In 2014, more than 130 drugs have been described to potentially induce immune hemolytic anemia (supplemental Table 1).5
Some cases of DIIHA have been experimentally demonstrated by the serological detection of specific drug-dependent antibodies. However, most drugs are suspected from case reports or retrospective series. These drugs were identified as responsible for DIIHA by the absence of other causes of secondary AIHA, the compatible time from drug initiation to immune hemolytic anemia onset, and the recovery after drug withdrawal. However, comparative studies assessing the risk of DIIHA in the population are lacking. Only 1 case-control study has been conducted: the Berlin Case Control Study (FAKOS), which included 124 patients with immune hemolytic anemia and 731 matched controls from the general population between 2000 and 2009.6 In this study, the exposure to drugs within the 4 weeks before the index date (immune hemolytic anemia diagnosis for cases and similar date for their matched controls) was compared between cases and controls. Associations of immune hemolytic anemia occurrence with some antibiotics, lorazepam, fludarabine, and diclofenac were found. The study faced a lack of power to provide precise estimates and to investigate other drugs. Moreover, some differences between the cases and the controls might have influenced the exposure to drugs.
The aims of this 2-step study were (1) to detect new signals of drugs potentially responsible for DIIHA, excluding vaccines, and (2) to assess the association between all the previous suspected drugs and the occurrence of immune hemolytic anemia in a nationwide comparative study. We did not include vaccines in this study because vaccine-induced autoimmune diseases are a particular question needing specific methodological considerations.7,8
Methods
Step 1: signal detection of potential DIIHA
Data source
The first step of the study was conducted using the World Health Organization (WHO) pharmacovigilance database, Vigibase, that records all cases of suspected adverse drug reactions (ADRs) reported to the national pharmacovigilance systems from >148 countries.9 We selected the reports from January 1967 (creation of the database) to February 2020 (>20 million of ADR reports). For each report, the registered data are the reporter qualification, the patient’s age and sex, the seriousness of the case, the suspected and concomitant drugs (ie, nonsuspected drugs), and the suspected ADR. The ADRs were encoded using the Medical Dictionary for Regulatory Activities, which is organized into 5 hierarchical levels from the largest to the more precise (system organ class, high-level group term, high-level term, preferred term, and lowest level term).10
Study design
We performed a disproportionality analysis using a case/noncase design. This method is the gold standard for the identification of new pharmacovigilance signals. The principle is to highlight an excess in reporting a specific ADR (here, immune hemolytic anemia) associated with a suspected drug, compared with the randomly expected cases reported whether the drug is not associated with this ADR.11 To detect a new signal, the frequency of exposure to a drug among immune hemolytic anemia reports (cases) was compared with the frequency of exposure of this drug in all other reports (noncases).
Cases
Cases of immune hemolytic anemia were selected using the 5 preferred terms autoimmune anemia, autoimmune hemolytic anemia, Coombs–positive hemolytic anemia, Evans syndrome, and warm-type hemolytic anemia as well as the 6 lowest level terms hemolytic autoimmune anemia direct Coombs–negative, hemolytic autoimmune anemia direct Coombs–negative, hemolytic anemia with antidrug antibody, hemolytic anemia with antidrug antibody, immune hemolytic anemia drug-induced, and immune hemolytic anemia drug-induced. These terms were selected by 3 authors: 2 internal medicine physicians specializing in autoimmune cytopenia (J.M. and G.M.) and 1 pharmacologist (M.L.).
Exposures
All drugs that were recorded as suspected in at least 1 case were tested. Drugs used as first-line therapy in AIHA (systemic corticosteroids) and those used as symptomatic treatment of hemolysis (folinic acid and erythropoietin) were excluded to overcome indication bias.12 Moreover, because classification bias was expected for some cases (ie, nonimmunological hemolysis wrongly reported as immune hemolytic anemia), all the drugs identified as new signals were screened to exclude those with well-known other mechanisms of hemolysis (eg, dapsone).
Statistical analysis
Univariate logistic regression was used to conduct disproportionality analyses, resulting in the calculation of reporting odds ratios (RORs) with their 95% CI.11 Drugs with at least 3 ADR reports of immune hemolytic anemia and a lower boundary of 95% CI of the ROR >1 were defined as signals of potential DIIHA.13
Disproportionality analyses were performed using R Studio V1.2.5033.
