Administration of tagraxofusp, azacitidine, and venetoclax is feasible without evidence of increased capillary leak syndrome or infection.
TAG-AZA-VEN has encouraging activity in older patients with previously untreated, adverse-risk AML, including with somatic TP53 mutations.
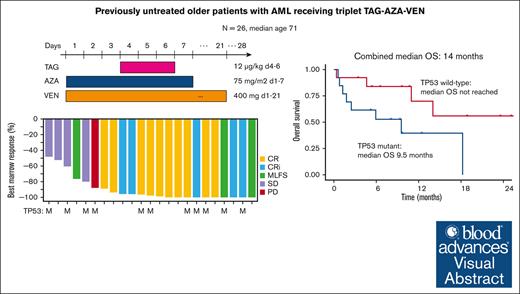
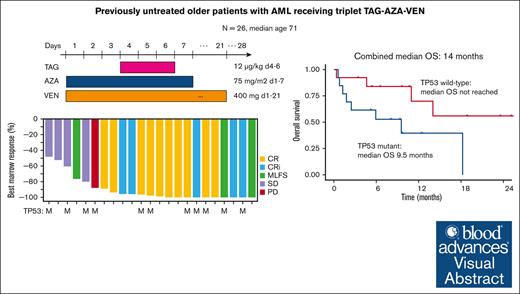
Visual Abstract
CD123, a subunit of the interleukin-3 receptor, is expressed on ∼80% of acute myeloid leukemias (AMLs). Tagraxofusp (TAG), recombinant interleukin-3 fused to a truncated diphtheria toxin payload, is a first-in-class drug targeting CD123 approved for treatment of blastic plasmacytoid dendritic cell neoplasm. We previously found that AMLs with acquired resistance to TAG were re-sensitized by the DNA hypomethylating agent azacitidine (AZA) and that TAG-exposed cells became more dependent on the antiapoptotic molecule BCL-2. Here, we report a phase 1b study in 56 adults with CD123-positive AML or high-risk myelodysplastic syndrome (MDS), first combining TAG with AZA in AML/MDS, and subsequently TAG, AZA, and the BCL-2 inhibitor venetoclax (VEN) in AML. Adverse events with 3-day TAG dosing were as expected, without indication of increased toxicity of TAG or AZA+/−VEN in combination. The recommended phase 2 dose of TAG was 12 μg/kg/day for 3 days, with 7-day AZA +/− 21-day VEN. In an expansion cohort of 26 patients (median age 71) with previously untreated European LeukemiaNet adverse-risk AML (50% TP53 mutated), triplet TAG-AZA-VEN induced response in 69% (n=18/26; 39% complete remission [CR], 19% complete remission with incomplete count recovery [CRi], 12% morphologic leukemia-free state [MLFS]). Among 13 patients with TP53 mutations, 7/13 (54%) achieved CR/CRi/MLFS (CR = 4, CRi = 2, MLFS = 1). Twelve of 17 (71%) tested responders had no flow measurable residual disease. Median overall survival and progression-free survival were 14 months (95% CI, 9.5-NA) and 8.5 months (95% CI, 5.1-NA), respectively. In summary, TAG-AZA-VEN shows encouraging safety and activity in high-risk AML, including TP53-mutated disease, supporting further clinical development of TAG combinations. The study was registered on ClinicalTrials.gov as #NCT03113643.
Introduction
CD123 is the alpha subunit of the interleukin 3 (IL-3) receptor and is expressed on the surface of myeloblasts in ∼80% of cases of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).1-4 CD123 expression is often higher on leukemia cells than on normal stem and progenitor cells and may be enriched in residual AML cells surviving chemotherapy.5 CD123 is also a marker of the rare subpopulation of leukemia initiating cells or leukemia stem cells (LSCs) that have the capacity to repopulate disease in model systems.6 Uniformly high CD123 expression is characteristic of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare aggressive acute leukemia derived from cells of the plasmacytoid dendritic cell lineage.7 For these reasons, CD123 is an attractive therapeutic target in multiple hematologic malignancies.
Tagraxofusp (TAG, SL-401) is a recombinant protein drug consisting of IL-3, the ligand for the IL-3 receptor/CD123, fused to a truncated diphtheria toxin (DT) payload.8 TAG targets cells by binding to CD123 on the cell surface, followed by internalization and endosomal escape of the DT component. DT inhibits protein synthesis by catalyzing ADP ribosylation of a modified histidine residue on eukaryotic elongation factor 2, called diphthamide, which leads to caspase-dependent apoptosis.9,10 Approved for the treatment of patients with BPDCN, single agent TAG leads to high remission rates and long-term disease-free survival, particularly when followed by stem cell transplantation.11,12 In addition, single agent TAG was effective in a small proportion of patients with relapsed/refractory (R/R) AML,13 which prompted us to study mechanisms of resistance and potential combination strategies.
We previously reported that CD123 cell surface expression is preserved on resistant cells after TAG treatment, including from patients, suggesting alternative mechanisms of escape.14 TAG resistance in AML and BPDCN cells is instead mediated by DNA methylation and downregulation of diphthamide genes (eg, DPH1), which eliminates the diphthamide target for DT. TAG resistance is reversed by treatment with the hypomethylating agent azacitidine (AZA), which increases DPH1 expression and restores the DT target, and TAG plus AZA improved survival compared to either agent alone in xenograft models.14 Furthermore, we found that cells escaping TAG therapy had an altered mitochondrial apoptosis threshold and increased propensity to undergo cell death in the setting of BCL-2 inhibition by venetoclax (VEN).14 Given this mechanistic rationale for synergy, we performed a phase 1b trial of TAG with AZA in AML or MDS, and subsequently TAG with AZA-VEN in AML.
