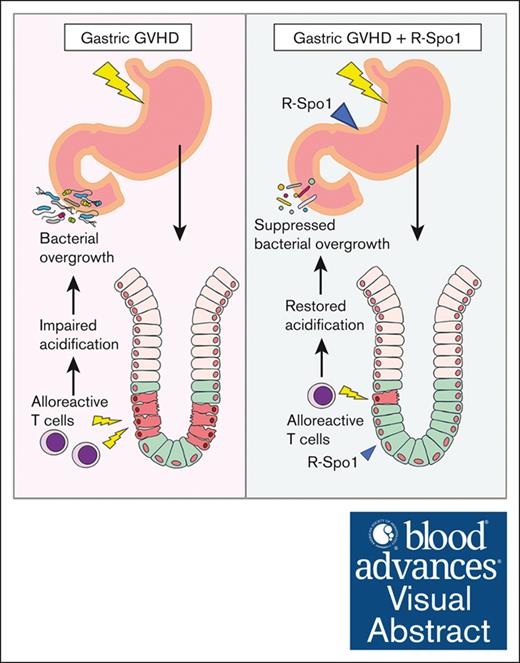
GVHD damages Lgr5+ gastric tissue stem cells and causes aerobic bacterial overgrowth in the duodenum.
R-Spondin1 protects Lgr5+ gastric tissue stem cells and ameliorates gastric GVHD.
Visual Abstract
Graft-versus-host disease (GVHD) is the major obstacle to performing allogeneic hematopoietic cell transplantation (allo-HCT). We and others have shown that intestinal stem cells are targeted in lower gastrointestinal GVHD. A leucine-rich repeat–containing G-protein coupled receptor 5 (Lgr5)–expressing gastric stem cells (GSCs) reside at the base of the gastric glands in mice. After experimental allo-HCT, Lgr5+ GSCs significantly decreased. Parietal cells, which underwent continuous renewal by GSCs, were injured in gastric GVHD, leading to failure of gastric acidification and aerobic bacterial overgrowth in the duodenum. Fate-mapping analysis demonstrated that administration of R-Spondin1 (R-Spo1) that binds to Lgr5 for 6 days in naïve mice significantly increased proliferating epithelial cells derived from Lgr5+ GSCs. R-Spo1 administered on days −3 to −1 and from days +1 to +3 of allo-HCT protected GSCs, leading to amelioration of gastric GVHD and restoration of gastric acidification, and suppression of aerobic bacterial overgrowth in the duodenum. In conclusion, Lgr5+ GSCs were targeted by gastric GVHD, resulting in disruption of the gastric homeostasis, whereas R-Spo1 protected Lgr5+ GSCs from GVHD and maintained homeostasis in the stomach.
Introduction
Graft-versus-host disease (GVHD) is the major complication of allogeneic hematopoietic cell transplantation (allo-HCT). Upper gastrointestinal (GI) GVHD presents symptoms such as anorexia, dyspepsia, nausea, and vomiting and significantly impairs the quality of life of the patients.1 In the stomach, leucine-rich repeat–containing G-protein coupled receptor 5 (Lgr5)–positive gastric stem cells (GSCs) residing at the base of antral and pyloric glands play a critical role in mucosal tissue regeneration after injury.2-4 We and others have shown that intestinal stem cells (ISCs) were targeted in lower GI GVHD,5,6 leading to impaired tissue regeneration and homeostasis.7 However, it remains to be investigated whether GSCs could be also targeted in upper GI GVHD. In this study, we evaluated whether Lgr5+ GSCs could be damaged in GVHD in a murine model of allo-HCT using Lgr5-reporter mice. We also evaluated whether Wnt agonist R-Spondin1 (R-Spo1), which binds to Lgr5, could protect Lgr5+ GSCs and ameliorate gastric GVHD.
