Key Points
Patients with BDUC showed a disrupted kinetic profile for PG, which might be associated with altered clot structure.
PG parameters and fibrinogen showed a high predictive power in discriminating between patients with BDUC and HCs.
Visual Abstract
Bleeding disorder of unknown cause (BDUC) is a diagnosis of exclusion after evaluation of plasma coagulation and platelet function. Patients with BDUC (n = 375) recorded in the Vienna Bleeding Biobank were analyzed in comparison with healthy controls (HCs; n = 100) in this case-control study. Plasmin generation (PG) parameters were analyzed using calibrated fluorescence detection in citrated plasma. Turbidimetric plasma clot formation/lysis of 293 (78%) patients with BDUC and confocal microscopy of clots from representative patients with BDUC (n = 6) and HCs (n = 9) were assessed. In the PG analysis, patients with BDUC exhibited lower velocity and peak plasmin levels but a higher endogenous plasmin potential than HCs. Peak plasmin levels correlated with maximum clot absorbance but not with clot lysis time. Clot absorbance is an indicator of clot fiber density. Confocal microscopy analysis revealed a tendency towards thicker fibers in clots of patients with BDUC, which negatively correlated with peak plasmin (r = −0.561; P = .030). Peak plasmin correlated weakly with factor XIII, but not with other fibrinolytic factors (alpha2-antiplasmin, thrombin activatable fibrinolysis inhibitor, or plasminogen activator inhibitor 1) or bleeding severity. A model comprising fibrinogen and parameters of PG yielded high predictive power in discriminating between patients with BDUC and HCs across a fivefold stratified cross validation (80% of data; mean area under the curve [AUC], 0.847). The model generalized well to unseen data (20% of data; AUC, 0.856). Overall, patients with BDUC counterintuitively exhibited reduced peak plasmin levels, potentially related to altered clot structure.
Introduction
Bleeding disorder of unknown cause (BDUC) is an established diagnosis that is characterized by a nontrivial bleeding tendency and normal findings in plasma coagulation and platelet function assays.1,2 Patients with BDUC exhibit a bleeding phenotype similar to individuals with mild-to-moderate established bleeding disorders (MBDs), such as platelet function defects, von Willebrand disease, or mild coagulation factor deficiencies, and face an increased risk for recurrent bleeding.3-5
The underlying mechanisms that drive BDUCs remain uncertain. Our recent investigations have uncovered an impaired hemostatic capacity in patients with BDUC that is manifested as decreased peak thrombin levels and endogenous thrombin generation, slower plasma clot formation with increased final clot turbidity, and shortened clot lysis time.6 In addition, the fibrinolytic system’s role in the pathophysiology of BDUCs has garnered increasing attention as evidenced by a body of literature of smaller studies7,8 and the phenotypic parallels seen in patients with hereditary hyperfibrinolytic conditions.9,10 The fibrinolytic system counterbalances clot formation and is challenging to measure in vitro.11 Tissue plasminogen activator (tPA) converts plasminogen to plasmin, the pivotal fibrinolytic protease. Established assays to assess clot lysis capacity include the euglobulin clot lysis time, rotational thromboelastometry, and clot turbidity.10 However, these tests do not provide information on the plasmin generation (PG) kinetics.10 A PG assay that uses a fluorogenic substrate and that enables correction for the inner filter effect and substrate consumption12 was recently developed for use in mouse and human plasma.13,14 This PG assay yields parameters that describe the quantitative dynamics of PG, and it is sensitive to the antifibrinolytic effects of tranexamic acid on PG.14
Previous studies, including data from our group, have identified altered levels of plasma proteins that may (in)directly regulate PG in bleeding cohorts, including tPA, alpha2-antiplasmin, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor (PAI-1).7,15-20 We found higher levels of tPA but, paradoxically, also higher levels of TAFI and alpha2-antiplasmin in patients with BDUCs when compared with healthy controls (HCs), which was suspected to reflect a counter-regulatory mechanism for hyperfibrinolysis, for example, by balancing the augmented activity of tPA.7 Alterations in PG have been described in patients with rare coagulation factor deficiencies,21 but PG has not been investigated in patients with BDUCs.