Methods
Study design and patients
This study was registered on ClinicalTrials.gov (NCT03113643). All research was approved by each institution’s review board and all human participants provided written informed consent. Eligibility for cohorts testing doublet TAG-AZA and triplet TAG-AZA-VEN included patients aged 18 or higher with newly diagnosed AML, excluding acute promyelocytic leukemia, who declined or were ineligible for intensive induction chemotherapy due to age ≥75 or comorbidity, or had investigator perceived futility of standard chemotherapy, or had R/R AML. Eligibility for doublet TAG-AZA also included higher-risk MDS, defined as 10% or higher blasts in the bone marrow (MDS-EB2). Myeloblasts were required to demonstrate CD123 expression in marrow or blood by flow cytometry or immunohistochemistry, without specified minimum values for staining intensity or percentage-positive cells, as determined per each site’s local hematopathology standard. Other eligibility included Eastern Cooperative Oncology Group performance status ≤2, and adequate organ function, including albumin ≥3.2 g/dL, creatinine ≤1.5× the upper limit of normal (ULN), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <2.5× ULN, total bilirubin <1.5× ULN, and left ventricular ejection fraction greater than or equal to institutional normal. Patients receiving VEN needed to have white blood cell count of ≤20 x103/μL on day 1 of treatment (pretreatment hydroxyurea was permitted) and avoid strong CYP3A inducers. Prophylactic antibiotics were allowed per institutional standards. Certain strong and moderate CYP3A inhibitors were allowed, with modifications to the VEN dose, following VEN prescribing information.
The study followed a 3+3 dose escalation plus expansion cohorts with 28-day cycles of TAG and fixed doses of AZA or AZA-VEN (Figure 1). Patients were hospitalized during cycle 1, at least through 1 day after the last TAG dose, to monitor and mitigate risks of capillary leak syndrome (CLS). CLS monitoring included daily weight and examination, and laboratory monitoring of serum albumin, liver enzymes, and renal function. Management of findings potentially consistent with CLS (eg, hypoalbuminemia, edema, hemodynamic instability) were managed per the TAG prescribing information and included holding TAG doses (which could be made up before day 10), intravenous albumin supplementation, diuresis or volume repletion based on examination and laboratories, and corticosteroids. Bone marrow examination was required at the end of cycles 1, 2, 4, and 6, and later at investigator discretion. In patients receiving TAG-AZA-VEN, a bone marrow examination was performed on day 21 of cycle 1 to determine if cycle 2 would be delayed for count recovery in the absence of morphologic leukemia (<5% blasts). There was no limit on the number of cycles received; patients could remain on the study provided they did not have unacceptable toxicity or overt disease progression. Patients were allowed to proceed to stem cell transplantation at any time per their provider’s recommendation and were followed for disease progression and survival after transplant.
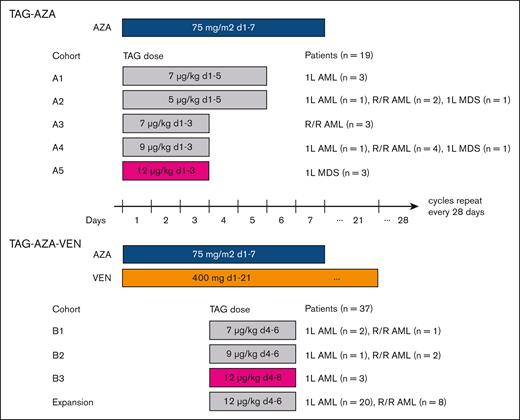
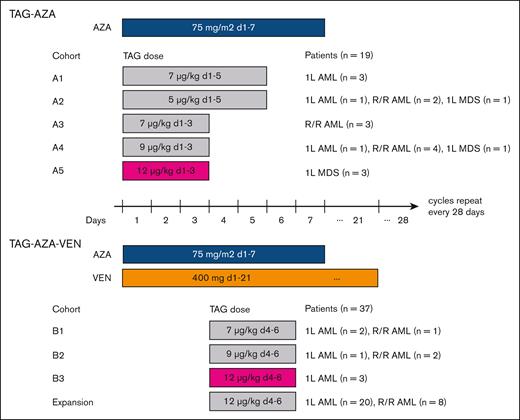
Treatment schema with dose levels and schedules tested. Diagram of the study design and participants. (Top) TAG and AZA were first tested as a doublet combination at 5 different doses/schedules of TAG with 7-day dosing of AZA. The RP2D of TAG was determined to be 12 μg/kg daily for 3 days (d1, 2, 3; in magenta) in combination with AZA. Patients with AML or MDS were eligible in Cohorts A1-A4. While cohort A4 was being enrolled, AZA-VEN was approved for AML. Therefore, the study was amended and cohort A5 was limited to MDS. (Bottom) 3 doses of TAG were tested with AZA-VEN as a triplet in patients with AML. In the first cycle, VEN was given as 100 mg on day 1, 200 mg on day 2, and 400 mg on day 3, followed by 400 mg on days 4 to 21. In subsequent cycles, VEN was given as 400 mg on days 1 to 21. The RP2D of TAG was determined to be 12 μg/kg daily for 3 days (d4, 5, 6; in magenta) in combination with AZA-VEN. Patients with 1L or R/R AML were eligible in dose escalation, and then separate expansion cohorts in 1L and R/R AML were enrolled.