Methods
Mice
Female C57BL/6 (B6: H-2b, CD45.2+), B6D2F1 (H-2b/d, CD45.2+), and DBA/2 (H-2d, CD45.2+) mice were purchased from Clea Japan (Tokyo, Japan). B6-Lgr5-EGFP-creER (Lgr5-EGFP-IRES-creERT2), B6-Lck-cre, and B6-R26tdTomato (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTOMATO)Hze/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Lgr5-EGFP-creER × R26tdTomato and B6-Lck-cre × R26tdTomato mice were generated by crossing B6-R26tdTomato female mice with B6-Lgr5-EGFP-creER and B6-Lck-cre males, respectively. B6D2F1-Lgr5-EGFP-creER mice were generated by crossing B6-Lgr5-EGFP-creER male with DBA/2 female.8 All animal experiments were performed under the auspices of the Institutional Animal Care and Research Advisory Committee (approval number 17-0026). Experiments were performed in a nonblinded fashion.
HCT
Mice received transplantation as previously described.8 In brief, after lethal X-ray total body irradiation (12 Gy) delivered in 2 doses at 4-hour intervals, mice were IV injected with 5 × 106 bone marrow cells with 5 × 106 splenocytes.
Reagent
Naïve mice were treated with 200 μg per day R-Spo1 (Kyowa Kirin Co, Ltd, Tokyo, Japan) for 6 days. A group of recipients were IV injected with 100 μg per day of R-Spo1 from days −3 to −1 and days +1 to +3 after allo-HCT, as previously described.8 Mice were maintained in specific pathogen–free condition and received normal chow and autoclaved water after HCT.
Histological and immunofluorescent analyses
For pathological analysis, samples of the stomach were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and subjected to hematoxylin and eosin or periodic acid–Schiff (PAS) staining. Immunofluorescent analysis was performed using primary antibodies (Abs), including chicken anti-EGFP (ab13970; Abcam, Tokyo, Japan), rabbit anti–red fluorescent protein (ab34771; Abcam), rabbit anti–Ki-67 (MA5-14520; Thermo Fisher Scientific), and rabbit anti-cleaved caspase-3 (9661; Cell Signaling), and visualized with Alexa Fluor-488 or -555–conjugated secondary Abs. Pictures of tissue sections were taken at room temperature using a digital camera (DP20; Olympus, Tokyo, Japan) mounted on a microscope (BX50; Olympus) and confocal laser microscope (FV-1000D; Olympus).
Fate-mapping of Lgr5+ GSCs
Lgr5-EGFP-creER × R26tdTomato mice were intraperitoneally injected with 40 mg/kg tamoxifen (Sigma Aldrich) daily for 3 days to label GSCs, followed by IV injection of R-Spo1 of phosphate-buffered saline (PBS) daily for 6 days. The stomach was harvested on the next day of the last R-Spo1 injection.
Lamina propria lymphocytes dissociation
Stomach was isolated and opened with scissors. Samples were then incubated on a shaker in complete medium (CM; 2% fetal calf serum in PBS) in the presence of 1 mM DL-dithiothreitol (Sigma Aldrich) at 37°C for 20 minutes and subsequently incubated with 1.3 mM ethylenediaminetetraacetic acid (Nippon Gene, Toyama, Japan) in CM at 37°C for 40 minutes. Then, samples were rinsed twice in CM and digested with 0.3 mg/ml of type IV collagenase (Sigma Aldrich) at 37°C for 45 minutes, homogenized, filtered, and washed.
Flow cytometric analyses
Monoclonal Abs conjugated with phycoerythrin, phycoerythrin-Cyanine7 (Cy7), allophycocyanin, or allophycocyanin-Cy7 were purchased from BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA), or Biolegend (San Diego, CA,). Lamina propria cells in stomach were stained with the antibodies against murine CD45, TCR-β, CD4, and CD8. Samples were analyzed using FACSCantoII (BD Biosciences, Tokyo, Japan) and FlowJo software (Tree Star, OR).
Measurement of pH in the stomach
pH of the mucosal surface was measured using pH indicator strips, pH-FIX 0 to 14 (Sigma Aldrich).