This study aimed to analyze the kinetics of PG and the relationship of PG with clot structure, fibrinolytic factors, and the bleeding phenotype in patients with BDUCs from the Vienna Bleeding Biobank (VIBB), a single-center cohort study on patients with MBD, in comparison with HC.
Methods
Patients and study design
The VIBB was a prospective, single-center cohort study that has been enrolling patients with a bleeding tendency without a previously established diagnosis of an inherited or acquired bleeding disorder since October 2009. Patients who presented at our outpatient clinic with a nontrivial bleeding tendency were enrolled by experienced hematologists and hemostasis experts. For comparison in this case-control study, a cohort of age- and sex-matched HCs was selected from the Vienna Bleeding Study (VIBS).4
Patients and HCs with the following characteristics were excluded from the VIBB and VIBS: active pregnancy; ongoing malignancy; surgical procedures within the previous 6 weeks; bacterial infections within the previous 2 weeks; continuous therapy with anticoagulants, antiplatelet- or anti-inflammatory drugs within the previous 10 days; thrombocytopenia with a platelet count below 100 × 109/L; and impaired liver (prothrombin time <75% of normal owing to deficiency in vitamin K–dependent clotting factors) and kidney functions (Glomerular filtration rate [GFR] <60 mL/min per 1.73 m2). Details of the study and its inclusion and exclusion criteria have been published previously and are shown in supplemental Table 1.4
In this case-control study, 375 patients with BDUC who were included from June 2021 in the VIBB were compared with 100 age-matched HCs selected from the VIBS.4 The remaining patients from the VIBB, including 144 patients with platelet function disorders, 63 patients with von Willebrand disease, 18 patients with coagulation factor deficiencies, and 4 patients with other diagnoses, were not analyzed.
At enrollment, all study participants provided written informed consent and underwent a structured interview on their medical and bleeding history and their family history of bleeding. Both studies were approved by the ethics committee of the Medical University Vienna (VIBB: EC no. 603/2009; VIBS: Ethics Commission [EC] no. 039/2006) and were conducted in accordance with the tenets of the Declaration of Helsinki of 1975 and its later amendments.
Biomaterial was processed and stored according to standard operating procedures of the MedUni Wien Biobank in an ISO 9001:2015-certified environment.22
Diagnostic criteria for BDUC
Patients with BDUC were defined as those with a clinically relevant and nontrivial bleeding tendency for which no underlying cause could be identified. To classify patients with BDUC, a standardized and comprehensive diagnostic workup was performed according to the recently published Scientific and Standardization Committee (SSC) communication by Baker et al2 and as shown in supplemental Table 2. The diagnostic criteria for BDUC and other established MBD diagnoses are described in detail in supplemental Table 3.
Bleeding phenotype and severity
Bleeding severity was recorded using the Vicenza bleeding score, a standardized bleeding assessment tool with cutoffs for pathologic bleeding of ≥3 points for males and ≥5 points for females.23,24 The bleeding score based on the bleeding assessment tool of the International Society for Thrombosis and Haemostasis (ISTH Bleeding Assessment Tool [BAT]) was additionally recorded for patients after June 2013 and showed a good correlation with the Vicenza bleeding score in our cohort.3 The ISTH BAT cutoffs for pathologic bleeding were ≥4 points for men and ≥5 for women aged 18 to 30 years, >6 for women aged 31 to 51 years, and ≥7 for women aged ≥52.
PG assay
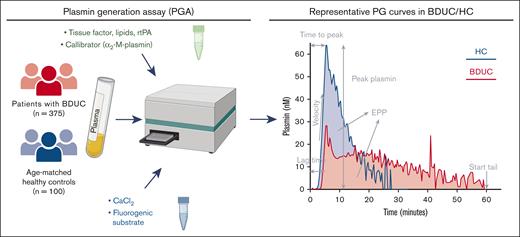
A detailed description of the PG assay is included in the supplemental Materials and depicted in Figure 1. Citrated platelet-poor plasma (PPP) samples (stored at −80°C and not previously thawed) were analyzed. Briefly, procoagulant activity was initiated by the addition of exogenous tissue factor (TF; PPP-reagent LOW, ∼1 pM TF; Diagnostica Stago, Asnières sur Seine, France) to recalcified plasma, and PG was triggered by the addition of exogenous tPA (0.62 μg/mL). PG was determined using fluorogenic substrate cleavage over time.