Treatment schema with dose levels and schedules tested. Diagram of the study design and participants. (Top) TAG and AZA were first tested as a doublet combination at 5 different doses/schedules of TAG with 7-day dosing of AZA. The RP2D of TAG was determined to be 12 μg/kg daily for 3 days (d1, 2, 3; in magenta) in combination with AZA. Patients with AML or MDS were eligible in Cohorts A1-A4. While cohort A4 was being enrolled, AZA-VEN was approved for AML. Therefore, the study was amended and cohort A5 was limited to MDS. (Bottom) 3 doses of TAG were tested with AZA-VEN as a triplet in patients with AML. In the first cycle, VEN was given as 100 mg on day 1, 200 mg on day 2, and 400 mg on day 3, followed by 400 mg on days 4 to 21. In subsequent cycles, VEN was given as 400 mg on days 1 to 21. The RP2D of TAG was determined to be 12 μg/kg daily for 3 days (d4, 5, 6; in magenta) in combination with AZA-VEN. Patients with 1L or R/R AML were eligible in dose escalation, and then separate expansion cohorts in 1L and R/R AML were enrolled.
Adverse events were defined as in the revised NCI Common Terminology Criteria for Adverse Events version 4.0. Dose-limiting toxicities (DLTs) were predefined as grade 3 or higher nonhematologic toxicity (excluding fatigue) considered at least possibly related to study treatment that did not resolve to grade 1 or lower by day 28 of cycle 1. Hematologic DLTs were defined as neutropenia with absolute neutrophil count <500/μL or thrombocytopenia with platelet count <50 x103/μL present at day 28 and lasting 42 days or more from the start of cycle 1, in the setting of a bone marrow biopsy showing less than 5% blasts. Patients experiencing neutropenia with absolute neutrophil count <500/μL or thrombocytopenia with platelet count <50 x103/μL entering the study were unevaluable for a hematologic-related DLT. All participants who received at least 1 dose of study treatment were evaluable for toxicity.
Endpoints and assessments
The primary objective was to determine the maximum tolerated or recommended phase 2 dose (RP2D) of TAG in combination with AZA or AZA-VEN and evaluate the safety of these regimens. Secondary objectives were to estimate the response rate, duration of response, and progression-free and overall survival (OS) with these regimens. Responses were based on European LeukemiaNet (ELN) AML and International Working Group MDS criteria.15,16 Exploratory objectives included characterization of CD123 expression on leukemia cells and evaluation of association with treatment effect. All analyses were intention-to-treat.
Correlative laboratory studies
Next-generation sequencing was performed locally, at variant allele frequency sensitivity thresholds of 1%-5% depending on gene and assay.17 Measurable residual disease (MRD) testing was not mandated in the protocol; each site measured flow MRD per their local standard-of-care, and here we report MRD positivity using 0.1% of CD45-expressing cells as a threshold.18 Central laboratory quantitation of CD123 on the surface of leukemia blasts was performed as an exploratory analysis retrospectively on cryopreserved samples from the time of screening using the Quantibrite PE kit (BD Biosciences, number 340495) per the manufacturer’s instructions. Blasts were gated by CD45+/SSClow and the amount of CD123 staining by flow cytometry on blasts was normalized to the standards provided. In each run, CAL1 BPDCN (high CD123) and U937 AML (low CD123) cell lines were used as positive and negative controls for batch standardization. Pharmacokinetics (PK) samples were collected during cycles 1 and 2 on the days of first and last TAG administration at: predose, immediately after the end of infusion, 15, 30, 45, 60, 90, 120, 180, and 240 minutes post-end of infusion. Plasma concentrations of free TAG were quantified using a validated noncompetitive sandwich immunoassay by the drug manufacturer. PK data analysis was conducted using classic noncompartmental methods for intravenous infusion administration using Phoenix WinNonLin (version 8.3.5) and plotted with R (version 4.2.2) using geom_boxplot() from the ggplot2 library.
Statistical methods
OS was estimated using the method of Kaplan and Meier calculated from the first treatment day to death or last known follow-up. Progression-free survival (PFS) was similarly estimated from the first treatment day to first progression or death from any cause and were censored at the last response assessment date. The response duration was estimated from the date of first response (CR/CRi/MLFS) to the date of progression or death using the method of Kaplan and Meier. No censoring was performed at the time of transplant.
Results
Patients and treatment cohorts
First, we tested escalating doses of TAG as a doublet with AZA (75 mg/m2 days 1-7) in newly diagnosed AML (1L AML), R/R AML, or MDS with ≥10% blasts (MDS-EB2) (Figure 1). In the initial cohort of 3 patients receiving TAG 7 μg/kg for 5 days, 2 patients had grade 2 CLS and 1 patient had a DLT of prolonged hyperbilirubinemia. There were no DLTs in cohorts of 5 μg/kg daily for 5 days or 7, 9, or 12 μg/kg for 3 days. TAG 12 μg/kg daily for 3 days was selected as the RP2D for the TAG-AZA doublet.
Next, we tested 3 doses of TAG (7, 9, or 12 μg/kg per day for 3 days on days 4-6) with AZA (75 mg/m2 days 1-7) and VEN (400 mg days 1-21; ramp up 100 mg, 200 mg, 400 mg on days 1-3 in cycle 1) in 1L or R/R AML. TAG was started on day 4 to avoid overlap with VEN ramp up in cycle 1 and because we previously found that AZA pretreatment sensitizes AML cells to TAG.14 There were no DLTs observed at any dose level. TAG at 12 μg/kg daily for 3 days was selected as the expansion dose/RP2D for the TAG-AZA-VEN triplet. Expansion cohorts were then enrolled to a combined total of 26 patients with 1L AML and 11 patients with R/R AML receiving TAG-AZA-VEN. Demographics of all 56 patients in the study are shown in Table 1, comprising 19 patients who received TAG-AZA and 37 patients who received TAG-AZA-VEN. This included a subcohort of 26 patients with 1L AML who received TAG-AZA-VEN as their initial treatment (n = 23 at 12 μg/kg, 1 at 9 μg/kg, and 2 at 7 μg/kg).