Fluorescence in situ hybridization
Stomach containing gastric contents were fixed in Carnoy fixative for 2 hours, and the 8-μm thin sections were made as previously reported.9 Briefly, sections were incubated with 1 μg of Cyanine5 (Cy5)-conjugated EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′) for detection of all bacteria in hybridization buffer (750 mM NaCl, 100 mM tris(hydroxymethyl)aminomethane HCl [pH 7.4], 5 mM EDTA, 0.01% bovine serum albumin, and 10% dextran sulfate) at 40°C for overnight. Sections were rinsed in wash buffer (50 mM NaCl, 4 mM tris(hydroxymethyl)aminomethane HCl [pH 7.4], and 0.02 mM EDTA), washed at 45°C for 20 minutes and counterstained with 4′,6-diamidino-2-phenylindole. Photographs of sections were obtained using confocal laser microscope (FV-1000D; Olympus).
Bacterial colony counts in duodenum and gastric draining lymph nodes
The contents from 1 cm of duodenum were harvested in 500 μL of PBS. Homogenized duodenum contents were diluted and cultured on Lysogeny Broth (LB) plates containing yeast extract for overnight at 37°C. Gastric draining lymph nodes were harvested from mice and homogenized in PBS and cultured on Columbia blood agar plates (BD) for 48 hours at 37°C. Colony-forming units were counted and adjusted per total contents in 1 cm of duodenum or per organ.
Quantification of bacterial density
Genomic DNA was isolated from duodenum content or gastric draining lymph nodes as previously described.8,9 16S ribosomal RNA (rRNA) copies of duodenum content or gastric draining lymph nodes were quantified using quantitative polymerase chain reaction (qPCR), and fold differences relative to the quantities of syngeneic controls were calculated as previously described.9 In brief, 16S rRNA gene sequences were amplified using the primers 5′-AGAGTTTGATCCTGGCTCAG-3′ for the forward primer, 5′-CTGCTGCCTYCCGTA-3′ for the reverse primer (Y = C + T), and FAM- TAACACATGCAAGTCGA-BHQ1 for the probe. qPCR was carried out on Applied Biosystems StepOnePlus (Thermo Fisher Scientific).
Statistical analysis
Mann-Whitney U tests or 1-way analysis of variance was used to compare data between the groups. Analyses were performed using Prism version 10.0 (GraphPad Software, San Diego, CA). P < .05 was considered statistically significant.
Results
R-Spo1 stimulates differentiation of GSCs into mucosal epithelial cells in naïve mice
First, we evaluated the effects of a Wnt agonist R-Spo1 on Lgr5+ GSCs in naïve mice. When injected to B6D2F1-Lgr5-EGFP mice for 6 days, R-Spo1 significantly elongated depth of the gastric glands and increased the numbers of Ki-67+ proliferating epithelial cells in the isthmus zone but not those of Lgr5+ GSCs (Figure 1A-F). R-Spo1 did not alter mucus layer on the surface of gastric epithelium (supplemental Figure 1). Fate-mapping analysis using B6-Lgr5-EGFP-creER × Rosa26tdTomato mice, in which Lgr5+ GSCs and their progenies were labeled with tdTomato upon tamoxifen treatment, showed that R-Spo1 significantly increased in numbers of gastric glands with tdTomato expression in nearly all epithelial cells without increasing enhanced green fluorescent protein positive (EGFP+) GSCs (Figure 1G-H). Altogether, R-Spo1 promotes GSCs to differentiate to epithelial cells in the gastric glands without stimulating proliferation of GSCs.
R-Spo1 promotes proliferation of gastric epithelium in naïve mice. (A-E) B6D2F1-Lgr5-EGFP-creER mice were IV injected daily with R-Spo1 (200 μg/d) or PBS for 6 days. (A) Hematoxylin and eosin (H&E staining) of the fundic glands in the stomach. (B) Gland depth of fundic glands (n = 6 per group). (C) Immunofluorescent images of Ki-67 (red) with 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue). (D) Numbers of Ki-67+ cells per gland (n = 7 per group). (E) Immunofluorescent images of EGFP (green) with DAPI nuclear staining (blue). (F) Numbers of EGFP+ cells per gland (n = 3 per group). (H-I) Lgr5-EGFP-creER × R26tdTomato mice were IP injected with 40 mg/kg of tamoxifen for 3 days to label GSCs, followed by IV injection of R-Spo1 for 3 days. (G) Immunofluorescent images of red fluorescent protein (tdTomato/red fluorescent protein [RFP]; red) and EGFP (green). (H) Numbers of glands (per 1.5 mm) in which RFP was expressed from base to top (n = 3 per group). (A,C,E,G) Bars represent 100 μm. (B,D,F,H) Data are shown as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. IP, intraperitoneally.