PG assay experiment setup and representative curves of PG in patients with BDUC and HCs. Summary of the setup of the PG assay. PG curves represent single measurements of 1 representative patient with BDUC and 1 representative HC. rtPA, recombinant tPA. Figure created with BioRender.com
PG assay experiment setup and representative curves of PG in patients with BDUC and HCs. Summary of the setup of the PG assay. PG curves represent single measurements of 1 representative patient with BDUC and 1 representative HC. rtPA, recombinant tPA. Figure created with BioRender.com
The kinetic parameters that were investigated included the lag time, time to peak (TTP) levels, rate of PG (velocity), peak plasmin level, and endogenous plasmin potential (EPP) and were calculated from the derivates of the obtained fluorescence curve (Figure 1).13 To calculate PG parameters using the Thrombinoscope software (version 5.0.0.742, Fluoroskan Ascent; Thrombinoscope, Maastricht, The Netherlands), the end of the PG curve (start tail time, ST) was set at 60 (±1) minutes, if possible, or otherwise was set at the earliest possible time point.
Turbidimetric plasma clot analysis
Plasma clot formation and lysis were turbidimetrically measured in 293 (78%) patients with BDUC, as previously described.6,25 In short, calcium chloride (20 mM, final), phospholipids (4 μmol/L, final), TF (Innovin, 2 pmol/L final), and tPA (333 ng/mL final) were added to citrated plasma. Turbidity was monitored by absorbance (optical density [OD]) on a Thermo Scientific microplate reader. In this study, we correlated PG data with maximum absorbance at plateau (OD405nm) and clot lysis time (CLT) (time from 50% of peak OD during clot formation to 50% decrease in turbidity from peak OD in minutes).
Plasma clot structure analysis
The clot structure was analyzed using confocal microscopy (Zeiss LSM700) with a 63×/1.4 oil immersion objective. The settings included a pinhole aperture of 1 Airy unit (0.7 μm section), an image/frame size of 101.5 μm × 101.5 μm at a resolution of 1024 × 1024 pixels, and a pixel size of 0.1 μm. For this experiment, plasma from randomly selected groups of patients with BDUC (n = 6) and from HCs (n = 9) with distinct peak plasmin levels were analyzed. PPP was spiked with AlexaFluor488–conjugated fibrinogen (80 μg/mL; final, 2.6% of total fibrinogen) and clots were formed with TF, calcium chloride, and phospholipids in Laboratory-Tek II chamber cover glass slides (Thermo Fisher Scientific, Waltham, MA), and after 3 hours of incubation, were imaged as described previously.26 For each well, a 30-slice Z-stack of 10 μm each was performed in 4 different areas to determine the median fibrin fiber density and the median diameter of the fibers. The density of fibrin fibers was estimated as a percentage by dividing the area covered by fibrin fibers with the area of 1 reading spot. The diameter of fibrin fibers (in μm) was determined by manual measurement of 20 different widths of fibers on a 2-dimensional reading spot. Fibers were visualized using ImageJ, version 1.410. Representative samples from patients with BDUC and from HCs with different ages, sexes, bleeding scores, and family histories were selected for the confocal microscopy experiments (supplemental Table 4).
Assessment of fibrinolytic factors
As previously described,7,27 the following fibrinolytic factors were measured using commercially available enzyme-linked immunosorbent assays (ELISAs) according to manufacturer’s manuals: fibrinogen (STA Fibrinogen, Roche Diagnostics and Diagnostica Stago, Asnières sur Seine, France), alpha2-antiplasmin activity (Diagnostica Stago), activated TAFI antigen (Stachrom TAFI, Asnières sur Seine, France), soluble thrombomodulin antigen (sTM; ab46508; Thrombomodulin [CD141] Human ELISA Kit; Abcam, Cambridge, United Kingdom), active PAI-1 antigen (PAI-1 Actibind ELISA; Technoclone, Vienna, Austria), and D-Dimer (Asserachrom D-Dimer, Diagnostica Stago). Factor XIII (FXIII) activity was analyzed using a chromogenic assay (Berichrom Factor XIII assay; Siemens Healthineers, Erlangen, Germany).