To assess whether TAG plasma exposure was altered by combination treatment, we compared PK data for TAG-AZA-VEN in this study with data from single agent TAG in the pivotal STML-401-0114 trial, which led to approval for BPDCN and also included patients with AML.11,19 We saw no evidence that TAG exposure (Cmax and AUClast) was different when administered in combination or as a single agent (supplemental Figure 1).
Safety
Adverse events were largely similar as for single agent TAG and for AZA or AZA-VEN, as reported previously in similar patient populations.11,20 The most frequent grade 3 or higher adverse events in each cohort were related to cytopenias and febrile neutropenia (Table 2). Toxicities related to TAG were also as expected, including elevated ALT/AST of grade 2 or higher in 52.7%/16.8% and 10.8%/10.8% with TAG-AZA or TAG-AZA-VEN, respectively. In the 1L AML TAG-AZA-VEN cohort, grade 3 or higher adverse events were similar to the combined 1L and R/R triplet group, and included thrombocytopenia in 53.9%, febrile neutropenia in 34.6%, ALT elevation in 11.5%, and AST elevation in 7.6%. In the 1L AML TAG-AZA-VEN cohort, 3 of 26 patients had VEN dose reductions (to 14 days of treatment) for cytopenias in prior cycles. There were no TAG or AZA dose modifications for cytopenias. We also analyzed time from cycle 1 to cycle 2 as a surrogate for recovery from possible regimen-related toxicities, including cytopenias. Median number of days from start of cycle 1 to start of cycle 2 was 28.5 for TAG-AZA and 34 for TAG-AZA-VEN. Among patients receiving TAG-AZA-VEN for at least 2 cycles, the median time from start of cycle 1 to start of cycle 2 was 30 days at 7 μg/kg per day TAG (n = 3), 29 days at 9 μg/kg per day TAG (n = 3), and 37 days at 12 μg/kg per day TAG (n = 23).
CLS occurred in 9 of 19 patients who received TAG-AZA (47%; 7 grade 2, 2 grade 3) and in 7 of 37 patients who received TAG-AZA-VEN (18.9%; 5 grade 2, 1 grade 3, 1 grade 4). Nearly all CLS occurred in cycle 1 and included hypoalbuminemia in all cases, accompanied by 1 or more of weight gain, edema, or hypotension. A 2-sided Fisher exact test comparing CLS incidence with TAG-AZA and TAG-AZA-VEN suggested a lower rate with the triplet (P = .059). In the 1L AML TAG-AZA-VEN cohort, 5 of 26 patients experienced CLS (19.2%; 3 grade 2, 1 grade 3, 1 grade 4), which was similar to that reported with single agent 5-day dosing of TAG.11,12 All 5 patients with CLS received intravenous albumin, as did an additional 6 patients who had hypoalbuminemia without CLS, all occurring in cycle 1. Three of 5 with CLS remained on study and received treatment in cycle 2. There were no deaths attributed solely to CLS. One patient with AML who received TAG-AZA-VEN experienced a complicated series of potentially treatment-related events in the first cycle that included early tumor lysis syndrome, then later CLS and non-ST elevation myocardial infarction, with eventual death attributed to multiorgan system failure.
Among patients who received TAG-AZA (n = 19), the 30-day all-cause mortality rate was 10.5% (95% CI, 0-23.3). The cause of death in each of these 2 cases was AML disease progression. Among patients who received TAG-AZA-VEN (n = 37), the 30-day all-cause mortality rate was 10.8% (95% CI, 0.2-20.3). The causes of death within 30 days were sepsis (n = 2), multiorgan system failure (n = 1), and AML disease progression (n = 1).