R-Spo1 promotes proliferation of gastric epithelium in naïve mice. (A-E) B6D2F1-Lgr5-EGFP-creER mice were IV injected daily with R-Spo1 (200 μg/d) or PBS for 6 days. (A) Hematoxylin and eosin (H&E staining) of the fundic glands in the stomach. (B) Gland depth of fundic glands (n = 6 per group). (C) Immunofluorescent images of Ki-67 (red) with 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue). (D) Numbers of Ki-67+ cells per gland (n = 7 per group). (E) Immunofluorescent images of EGFP (green) with DAPI nuclear staining (blue). (F) Numbers of EGFP+ cells per gland (n = 3 per group). (H-I) Lgr5-EGFP-creER × R26tdTomato mice were IP injected with 40 mg/kg of tamoxifen for 3 days to label GSCs, followed by IV injection of R-Spo1 for 3 days. (G) Immunofluorescent images of red fluorescent protein (tdTomato/red fluorescent protein [RFP]; red) and EGFP (green). (H) Numbers of glands (per 1.5 mm) in which RFP was expressed from base to top (n = 3 per group). (A,C,E,G) Bars represent 100 μm. (B,D,F,H) Data are shown as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. IP, intraperitoneally.
Gastric GVHD targets GSCs
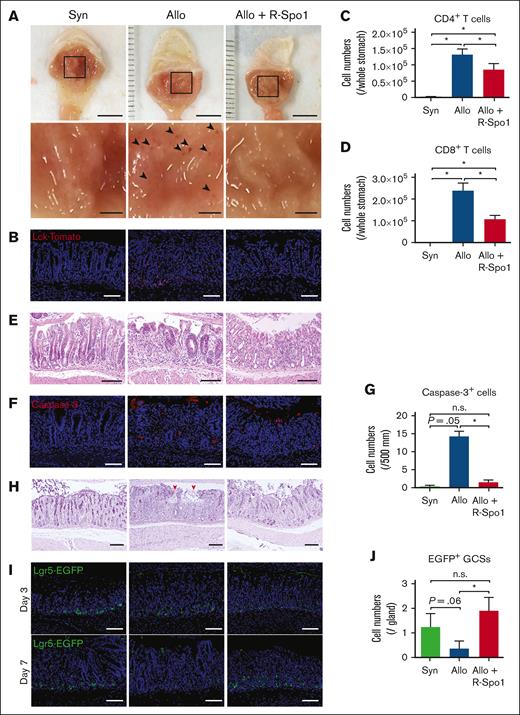
Next, we evaluated whether GSCs are damaged in GVHD after allo-HCT. Lethally irradiated B6D2F1 (allogeneic) or B6 (syngeneic) mice received transplantation with bone marrow cells and splenocytes from B6-Lck-cre × R26tdTomato donor mice, in which T cells are labeled with tdTomato. Macroscopic images demonstrated that there were multiple small erosions in allogeneic recipients, whereas much less erosions were observed in syngeneic mice (Figure 2A). Immunofluorescent and flow cytometric analyses showed that donor T cells were preferentially infiltrated around the base of gastric glands in allogeneic recipients, whereas very few T cells were found in the syngeneic controls (Figure 2B-D). Hematoxylin and eosin staining demonstrated that inflammatory cell infiltration in the lamina propria and apoptosis of epithelial cells in the gastric glands in allogeneic animals (Figure 2E). Immunofluorescent studies targeting cleaved caspase-3 showed that apoptotic gland epithelial cells were markedly increased in allogeneic recipients compared with syngeneic controls (Figure 2F-G). PAS staining showed disruption of mucus layer of the stomach in allogeneic mice (Figure 2H). These pathological changes were not observed in syngeneic controls, indicating that these changes were associated with GVHD. To test whether gastric GVHD targets Lgr5+ GSCs, B6D2F1-Lgr5-EGFP, mice received transplantation from syngeneic B6D2F1 or allogeneic B6 mice. Confocal images of Lgr5+ EGFP showed Lgr5+ GSCs were reduced in allogeneic mice compared with syngeneic controls, indicating that gastric GVHD targets Lgr5+ GSCs (Figure 2I-J).