Statistical analysis
The statistical analysis was conducted using the free open-source software GNU R (www.r-project.org/), version 4.2.2. Continuous variables were described using median and interquartile ranges (IQRs), whereas categorical variables were presented as counts and percentages. For normally distributed continuous clinical and laboratory characteristics, a Student t test was used to compare groups. In cases of nonnormal distributions, the nonparametric Wilcoxon rank sum test was employed. Linear regression was used to analyze effect size with adjustment for confounding variables. Correlations between parameters were assessed using Spearman rho correlations. Given the exploratory and hypotheses-generating nature of this study, no correction for multiple testing was performed. Our study on 375 patients with BDUC in comparison with 100 HCs yielded a power of 0.9997 when a mean difference in peak plasmin of 1.10 nM was considered with a standard deviation of 1.81 based on previously published data.28
Model to analyze the predictive power of PG and fibrinogen to discriminate between patients with BDUC and HCs
To assess whether BDUC (vs HC) status can be predicted by PG parameters and fibrinogen, a multiple logistic regression model was trained (supplemental Paragraph 2). Initially, only PG parameters that were found to be significant in the multivariable logistic regression (testing 1 PG parameter at a time, together with fibrinogen, sex, age, blood group O vs non-O) were included. Parameter selection was then performed by backward elimination, and all parameters with a P value ≥.05 were excluded. The data were divided into training and test sets at a ratio of 0.8 with adjustment for patient groups (BDUC, HC). Areas under the curve (AUC) of the receiver operator characteristic (ROC) curves were evaluated in a fivefold stratified cross validation on the randomly selected training set to assess the predictive power of the models during backward elimination. The generalizability of the final model was assessed on the test set using the AUC of the ROC curve.
Results
Patients’ clinical and laboratory characteristics
The clinical and laboratory characteristics of the 375 patients with BDUC and the 100 HCs are shown in Table 1. Female sex and blood group O were more prevalent in the groups of patients with BDUC than in the groups of HCs. A family history of a bleeding tendency was present in 35% of patients. Patients with BDUC had a median Vicenza and ISTH BAT bleeding score of 5.0 (IQR, 4.0-8.0) and 6.0 (IQR, 4.0-8.0), respectively. The median number of bleeding manifestations in patients was 3.0 (IQR, 2.0-5.0). Of all patients, 67% had a pathologic Vicenza bleeding score based on the pathologic cutoffs for men and women. When compared with HCs, patients had lower hemoglobin levels and a prolonged activated partial thromboplastin time and prothrombin time (Table 1). Patients had significantly higher levels of fibrinogen, albeit within the normal range, however, there was no difference in the plasminogen levels.
PG is impaired in BDUC
The kinetic profiles for PG were compared between those with BDUC and HCs (Table 2; Figure 2). Patients with BDUC had significant alterations in PG, reflected by a longer lag time and TTP and lower velocity and peak plasmin levels but higher EPP. Except for the lag time and TTP, these differences persisted after adjusting for fibrinogen levels, age, sex, and blood group O (Table 2).
PG parameters in patients with BDUC and HCs. Procoagulant activity was initiated by the addition of exogenous TF (PPP-reagent LOW, ∼1pM TF, Diagnostica Stago) to recalcified plasma, and PG was triggered by the addition of exogenous tPA (0.62 μg/mL). PG was detected via fluorogenic substrate cleavage over time. Black lines represent the median and IQR. Significance (adjusted for age, sex, blood group O, and fibrinogen levels) is indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
PG parameters in patients with BDUC and HCs. Procoagulant activity was initiated by the addition of exogenous TF (PPP-reagent LOW, ∼1pM TF, Diagnostica Stago) to recalcified plasma, and PG was triggered by the addition of exogenous tPA (0.62 μg/mL). PG was detected via fluorogenic substrate cleavage over time. Black lines represent the median and IQR. Significance (adjusted for age, sex, blood group O, and fibrinogen levels) is indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
In 184 (49.1%) patients with BDUC and 81 (81.0%) HCs, the standard ST time produced an error in the analysis software in that the software reported too high PG and could not calculate the PG parameters. Therefore, we manually set the ST time for these samples at the latest time point that allowed the software to calculate the parameters. This manual procedure was performed blindly without knowledge of the participant phenotype (BDUC or HC). To validate this approach, we performed an additional subanalysis in which we only compared the PG parameters between patients with BDUC and HCs who had a start time at 60 (±1) minutes. The trends in all parameters, including the significant differences in TTP, velocity, and peak plasmin levels, were preserved, whereas no statistical difference prevailed for EPP (supplemental Figure 1).