Step 2: association between drugs potentially associated with DIIHA and immune hemolytic anemia occurrence in a nationwide comparative study
Data source
The source of data was the French National health database (Système National des Données de Santé [SNDS]).14-16 SNDS prospectively collects health data for all insured individuals in France since 2009, covering virtually the entire population (67 million habitants). It contains sociodemographic data (age, sex, place of residency, and date of death [if any]); reimbursed out-of-hospital health expenditures, including drugs dispensed at community pharmacies (prescription data are not recorded); long-term diseases (ie, chronic diseases that give right to the full reimbursement of health care, recorded by general practitioners); and hospital data (dates of hospitalization, discharge diagnoses, procedures, and expensive drugs dispensed during the stay). Long-term diseases and hospital discharge diagnoses are encoded with the international classification of diseases, version 10 (ICD-10). Importantly, out-of-hospital medical records as well as nonexpensive drugs dispensed during hospital stays are not registered in the SNDS.
Study population
The study population was the AHEAD (Autoimmune HEmolytic Anemia: a population-baseD study) cohort, built in the SNDS, which includes all patients aged ≥15 years in France with an incident AIHA between 2012 and 2018.2 The patients were identified with the D59.0 (DIIHA) and D59.1 (AIHA) ICD-10 codes as hospital discharge diagnosis or long-term disease. In a previous validation study, the D59.1 codes yielded to a positive predictive value of 90% (95% CI, 80-96).17 The date of immune hemolytic anemia diagnosis was defined based on the first occurrence of D59.0 or D59.1 code after a prior observation period of at least 2 years in the SNDS. We excluded patients with secondary AIHA, defined by a hospital or a long-term disease diagnosis code of disease that causes secondary AIHA in the year before the diagnosis of immune hemolytic anemia (codes are listed in the supplemental Table 2).
Each patient was matched for the year of birth, sex, and place of residency with 5 controls randomly selected from the general population. The index date was the date of immune hemolytic anemia diagnosis for cases and the same date for their corresponding controls.
Study design
We used 2 complementary designs: case-control and case-crossover (CCO) designs.18
Outcome
The outcome was the incident diagnosis of immune hemolytic anemia.
Exposure
Exposures to the drugs marketed in France during the study period and potentially associated to DIIHA, that is, those previously listed by Garratty et al5 as well as those identified as new signals at the first step of this study, were searched in the out-of-hospital dispensing data. Patients and controls hospitalized during the periods of interest for drug exposure (defined later in the article as the case period and control period in the CCO design and the period preceding the index date in the case-control design) were excluded to avoid measurement bias.
Confounding variables
Comorbidities present before the index date and were included in the Charlson comorbidity index were identified in the SNDS using the algorithm by Bannay et al.19
Analytic methods
Case-control design
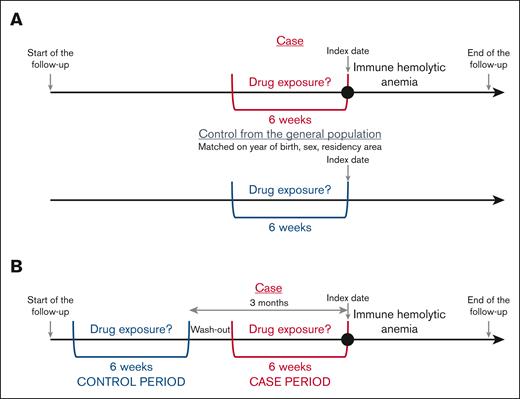
The drug exposures were searched during the 6 weeks before the index date (immune hemolytic anemia diagnosis) in cases and controls (Figure 1A). The analyses were conducted for drugs with at least 5 cases exposed.
CCO design
This self-controlled design, derived from the case-control approach, was first described by Maclure et al.18 Each case serves as its own control. Consequently, non–time-dependent confounding factors are controlled. Only the patients with immune hemolytic anemia were included in the analysis. The frequency of exposure to each drug during the case period, that is, the 6 weeks preceding immediately the immune hemolytic anemia diagnosis, was compared with the frequency of exposure during a 6-week control period, 3 months before immune hemolytic anemia diagnosis (Figure 1B). The analyses were conducted for drugs with at least 5 patients exposed in the case period and unexposed in the control period (discordant pairs).
Statistical analyses
Conditional logistic regressions were performed for the 2 designs, computing odds ratios (ORs) and their 95% CI. The case-control analysis was adjusted for the Charlson comorbidity index score.
Data management and statistical analyses were carried out using SAS Entreprise Guide version 4.3 software (Cary, NC).