R-Spo1 mitigates GVHD-induced GSC injury. (A-D) Lethally irradiated B6 (Syn) or B6D2F1 (allo) mice received transplantation with bone marrow cells plus splenocytes from B6-Lck-cre donors on day 0. Allogeneic recipients were treated with R-Spo1 or PBS on days −3 to −1 and days +1 to +3. The stomach was harvested on day +7. (A) Macroscopic images of the gastric mucosa (top). The area in the black squares were magnified and shown in the bottom of the original images. Arrow heads indicate the erosions observed macroscopically. Bars, 5 mm (top) and 1 mm (bottom). (B) Immunofluorescent images of LcktdTomato+ T cells (red) in the gastric mucosa with DAPI nuclear staining (blue). (C-D) Numbers of CD4+ and CD8+ T cells infiltrated in the gastric mucosa. (E-J) Lethally irradiated B6D2F1-Lgr5-EGFP-creER mice received transplantation from B6D2F1 (syn) or B6 (allo) donors on day 0 and treated with R-Spo1 or PBS, as in panel A. On day +7, stomach was harvested. H&E staining (E), immunofluorescent images of cleaved caspase-3 (F) (red) with DAPI nuclear staining (blue), numbers of cleaved caspase-3 (G), PAS staining (H), immunofluorescent images of EGFP+ Lgr5+ GSCs (green) (I), and numbers of Lgr5+ GSCs (J) in the stomach. (H) Red arrow heads represent disrupted mucin layer. (B,E,F,H,I) Bars represent 100 μm. Data from 2 experiments were combined and shown as means ± standard error of the mean (n = 4-5 per group). ∗P < .05.
R-Spo1 mitigates GVHD-induced GSC injury. (A-D) Lethally irradiated B6 (Syn) or B6D2F1 (allo) mice received transplantation with bone marrow cells plus splenocytes from B6-Lck-cre donors on day 0. Allogeneic recipients were treated with R-Spo1 or PBS on days −3 to −1 and days +1 to +3. The stomach was harvested on day +7. (A) Macroscopic images of the gastric mucosa (top). The area in the black squares were magnified and shown in the bottom of the original images. Arrow heads indicate the erosions observed macroscopically. Bars, 5 mm (top) and 1 mm (bottom). (B) Immunofluorescent images of LcktdTomato+ T cells (red) in the gastric mucosa with DAPI nuclear staining (blue). (C-D) Numbers of CD4+ and CD8+ T cells infiltrated in the gastric mucosa. (E-J) Lethally irradiated B6D2F1-Lgr5-EGFP-creER mice received transplantation from B6D2F1 (syn) or B6 (allo) donors on day 0 and treated with R-Spo1 or PBS, as in panel A. On day +7, stomach was harvested. H&E staining (E), immunofluorescent images of cleaved caspase-3 (F) (red) with DAPI nuclear staining (blue), numbers of cleaved caspase-3 (G), PAS staining (H), immunofluorescent images of EGFP+ Lgr5+ GSCs (green) (I), and numbers of Lgr5+ GSCs (J) in the stomach. (H) Red arrow heads represent disrupted mucin layer. (B,E,F,H,I) Bars represent 100 μm. Data from 2 experiments were combined and shown as means ± standard error of the mean (n = 4-5 per group). ∗P < .05.
R-Spo1 ameliorates gastric GVHD and protects Lgr5+ GSCs
We previously showed that R-Spo1 suppresses donor T-cell activation and ameliorates GVHD in the intestine and liver by protecting Lgr5+ ISCs and preventing the development of dysbiotic gut microbiome in murine GVHD models.5,8 We next evaluated whether GVHD prophylaxis with R-Spo1 could ameliorate gastric GVHD after allo-HCT. When the recipient mice were IV injected with R-Spo1 on days −3 to −1 and from days +1 to +3 after allo-HCT, donor T-cell infiltration in the stomach was significantly reduced compared with that of the allogeneic controls (Figure 2B-D). R-Spo1 suppressed inflammatory cell infiltration in the lamina propria and epithelial apoptosis in the gastric glands (Figure 2E-G). R-Spo1 mitigated damages in the mucus layer covering the gastric mucosal surface and reduced macroscopic erosion in the gastric mucosa (Figure 2A,F-H). Importantly, there were significantly greater numbers of Lgr5+ GSCs persisting in R-Spo1–treated allogeneic mice than in vehicle-treated allogeneic controls, indicating that R-Spo1 protected GSCs against GVHD (Figure 2I-J).