Association of peak plasmin with plasma clot characteristics in patients with BDUC
Because the peak plasmin level may be the most accurate indicator of PG capacity and the significant difference in peak plasmin between groups was independent of the ST time, we focused on this parameter in subsequent analyses. We previously found that clots from patients with BDUC had a higher maximum absorbance and a shorter clot lysis time in a turbidimetric plasma clot formation and lysis assay when compared with HCs.6 Because PG may be influenced by clot characteristics, we subsequently analyzed the association between the peak plasmin level and plasma clot structure.29,30 Both PG and clot formation and lysis assay measurements were available for 293 (78%) patients with BDUC.
Peak plasmin correlated with the maximum absorbance (r = 0.413; P < .001), whereas no correlation was found for clot lysis time (r = 0.111; P = .058), as shown in Figure 3.
Association between peak plasmin level and maximum plasma clot absorbance and clot lysis time. After addition of calcium chloride (20 mM, final), phospholipids (4 μmol/L, final), TF (Innovin, 2 pmol/L final), and tPA (333 ng/mL final) to citrated plasma, turbidity was monitored by absorbance (OD) on a Thermo Scientific microplate reader. Clot lysis time was defined as the time from 50% of the peak OD during clot formation to 50% decrease in turbidity from peak OD.
Association between peak plasmin level and maximum plasma clot absorbance and clot lysis time. After addition of calcium chloride (20 mM, final), phospholipids (4 μmol/L, final), TF (Innovin, 2 pmol/L final), and tPA (333 ng/mL final) to citrated plasma, turbidity was monitored by absorbance (OD) on a Thermo Scientific microplate reader. Clot lysis time was defined as the time from 50% of the peak OD during clot formation to 50% decrease in turbidity from peak OD.
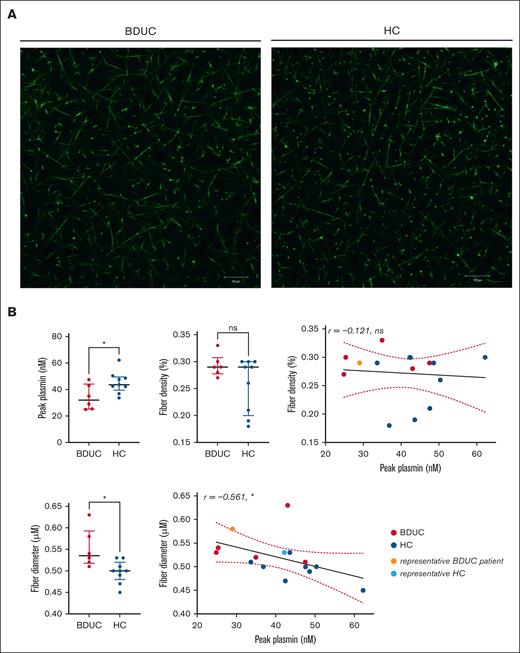
Subsequently, we examined the association between peak plasmin and the fibrin network density and the fibrin fiber structure. Fibrin fiber diameter and density were assessed in clots from 6 patients with BDUC and 9 HCs using confocal microscopy. The characteristics of the randomly selected patients with BDUC and HCs are shown in supplemental Table 4. Consistent with all patients with BDUC, the peak plasmin was lower in the patients than in the HCs (Figure 4). Fibrin fibers were thicker in patients with BDUC than in the HCs. Furthermore, there was a negative correlation between fiber diameter and peak plasmin in both the patients and HCs (r = −0.561; P = .030; Figure 4). Fiber density exhibited a tendency to be higher in patients with BDUC than in the HCs, however, it did not correlate with peak plasmin (r = −0.121; P = .67; Figure 4).