Ethical issues
The access to Vigibase data was permitted by the Uppsala Monitoring Center and the WHO collaborating center and was accessed by our clinical pharmacology department, which is part of the WHO pharmacovigilance network.
According to the French law, authorizations regarding the AHEAD cohort were obtained from the Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé on 11 July 2019 (TPS 532004) and the Commission Nationale de l’Informatique et des Libertés on 2 August 2019 (DR-2019-229).
The data contained in SNDS are anonymous. Because of the very nature of SNDS, we had no access to patients' identities, so consent was neither necessary nor possible.
Results
Step 1: signal detection of potential DIIHA
Cases of DIIHA
During the study period, 3371 cases of DIIHA were reported to Vigibase. Cases are described insupplemental Table 3. Among these cases, 789 different drugs were suspected. Methyldopa was the most frequent (n = 240 cases).
Disproportionality analysis
We found 64 associations using the case/noncase design in Vigibase. Five drugs were subsequently excluded because of another well-documented mechanism of hemolysis (dapsone, ribavirin, artemether-lumefantrine, and artesunate), resulting in a final list of 59 new signals (Table 1). Notably, we found new signals for some antibiotics (fixed association of sulfamethoxazole and trimethoprim and 2 cephalosporins: cefpodoxime and cefepime), antineoplastics, and immunomodulating agents. The highest ROR was for pentostatin: 90.2 (95% CI, 48.3-168.6).
Step 2: association between drugs potentially responsible for DIIHA and immune hemolytic anemia occurrence at the population level in the nationwide comparative study
Study population
Between 1 January 2012 and 31 December 2018, a total of 6553 patients aged ≥15 years with incident primary AIHA were included in the AHEAD cohort. Among them, 3728 (56.9%) were women, and the median age was 71 years (interquartile range: 55-82). The patients’ characteristics are presented in the supplemental Table 4.
After the exclusion of the patients hospitalized during the 6-week period before the index date, 4746 cases (15.1% identified using the D59.0 diagnosis code) matched with 22 447 controls from the general population were included in the case-control analysis (supplemental Table 4). The median age was 70 years, and 58.8% of cases were women. Comorbidities from the Charlson comorbidity index were more frequent in cases than in controls (64.6% vs 47.2%). For the CCO analyses, 4419 patients were included (supplemental Table 4).
Drugs potentially associated with DIIHA
Combining the list by Garratty et al and the new pharmacovigilance signals identified at “Step 1” of this study, 198 drugs were identified as potentially associated to DIIHA, including 146 marketed in France. Among them, 112 were available in the out-of-hospital dispending data of the SNDS (supplemental Table 5). Of note, some of these drugs are only marketed or prescribed in France as fixed association (carbidopa and benserazide are in association with levodopa and sulfamethoxazole with trimethoprim).
Associations between drug exposures and immune hemolytic anemia occurrence
The results of the comparative analyses conducted in the AHEAD cohort are presented in Table 2. Using the 2 designs, we found an association with immune hemolytic anemia occurrence for 14 drugs: furosemide, amoxicillin with and without clavulanic acid, ceftriaxone, cefixime, cefpodoxime, the fixed association of sulfamethoxazole and trimethoprim, ciprofloxacin, norfloxacin, amphotericin B, azathioprine, ibuprofen, acetaminophen, and iomeprol. The highest risk was found for azathioprine: adjusted OR (aOR) in the case-control design was 8.6 (95% CI, 4.5-16.5), and OR in the CCO design was 4.0 (95% CI, 1.1-14.2).
For some other drugs, an association was found using the case-control analysis but was not confirmed with the CCO analysis: ranitidine, insulin, methyldopa, urapidil, hydrochlorothiazide, hydrocortisone, acyclovir, mycophenolic acid, ciclosporin, tacrolimus, hydroxycarbamide, diclofenac, etodolac, and apomorphine. On the contrary, an association was found in the CCO analysis only for fluconazole with an aOR in the case-control design of 1.4 (95% CI, 0.8-2.5) and an OR in the CCO design of 4.2 (95% CI, 1.6-11.1) as well as for cloxacillin with an aOR in the case-control design of 1.5 (95% CI, 0.7-3.2) and an OR in the CCO design of 11.0 (95% CI, 1.4-85.2).
Discussion
This 2-step study identified new signals of drugs potentially responsible for DIIHA using worldwide pharmacovigilance data and then assessed the risk of DIIHA at the population level for old and new signals using comparative analyses in a nationwide cohort. Even if case reports had previously demonstrated experimentally the possibility of DIIHA for some drugs, this study measured the association at the population level, resulting in a list of drugs that clinicians should search systematically upon encountering a patient who develops immune hemolytic anemia.