Efficacy and outcomes
First, we present outcomes in the 26 patients with 1L AML that received triplet TAG-AZA-VEN, given that this is the largest patient group in the study and provides the most robust analyses. The median age for this cohort was 71 (range 60-81). All patients were categorized as adverse risk based on the ELN 2022 classification (Table 1).21 All cases had either an MDS-related gene mutation or a mutation in TP53 (supplemental Table 1). Thirteen of 26 (50%) AMLs harbored a mutation in TP53 and 9/13 had “multi-hit” TP53 loss, with either 2 mutations in TP53 or concurrent deletion of chromosome 17p.22 Eight patients had secondary AML, occurring after MDS (n = 4), myelofibrosis (n = 3), or chronic myelomonocytic leukemia (n = 1). Five had therapy-related AML, occurring after treatment for Hodgkin lymphoma (n = 2), B-cell acute lymphoblastic leukemia, breast cancer, and lung cancer. Two had “pDC-AML” an AML subset with high CD123 expression, marked expansion of mature plasmacytoid dendritic cells, enrichment of RUNX1 mutations, and response to TAG in laboratory models.23
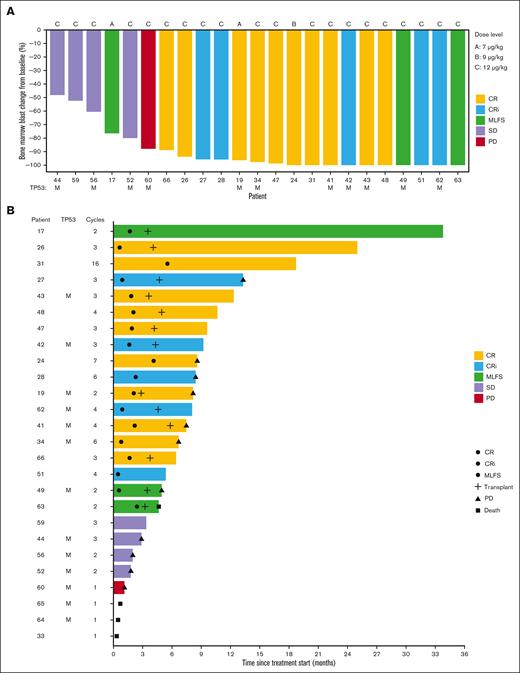
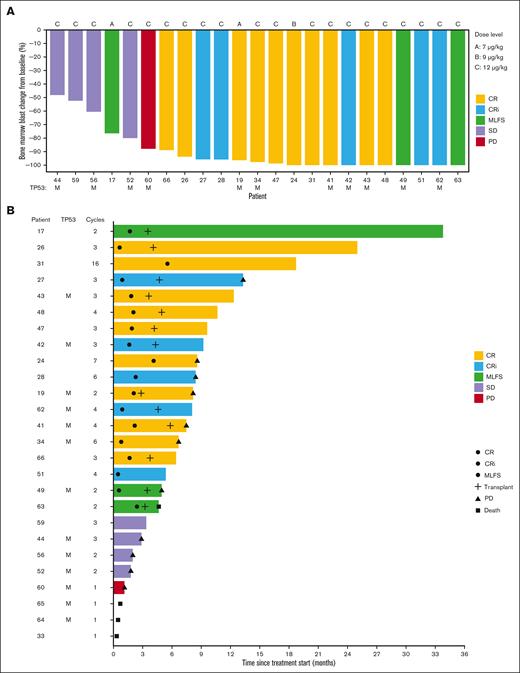
Eighteen of 26 patients (69%) achieved a best response of CR (10; 39%), CRi (5; 19%), or MLFS (3; 12%). Median time to best response among these 18 patients was 55 days. Of the 13 patients with a TP53 mutation, 7 (54%) achieved CR (n=4), CRi (n=2), or MLFS (n=1). Bone marrow blast percentage at the time of best response was decreased in all evaluated patients, including in those who did not achieve protocol-defined CR/CRi/MLFS (Figure 2A). In a post hoc exploratory analysis, we centrally analyzed CD123 on AML blasts. There was no association observed between baseline CD123 level and achieving CR/CRi/MLFS in the 1L AML cohort (P = .6 by Wilcoxon rank-sum test) (supplemental Figure 2).
Overview of bone marrow response and patient outcomes in the previously untreated AML cohort (n = 26) that received TAG-AZA-VEN. (A) Waterfall plot showing the best bone marrow blast response at any cycle as percentage change from baseline. Not shown are 3 patients who left the study prior to bone marrow assessment and 1 patient with secondary AML after myelofibrosis whose bone marrow was fibrotic and acellular at baseline. Two patients received 7 μg/kg TAG (dose level A), 1 received 9 μg/kg (dose level B), and the remaining 23 received 12 μg/kg (dose level C). Best response is annotated by color, as indicated. TP53 mutant status is annotated with an “M.” (B) Swimmer plot showing events and outcome for each patient over time from treatment start. Best response is annotated by the color of each bar, as in panel A. Circles indicate the time of first achieving CR, CRi, or MLFS, as indicated; cross is the time of allogeneic stem cell transplantation; triangle is the time of progressive disease (PD); square is the time of death. Annotation at the end of the bar is the status at end of study treatment. Patients without PD or death noted remained in remission at the last known follow-up. TP53 mutant status is annotated with an “M” and the number of cycles of treatment received is indicated.
Overview of bone marrow response and patient outcomes in the previously untreated AML cohort (n = 26) that received TAG-AZA-VEN. (A) Waterfall plot showing the best bone marrow blast response at any cycle as percentage change from baseline. Not shown are 3 patients who left the study prior to bone marrow assessment and 1 patient with secondary AML after myelofibrosis whose bone marrow was fibrotic and acellular at baseline. Two patients received 7 μg/kg TAG (dose level A), 1 received 9 μg/kg (dose level B), and the remaining 23 received 12 μg/kg (dose level C). Best response is annotated by color, as indicated. TP53 mutant status is annotated with an “M.” (B) Swimmer plot showing events and outcome for each patient over time from treatment start. Best response is annotated by the color of each bar, as in panel A. Circles indicate the time of first achieving CR, CRi, or MLFS, as indicated; cross is the time of allogeneic stem cell transplantation; triangle is the time of progressive disease (PD); square is the time of death. Annotation at the end of the bar is the status at end of study treatment. Patients without PD or death noted remained in remission at the last known follow-up. TP53 mutant status is annotated with an “M” and the number of cycles of treatment received is indicated.
Seventeen patients achieving CR/CRi/MLFS had MRD testing by multiparameter flow cytometry. MRD assessment was negative in 12/17 (71%). The median time to achieve MRD negativity was cycle 2 (range 1-4). Four of 7 (57%) patients with TP53 mutation who achieved CR/CRi/MLFS were MRD negative. Thirteen of 26 patients (50%) proceeded directly from this study to allogeneic stem cell transplantation; 6 of 13 patients who had undergone transplantation had TP53 mutation (Figure 2B). The median number of cycles received among all patients in the cohort was 3 (range 1-15). Patients who went to transplant received a median of 3 cycles (range 2-4) and those who did not received 2 cycles (range, 1-15). Among the 18 patients achieving CR/CRi/MLFS, median duration of response was 12.4 months (95% CI, 6.1-NA), including patients who underwent transplantation.