R-Spo1 suppresses bacterial translocation into the lamina propria of gastric mucosa
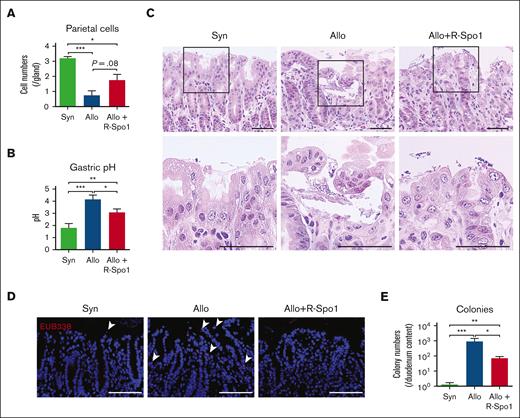
The fundic glands maintain acidic environment in the stomach and, thereby, suppress bacterial growth in the stomach and duodenum.10,11 We investigated whether GVHD could cause dysfunction of the fundic glands and cause bacterial growth in the duodenum.12 PAS staining demonstrated that parietal cells, which secrete hydrochloric acid, were significantly reduced after allo-HCT (Figure 3A). Consistent with histological damages of fundic glands, pH in the gastric lumen was significantly increased in allogeneic recipients compared with that in the syngeneic and naïve controls (pH, 1.4 ± 0.3; Figure 3B). Histological analyses demonstrated that there was obvious bacterial growth on the surface of gastric epithelial cells and bacterial translocation into the lamina propria of gastric mucosa in allogeneic recipients compared with in syngeneic mice (Figure 3C-D). However, there were no obvious bacterial transmigration into the gastric draining lymph nodes through gastric mucosa in both syngeneic and allogeneic mice (supplemental Figure 2A-B). The numbers of aerobic bacterial colony derived from the duodenal lumen were also increased in allogeneic mice compared with syngeneic controls (Figure 3E). In contrast to this result, bacterial densities of duodenum content quantified by 16S rRNA gene quantitative PCR (16S rRNA qPCR), which indicated the amount of both anaerobic and aerobic bacteria, were not significantly different between syngeneic and allogeneic mice, although the part of allogeneic recipient showed higher bacterial density even in 16S rRNA qPCR (supplemental Figure 2C). These data suggested that impaired acidification in the stomach caused by gastric GVHD led to less suppression of bacterial growth in the stomach and allowed bacteria, especially aerobic bacteria, to overgrow in the duodenum. Importantly, we found that administration of R-Spo1 mitigated the loss of parietal cells and restored acidification of the stomach, leading to suppression of aerobic bacterial overgrowth in the duodenum (Figure 3A-E; supplemental Figure 2C).
R-Spo1 suppresses GVHD-induced bacterial translocation into the gastric mucosa. Lethally irradiated B6D2F1 mice received transplantation and were treated with R-Spo1 as in Figure 2. The stomach and duodenum were harvested on day +7. (A-B) Numbers of parietal cells identified by PAS staining (A; n = 4 per group) and pH of the gastric lumen (B; n = 10-13 per group). (C) H&E staining of stomach. Areas in the black squares were magnified and shown in the bottom of the original images. Bars represent 50 μm. (D) FISH targeting a pan-bacterial marker, EUB338 (red), with DAPI nuclear staining (blue). Bars represent 100 μm. (E) Numbers of bacterial colony derived from the content of duodenum (n = 9-10 per group). (B,E) Data from 2 experiments were combined and shown as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. FISH, fluorescence in situ hybridization.