An association was confirmed using the 2 comparative designs for 14 drugs, including 3 drugs detected as new signals by the disproportionality analysis in Vigibase: the fixed combination of sulfamethoxazole and trimethoprim, cefpodoxime, and aziathioprine; the other drugs were previously identified by Garratty et al.5 This study adds new insight about the association with immune hemolytic anemia onset for these drugs combining in fine experimental data, case reports, and comparative data at the population level.
We found an association in the case-control analysis, but not in the CCO analysis, for 14 other drugs. These discrepancies can be explained by a lack of power in the CCO analysis, in which only discordant pairs (patients exposed during the case period and unexposed during the control period, or conversely) were used for the calculation of ORs (eg, for diclofenac with a lower boundary of the 95% CI = 1 in the CCO analysis). Consequently, we cannot exclude the reality of an association at the population level between these drugs and immune hemolytic anemia. Of note, in the case-control design, the exposure to drugs was measured before the index date regardless of the duration of the exposure, whereas many of these drugs are prescribed for chronic diseases (eg, insulin). In contrast, the CCO analysis is more appropriate to identify risks of recent, short-term exposures regarding the immunological mechanism in DIIHA, even if DIIHA has been reported with longer exposures and if hemolysis does not occur systematically at first exposure in case of short duration of exposure. The CCO analysis is also more accurate to control for non–time-dependent unmeasured confounders. This may explain that, conversely, an association was found for fluconazole and cloxacillin in the CCO analysis only.
We confirmed some associations measured in the FAKOS study: for β-lactams, ciprofloxacine, and the fixed combination of sulfamethoxazole and trimethoprim.6 This previous study described also associations with diclofenac, fludarabine, and lorazepam. As mentioned previously, we failed to identify a statistically significant association with diclofenac using the CCO design, probably owing to lack of power. Because of the contents of the SNDS database, we limited our study to out-of-hospital dispensed drugs. Consequently, we could not assess the association for fludarabine. Lorazepam was not listed by Garratty et al and not detected as a signal in the disproportionality analysis in Vigibase (data not shown; n = 2 reported cases). In the FAKOS study, those who were cases were older than those who were controls (mean age, 64 vs 58 years), and 52% of the cases had an underlying disease that cause secondary AIHA, notably malignancies and systemic autoimmune diseases. This may explain the higher frequency of exposure to lorazepam in cases than in controls. Conversely, the FAKOS study was underpowered to measure the risk associated with rarer drug exposures.
Interestingly, most signals identified in Vigibase were not confirmed by comparative analyses in the national cohort of patients with immune hemolytic anemia. Disproportionality analyses were influenced by various reporting biases. This reminds that the RORs measure the signal intensity of a possible association but not the association between the drug and the event.11,20,21
This study has several limitations. First, the cases of DIIHA reported in Vigibase are affected by the mandatory limits of reporting in pharmacovigilance: underreporting and selective reporting.11,22-24 The risks of misclassification bias (other types of drug-induced hemolysis) or confounding by indication have been limited by the review of all reports. Some signals may have been “diluted” and consequently not detected because of competition by drugs with a strong association with immune hemolytic anemia occurrence.25 The comparative study performed in the AHEAD cohort also has limitations because of the nature of the database. Patients were identified using diagnosis codes. Even if a huge majority of patients with immune hemolytic anemia were identified with the D59.1 code that had a positive predictive value of 90% (95% CI, 80%-96%) in a previous validation study,17 misclassification cannot be excluded. Because of the absence of specific codes in the ICD-10 classification, it was not possible to distinguish in the AHEAD cohort the patients with warm AIHA from those with cold AIHA.1,2 This is of particular concern with regard to cold agglutinin disease, accounting for 10% to 20% of the AIHA and for which a drug-related cause is unlikely.26 We could not assess the association for drugs not marketed or not reimbursed in France or for drugs available only in hospital. A specific study needs to be conducted to measure the risk associated with anticancer immunotherapies.27,28 Moreover, the association between the exposure to acetaminophen or to ibuprofen (which are also available over-the-counter in France) and immune hemolytic anemia occurrence may be underestimated because of measurement