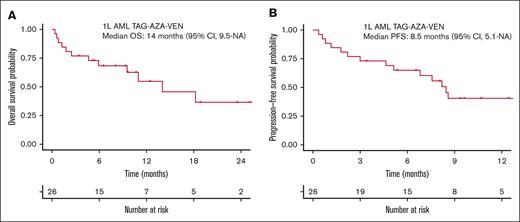
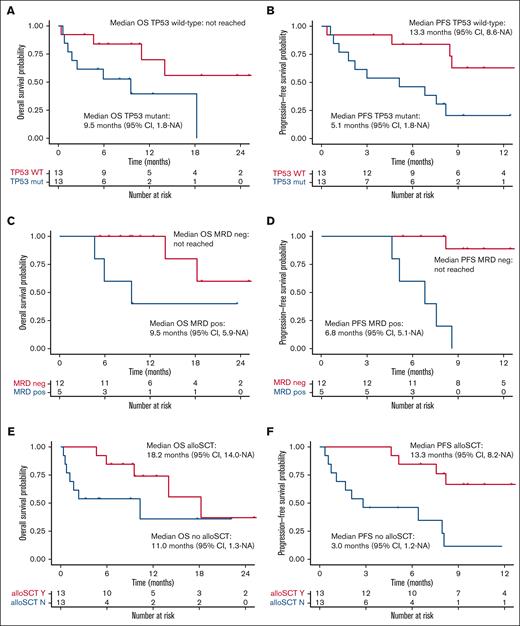
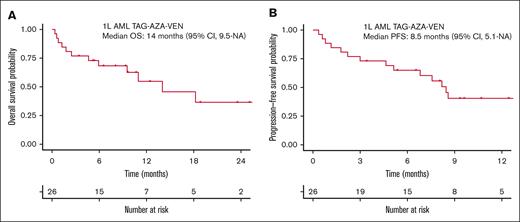
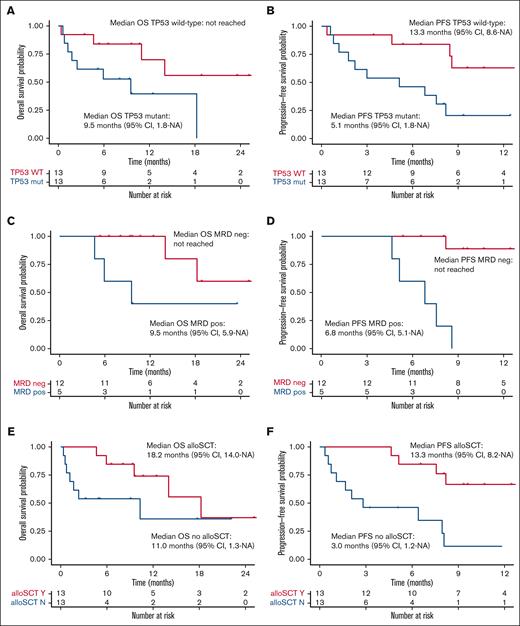
Median OS for the total 1L AML cohort receiving TAG-AZA-VEN was 14 months (95% CI, 9.5-NA) with median follow-up of 10.7 months (Figure 3A). Median PFS was 8.5 months (95% CI, 5.1-NA) (Figure 3B). In the 13 patients with TP53 mutation, median OS and PFS were 9.5 months (95% CI, 1.8-NA) and 5.1 months (95% CI, 1.8-NA), respectively (Figure 4A-B). In contrast, median OS for the patients without TP53 mutation was not yet reached and PFS was 13.3 months (95% CI, 8.6-NA) (Figure 4A-B).
Survival outcomes in the previously untreated AML cohort that received TAG-AZA-VEN. (A) OS probability by intention-to-treat analysis using the method of Kaplan-Meier for the 26 patients in the 1L AML TAG-AZA-VEN cohort. (B) PFS probability for the same cohort, calculated as in panel A.
Survival outcomes in the previously untreated AML cohort that received TAG-AZA-VEN. (A) OS probability by intention-to-treat analysis using the method of Kaplan-Meier for the 26 patients in the 1L AML TAG-AZA-VEN cohort. (B) PFS probability for the same cohort, calculated as in panel A.
Previously untreated AML receiving TAG-AZA-VEN, subgroup survival outcomes. (A) OS probability in the previously untreated AML TAG-AZA-VEN cohort, separated by TP53 mutant (n = 13, TP53 mut, blue) or TP53 wild-type (n = 13, TP53 WT, red). (B) PFS probability for the TP53 subgroups as in panel A. (C) OS probability for patients in the previously untreated AML TAG-AZA-VEN cohort that achieved CR/CRi/MLFS, separated by those determined to be MRD negative (n = 12, MRD neg, red) or positive (n = 5, MRD pos, blue). (D) PFS probability for the MRD subgroups as in panel C. (E) OS probability in the previously untreated AML TAG-AZA-VEN cohort, separated by those who received allogeneic stem cell transplant (n = 13, alloSCT Y, red) or those who did not (n = 13, alloSCT N, blue). (F) PFS probability for the alloSCT subgroups as in panel E. alloSCT, allogeneic stem cell transplantation.