R-Spo1 suppresses GVHD-induced bacterial translocation into the gastric mucosa. Lethally irradiated B6D2F1 mice received transplantation and were treated with R-Spo1 as in Figure 2. The stomach and duodenum were harvested on day +7. (A-B) Numbers of parietal cells identified by PAS staining (A; n = 4 per group) and pH of the gastric lumen (B; n = 10-13 per group). (C) H&E staining of stomach. Areas in the black squares were magnified and shown in the bottom of the original images. Bars represent 50 μm. (D) FISH targeting a pan-bacterial marker, EUB338 (red), with DAPI nuclear staining (blue). Bars represent 100 μm. (E) Numbers of bacterial colony derived from the content of duodenum (n = 9-10 per group). (B,E) Data from 2 experiments were combined and shown as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. FISH, fluorescence in situ hybridization.
Discussion
Upper GI GVHD is characterized by anorexia, nausea, and vomiting.13 Gastric acute GVHD is more responsive to immunosuppressive therapy than lower GI GVHD.13 Gastric GVHD refractory to immunosuppressive therapy often progress to lower GI GVHD.14 Systemic corticosteroids or oral administration of poorly absorbable corticosteroids have been used for the treatment of upper GI GVHD, but strategies to treat corticosteroid refractory upper GI GVHD have not been established.
Tissue stem cells play a critical role in maintaining tissue homeostasis and repairing tissue injury. We and others have shown that Lgr5+ ISCs and hair follicle stem cells are targeted by donor T cells, leading to impaired tissue regeneration and prolonged tissue injury after experimental HCT.5,6,15,16 However, impacts of GVHD on the GSCs remain to be elucidated.
Long-term ablation of Lgr5+ GSCs in Lgr5-DTR-EGFP mice treated with diphtheria toxin induces extensive apoptosis and a significant reduction of chief cells and impairs epithelial homeostasis.4 Here, we, to the best of our knowledge, for the first time demonstrated that Lgr5+ GSCs were targeted by alloreactive T cells, and gastric functions such as production and secretion of hydrochloric acid were significantly impaired in upper GI GVHD.
Lgr5+ GSCs have been identified to reside at the base of pyloric gland and contribute to long-term renewal of the gastric epithelium.2,17 In steady state, GSCs are largely quiescent and rarely contribute to the turnover of the gastric epithelium. Upon injury, GSCs proliferate rapidly and replenish the whole gland, the hallmark of a reserve stem cell population.17 In this study, we confirmed using fate-mapping analysis that R-Spo1 increased differentiation of Lgr5+ GSC and replenish the whole gland after GVHD-associated injury, suggesting that Wnt signaling stimulated by R-Spo1 contributes to regeneration of the gastric epithelium via GSCs. Although Wnt signaling pathway plays a critical role in carcinogenesis of gastric cancer,18 our previous study showed that R-Spo1 knockin transgenic mice did not show any carcinogenesis in the gut under specific-pathogen–free condition without gastric tumor/precursor.19 Brief administration of R-Spo1 for several days may not be sufficient to promote gastric cancer generation.
Impaired gastric homeostasis was evidenced by (1) an increased pH in the gastric lumen, (2) bacterial outgrowth on the surface of gastric epithelial cells, and (3) bacterial translocation into the lamina propria of gastric mucosa and increased numbers of bacterial colony in the duodenal luminal contents in allogeneic recipients. Small intestine, especially duodenum, has lower numbers of the intestinal bacteria than the terminal ileum and colon.20 Importantly, we found that impaired gastric acidification, which resulted by damaged parietal cells led to the increased bacterial numbers in the duodenum called as small intestinal bacterial overgrowth (SIBO).21 However, GVHD induces not only impaired gastric acidification but also decreased antimicrobial peptides from Paneth cells such as alpha-defensins in the intestinal lumen,8 suggesting that multiple factors are associated with gastric GVHD-induced SIBO. To elucidate what is a key mediator to induce SIBO in gastric GVHD and what is the exact mechanism of gastric GVHD-induced SIBO, further studies have been required.