bias. Analyses were not conducted for the drugs with too few patients exposed during the period of interest (n < 5). Therefore, we could not assess the association for drugs prescribed more scarcely. Similarly, our study does not account for coprescriptions because subgroup analyses of these patients would have led to hazardous estimates, owing to very few patients in each subgroup. Consequently, we cannot exclude that some measures of association may be influenced by coprescriptions (eg, for antibiotics). In contrast with the case-control design, in which the cases and the controls are matched for the index date, the CCO analysis can be affected by a bias resulting from temporal variations in the exposure, named exposure trend.29 Theses variations of exposure are mostly observed for anti-infective drugs with a higher exposure in winter. Immune hemolytic anemia may be triggered by acute infection, with a risk of confounding by indication for anti-infective drugs, nonsteroidal anti-inflammatory drugs, and acetaminophen. A protopathic bias cannot be excluded for some patients, in case of nonsteroidal anti-inflammatory drugs or acetaminophen prescribed for lumbar pain or fever due to intravascular hemolysis. Of note, an indication bias is unlikely for azathioprine because in the AHEAD cohort, the selected patients had incident diagnoses of immune hemolytic anemia whereas azathioprine is used as a third-line treatment of warm AIHA,1 and because we excluded patients with a cause of secondary AIHA such as systemic autoimmune diseases. Moreover, cases of immune hemolytic anemia because of 6-mercaptoputrine, the metabolite of azathioprine, have been previously reported.30 Regarding the statistical analysis, we did multiple testing and some false positive associations cannot be excluded, particularly in case of weak associations. Finally, a specific study with other designs, notably self-controlled case-series, is needed to measure the risk of immune hemolytic anemia associated with vaccines.8,31
Conclusions
This study identified associations of some drugs with the occurrence of immune hemolytic anemia at the population level: furosemide, amoxicillin with and without clavulanic acid, ceftriaxone, cefixime, cefpodoxime, the fixed combination of sulfamethoxazole and trimethoprim, ciprofloxacin, norfloxacin, amphotericin B, azathioprine, ibuprofen, acetaminophen, and iomeprol, which were identified using 2 study designs. Other drugs were identified using a single method: ranitidine, insulin, methyldopa, urapidil, hydrochlorothiazide, hydrocortisone, acyclovir, mycophenolic acid, ciclosporin, tacrolimus, diclofenac, etodolac, hydroxycarbamide, apomorphine, fluconazole, and cloxacillin. These 30 drugs should be searched systematically in case of new diagnosis of immune hemolytic anemia to consider for DIIHA.
Acknowledgments
The authors acknowledge the Uppsala Monitoring Centre, which provided and gave permission to use the data analyzed in this study. The authors are indebted to the National Pharmacovigilance Centers that contributed data. The authors also thank Marjorie Boussac and the Caisse Nationale de l'Assurance maladie engineers who performed raw data extraction from the SNDS database.
This study was academic, funded by the Société Nationale Française de Médecine Interne and the University Hospital of Toulouse.
Authorship
Contribution: J.M., M.L., M.M., M.L.-M., and G.M. designed the study and interpreted the results; J.M. conducted the data management and the statistical analyses; M.L., J.M., and G.M. wrote the manuscript; and all other authors critically reviewed the manuscript and gave final approval for publication.
Conflict-of-interest disclosure: G.M. received meeting attendance grants from Amgen, Grifols, and Novartis; is a coordinator of research studies granted by Amgen, CSL Behring, Grifols, Novartis, and Sanofi; has participated in educational sessions funded by Amgen, Grifols, and Novartis; and is on the boards of Amgen, Argenx, Grifols, Novartis, Sanofi, and Sobi. M.M. participated in educational sessions and boards of Amgen, Argenx, Novartis, Sobi, and UCB. The remaining authors declare no competing financial interests.
Correspondence: Julien Maquet, Toulouse University Hospital, Place du Docteur Baylac, TSA 40031, 31059 Toulouse, France; email: maquet.j@chu-toulouse.fr.
References
Author notes
∗J.M. and M.L. contributed equally to the work.
Vigibase data may be obtained from a third party and are not publicly available. Data may be obtained on request to the Uppsala Monitoring Center at https://who-umc.org/vigibase/vigibase-services/.
Système National des Données de Santé data may be obtained from a third party and are not publicly available. Fully anonymized data may be obtained on request to the Health-Data-Hub at https://www.health-data-hub.fr/depot.
The data management and statistical analysis codes are available on reasonable request from the corresponding author, Julien Maquet (maquet.j@chu-toulouse.fr).
The full-text version of this article contains a data supplement.