Previously untreated AML receiving TAG-AZA-VEN, subgroup survival outcomes. (A) OS probability in the previously untreated AML TAG-AZA-VEN cohort, separated by TP53 mutant (n = 13, TP53 mut, blue) or TP53 wild-type (n = 13, TP53 WT, red). (B) PFS probability for the TP53 subgroups as in panel A. (C) OS probability for patients in the previously untreated AML TAG-AZA-VEN cohort that achieved CR/CRi/MLFS, separated by those determined to be MRD negative (n = 12, MRD neg, red) or positive (n = 5, MRD pos, blue). (D) PFS probability for the MRD subgroups as in panel C. (E) OS probability in the previously untreated AML TAG-AZA-VEN cohort, separated by those who received allogeneic stem cell transplant (n = 13, alloSCT Y, red) or those who did not (n = 13, alloSCT N, blue). (F) PFS probability for the alloSCT subgroups as in panel E. alloSCT, allogeneic stem cell transplantation.
Median OS among the patients who were MRD negative was not reached at the time of analysis. Median OS in the patients who achieved CR/CRi/MLFS but were MRD positive was 9.5 months (95% CI, 5.9-NA) (Figure 4C). PFS for the CR/CRi/MLFS patients who were MRD negative was not reached; PFS for the MRD-positive patients was 6.8 months (95% CI, 5.1-NA) (Figure 4D). Among patients who achieved CR/CRi/MLFS and had follow-up DNA sequencing performed at the time of best response, most had clearance or significant decreases in mutation variant allele fraction compared with baseline, including in TP53 (supplemental Figure 3).
Median OS for the 13 patients with 1L AML receiving TAG-AZA-VEN who received allogeneic transplant was 18.2 months (95% CI, 14.0-NA); median OS for patients who did not undergo transplantation was 11.0 months (95% CI, 1.3-NA) (Figure 4E). Median PFS for patients who received transplant was 13.3 months (95% CI, 8.2-NA); median PFS for patients who did not undergo transplantion was 3.0 months (95% CI, 1.2-NA) (Figure 4F).
Among the other cohorts, the most encouraging activity was seen in MDS treated with TAG-AZA. Of the 5 patients with previously untreated MDS, 1 received TAG 5 μg/kg daily for 5 days, 1 at 9 μg/kg daily for 3 days, and 3 at 12 μg/kg daily for 3 days (Figure 1). Three of these 5 patients with MDS (60%) achieved a complete response (CR, n = 2; marrow CR, n = 1) and all 3 harbored a TP53 mutation. Among the 14 patients with 1L or R/R AML who received TAG-AZA, 1 patient with TP53-mutated 1L AML had best response of CRi. Among the 11 patients with R/R AML who received TAG-AZA-VEN, 1 achieved an MLFS. Response and survival data for patients in these cohorts (AML and MDS receiving TAG-AZA, and R/R AML receiving TAG-AZA-VEN) are summarized in supplemental Figure 4.
Discussion
We found that it is safe and feasible to add 3-day dosing of TAG to AZA (tested in AML and higher-risk MDS) or to AZA and VEN (tested in AML). The approved regimens of AZA alone for MDS and AZA with VEN for AML are effective in some cases. However, there are a substantial proportion of patients who do not respond initially or relapse shortly after achieving a response, which suggests that adding an additional agent with a nonoverlapping mechanism of action is 1 strategy to improve outcomes. CD123 is expressed in most AML/MDS and is enriched in cells surviving initial therapy as well as LSCs. TAG is an established CD123-targeted therapy and has an added benefit of not causing the myelosuppression seen with conventional chemotherapy or AZA-VEN. Furthermore, given that TAG resistance is caused by reversible DNA methylation-mediated loss of the DT target and that TAG-exposed cells are more sensitive to VEN,14 the biological rationale to combine TAG with AZA-VEN in AML is strong.
This is the first study to report treatment of AML using TAG in combination with other chemotherapies. We did not observe any signal of increased toxicity, including cytopenias, using 3-day dosing of TAG in TAG-AZA or TAG-AZA-VEN combinations. The most notable expected toxicities of TAG are transient liver enzyme elevations and CLS, whereas AZA-VEN causes myelosuppression and raises infection risk. The rates of those adverse events in this study were similar to those seen in patients receiving TAG (approved as a 5-day schedule for BPDCN) or AZA-VEN alone.11,20 As in prior studies of TAG, CLS occurred almost exclusively in cycle 1 and was manageable with albumin supplementation and diuresis. Notably, there was no apparent increase in infections.
The observed rate of CLS was lower in patients receiving TAG-AZA-VEN compared to TAG-AZA. This could be due to modulation of CLS risk by the addition of VEN or may be associated with differences between the groups related to disease burden, schedule of TAG, or increased recognition and intervention for early CLS over time during the study. CLS is linked to cytokine induced vascular permeability in the setting of inflammatory pathway activation24,25 and VEN is reported to inhibit cytokine production by targeting specific immune cell populations.26-28 An intriguing alternative possibility requiring further investigation is that the immunomodulatory properties of VEN might dampen CLS severity. Or the additional reduction in tumor burden with TAG-AZA-VEN might attenuate inflammatory pathway activation by leukemia cells themselves.