In summary, gastric GVHD targets Lgr5+ GSCs. The disrupted gastric mucosal integrity and function induced bacterial translocation into the lamina propria of gastric mucosa and bacterial overgrowth on the surface of gastric epithelial cell and in the duodenum. Administration of R-Spo1 stimulates the differentiation of GSCs into mucosal epithelial cells and protects GSCs from GVHD damage and suppresses bacterial overgrowth, indicating that R-Spo1 effectively ameliorated the gastric homeostasis in allo-HCT.
Acknowledgments
This study was supported by grants from MEXT/JSPS KAKENHI (20K21610 and 21H02944 [T.T.], 18K16104 [E.H.], 21K16259 [T.A.], and 20K17366 [H.O.]), and the Center of Innovation Program from JST (T.T.).
Authorship
Contribution: E.H. designed the experiments, conducted studies, analyzed data, and wrote the manuscript; T.A., Y.S., S.T., K.Y., H.O. R.O., E.Y., T.Y., K.E., and Y.H. conducted experiments; K.T., provided study reagents; and T.T. developed the conceptual framework of the study, designed the experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: T.T. is a recipient of research grants from Kyowa Kirin, Chugai, Fuji Pharma, Nippon Shinyaku, Asahi Kasei Pharma, Eisai, Sumitomo Pharma, ONO Pharmaceutical Co, Ltd, Astellas, Shionogi, Priothera SAS, LUCA Science, and Otsuka Co, Ltd; and has served as a consultant or advisory board member for Novartis, Meiji Seika Pharma, Daiichi Sankyo, Asahi Kasei Pharma, Astellas, AstraZeneca, Takeda, Janssen, Roche, Diagnostics, Sumitomo Pharma, Celgene, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Takanori Teshima, Department of Hematology, Hokkaido University Graduate School of Medicine, N15 W7, Kita-ku, Sapporo 060-8638, Japan; email: teshima@med.hokudai.ac.jp.
References
Author notes
All data generated in this study are available upon request from the corresponding author, Takanori Teshima (teshima@med.hokudai.ac.jp).
The full-text version of this article contains a data supplement.

![R-Spo1 promotes proliferation of gastric epithelium in naïve mice. (A-E) B6D2F1-Lgr5-EGFP-creER mice were IV injected daily with R-Spo1 (200 μg/d) or PBS for 6 days. (A) Hematoxylin and eosin (H&E staining) of the fundic glands in the stomach. (B) Gland depth of fundic glands (n = 6 per group). (C) Immunofluorescent images of Ki-67 (red) with 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue). (D) Numbers of Ki-67+ cells per gland (n = 7 per group). (E) Immunofluorescent images of EGFP (green) with DAPI nuclear staining (blue). (F) Numbers of EGFP+ cells per gland (n = 3 per group). (H-I) Lgr5-EGFP-creER × R26tdTomato mice were IP injected with 40 mg/kg of tamoxifen for 3 days to label GSCs, followed by IV injection of R-Spo1 for 3 days. (G) Immunofluorescent images of red fluorescent protein (tdTomato/red fluorescent protein [RFP]; red) and EGFP (green). (H) Numbers of glands (per 1.5 mm) in which RFP was expressed from base to top (n = 3 per group). (A,C,E,G) Bars represent 100 μm. (B,D,F,H) Data are shown as mean ± standard error of the mean. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. IP, intraperitoneally.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/3/10.1182_bloodadvances.2023011034/2/m_blooda_adv-2023-011034-gr1.jpeg?Expires=1768006321&Signature=HDh68OM1Y-ZArNRZzQysR3UtFGD1~4NEpWW5DW9TD2517kJT2PtDoWJA5iVyrrrlnVkcPiGM8LaGKR0DEkU1tm7Eg6tiwP1TyZHXjPC958KNf13VDXaVwEOxbKP46wVZuLq0rQcsBOWg21S2PHl7zsHd0LHNf3wcv6qCbU1nMhcMzXLzwCSkYeHG-n9-qOcwLH-r06ceVquw4KnpY7xd5HYOAJiqwhhX5Jz~ds5Jm0VnjjGXJmzQjMbxZxHUHoF3Cr2RFKkAdx~PNnoYd-1gkQGfpNX-mfMX8sxq64arFg7xAjII2XK3vaFStVbKkbZCjD-CtxjcVLM6klKsCouVxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)