Although eligibility for this study was not restricted to high-risk AML, all patients in the frontline cohort receiving triplet TAG-AZA-VEN had ELN 2022 adverse-risk disease, including half with a mutation in TP53. Effective therapy for AML with mutations in TP53 is lacking, as even fit patients respond poorly to intensive induction chemotherapy.29 Leukemia cells with TP53 mutations also have reduced sensitivity to BCL-2 inhibition,30 and TP53-mutated AMLs have poor outcomes with AZA and VEN.31 One hypothesis to explain the encouraging activity seen here with the addition of TAG in AML/MDS with mutated TP53 is that DT can induce potent cell death in certain contexts independent of TP53.32,33 This possible mechanism provides a direction for future laboratory investigation and additional rationale for exploring TAG combinations in myeloid malignancies with mutated TP53.
The outcomes observed with TAG-AZA-VEN here compare favorably to analyses of high-risk AMLs treated with hypomethylating agents (HMA) and VEN, albeit with some differences between populations. For example, OS was 12 months in patients with ELN adverse-risk AML receiving HMA plus VEN in the Beat AML study,34 compared with 14 months here with TAG-AZA-VEN. And, in combined analysis of patients with TP53 mutation and poor risk cytogenetics who received AZA and VEN in VIALE-A and its preceding phase 1b trial, OS was 5.2 months,31 compared to 9.5 months with TAG-AZA-VEN although not all patients had poor risk cytogenetics. Direct comparison is also complicated by clinical differences between the cohorts. For example, the median age was 77 in the high-risk VIALE-A subset and almost none went to allogeneic transplant, compared with median age 71 in the TAG-AZA-VEN cohort and half of them underwent transplantation. Our study also had albumin, bilirubin, and cardiac ejection fraction eligibility requirements that may have selected a fitter population. Ultimately, head-to-head randomized data comparing TAG-AZA-VEN to AZA-VEN are needed to confirm these results. Nonetheless, these data are encouraging and suggest that the additional targeting of CD123 with TAG may improve survival outcomes in patients with high-risk AML when given in combination with existing therapies.
We did not observe an association between the level of CD123 on AML blasts and response to TAG-AZA-VEN, which is consistent with other studies evaluating CD123-targeted agents in AML.35 This could be due to the lack of standardized CD123 measurements, or to other biologic factors like the contribution of CD123 on LSCs or CD123+ nonblast cells in the microenvironment. Moreover, in this study the number of samples tested for CD123 was small and all nonresponding AMLs available for assessment had a TP53 mutation. Uniformly assessed cell surface CD123 levels should be measured in larger treatment cohorts in future studies of TAG to better understand their relationship with efficacy. Additional biomarkers of sensitivity and resistance to TAG-AZA-VEN should also be the subject of future laboratory investigation.
We conclude that the safety and encouraging efficacy of TAG-AZA-VEN support continued development of this regimen in AML in the frontline setting. Despite several drug approvals in recent years, AML patients with high-risk genetic aberrations, including TP53 mutations, secondary-type genetics, and adverse chromosomal alterations still have inferior outcomes and represent an unmet clinical need.21 The addition of targeted agents with nonoverlapping toxicities and distinct mechanisms of action, such as TAG, to standard therapies may synergize to reduce overall disease burden and contribute to elimination of therapy-resistant leukemia cells.
Acknowledgments
A.A.L. is supported by the National Cancer Institute (NCI) (R37 CA225191), the Mark Foundation For Cancer Research, the Ludwig Center at Harvard, and the Bertarelli Rare Cancers Fund. A.A.L. is a Scholar of the Leukemia & Lymphoma Society. A.A.L. and N.P. are supported by the Department of Defense (W81XWH-20-1-0683). J.S.G. is supported by the NCI (K08 CA245209).
Authorship
Contribution: A.A.L., J.S.G., K.E.S., D.S.N., M.K., A.S., and N.P. designed the study; A.A.L., A.S., and N.P. led the trial at their sites; A.A.L., J.S.G., E.G.R., J.L.G., I.G., D.J.D., M.R.L., C.R.R., M.S., R.M.S., R.S.V., M.M.W., E.S.W., E.W.B., R.L., M.K., A.S., and N.P. performed the research; T.M., C.B., and I.V.G. provided critical reagents and interpreted results; A.A.L., K.E.S., D.S.N., S.R., and J.K. analyzed the data; and all authors participated in writing and editing the manuscript.
Conflict-of-interest disclosure: A.A.L. received research support from AbbVie and Stemline Therapeutics. A.A.L. received consulting fees from Cimeio Therapeutics, IDRx, Jnana Therapeutics, ProteinQure, and Qiagen, and has equity as an adviser for Medzown. J.S.G. served in advisory role for AbbVie, Astellas, Bristol Myers Squibb, Genentech, Gilead and Servier and had trial institutional funding from AbbVie, Genentech, Pfizer, Prelude and AstraZeneca. M.S. served on an advisory board for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, and Rigel; consulted for Boston Consulting and Dedham group, and participated in medical education activities for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. T.M. is a senior consultant for Stemline Therapeutics. C.B. and I.V.G. are employees of Stemline Therapeutics. N.P. received honoraria from Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer, Aptitude Health, NeoPharm, and CareDX; has a consulting or advisory role in Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb, ClearView Healthcare Partners, Astellas Pharma, Protagonist Therapeutics, Triptych Health Partners, and CTI BioPharma Corp; received research funding from Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon, and MustangBio; received travel, accommodation, and other expenses from Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, and MustangBio. The remaining authors declare no competing financial interests.
Correspondence: Andrew A. Lane, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 413, Boston, MA 02215; email: andrew_lane@dfci.harvard.edu.
References
Author notes
∗A.S. and N.P. contributed equally to this study.
Individual participant data will not be shared. Other data are available upon request from the corresponding author, Andrew A. Lane (andrew_lane@dfci.harvard.edu).
The full-text version of this article contains a data supplement.