Fibrin network structure and its correlations with peak plasmin in patients with BDUC (n = 6) and HCs (n = 9). (A) Fibrin network structure of a representative patient with BDUC and an HC. The clot structure was analyzed using confocal microscopy (Zeiss LSM700) with a 63×/1.4 oil immersion objective. The settings included a pinhole aperture of 1 Airy unit (0.7 μm section), an image/frame size of 101.5 μm × 101.5 μm at a resolution of 1024 × 1024 pixels, and a pixel size of 0.1 μm. (B) Comparison of the fibrinogen levels, fiber density, and fiber diameters and the association of fiber density and diameter with the peak plasmin level in 6 representative patients with BDUC and 9 HCs. The 2 representative participants showed in panel A are marked as light red (BDUC) and light blue (HC). Significance is indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, not significant.
Fibrin network structure and its correlations with peak plasmin in patients with BDUC (n = 6) and HCs (n = 9). (A) Fibrin network structure of a representative patient with BDUC and an HC. The clot structure was analyzed using confocal microscopy (Zeiss LSM700) with a 63×/1.4 oil immersion objective. The settings included a pinhole aperture of 1 Airy unit (0.7 μm section), an image/frame size of 101.5 μm × 101.5 μm at a resolution of 1024 × 1024 pixels, and a pixel size of 0.1 μm. (B) Comparison of the fibrinogen levels, fiber density, and fiber diameters and the association of fiber density and diameter with the peak plasmin level in 6 representative patients with BDUC and 9 HCs. The 2 representative participants showed in panel A are marked as light red (BDUC) and light blue (HC). Significance is indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, not significant.
Association between peak plasmin and fibrinolytic factors in patients with BDUC
To investigate the factors that contribute to a reduced peak plasmin in patients with BDUC, we analyzed the associations between peak plasmin levels and FXIII , alpha2-antiplasmin, TAFI, PAI-1, sTM, and D-dimer (supplemental Table 5). We observed a weak correlation between peak plasmin and FXIII (r = 0.22; P = .003). However, there were no significant correlations between the peak plasmin level and the levels of TAFI (r = 0.31; P = .190), alpha2-antiplasmin (r = 0.07; P = .360), sTM (r = 0.02; P = .082), PAI-1 (patients with levels <1 IU/mL vs ≥1 IU/mL; P = .480), or D-dimer (patients with levels <0.3 μg/mL vs ≥0.3 μg/mL; P = .120; supplemental Figure 2).
Association between peak plasmin and bleeding severity or phenotype among patients with BDUC
The peak plasmin level did not show any correlation with bleeding severity, as measured by the Vicenza or ISTH BAT bleeding scores, nor with the number of bleeding manifestations (Figure 5). Furthermore, the peak plasmin level, nor any other parameter of PG, among patients with BDUC with Vicenza or ISTH BAT bleeding scores above the pathologic cutoff did not differ from those with bleeding scores below the cutoff (Figure 5; supplemental Table 6). The peak plasmin was not associated with the presence of any specific bleeding symptom. Patients with oral mucosal bleeding had significantly increased peak plasmin levels, which did not prevail after correcting for multiple testing (Bonferroni-Holm-Correction, P > .5; supplemental Figure 3).
Association between peak plasmin level and bleeding severity. Correlation between PG potential and bleeding severity reflected by the Vicenza and ISTH BAT bleeding scores, as well as the number of bleeding manifestations in patients with BDUC. The pathologic Vicenza bleeding score cutoff is ≥5 for women and ≥3 for men; the pathologic ISTH BAT bleeding score cutoff is ≥5 for women aged 18 to 30 years, >6 for women aged 31 to 51 years, ≥7 for women aged ≥52 years, and ≥4 for men. BS, bleeding score; ns, not significant.
Association between peak plasmin level and bleeding severity. Correlation between PG potential and bleeding severity reflected by the Vicenza and ISTH BAT bleeding scores, as well as the number of bleeding manifestations in patients with BDUC. The pathologic Vicenza bleeding score cutoff is ≥5 for women and ≥3 for men; the pathologic ISTH BAT bleeding score cutoff is ≥5 for women aged 18 to 30 years, >6 for women aged 31 to 51 years, ≥7 for women aged ≥52 years, and ≥4 for men. BS, bleeding score; ns, not significant.
Predictive power of PG and fibrinogen to discriminate between patients with BDUC and HCs
Given the significant adjusted differences in several PG parameters (velocity, peak plasmin, EPP, and ST time) (Table 2) and the significant difference in fibrinogen levels between patients with BDUC and HCs, we evaluated if the PG assay, in combination with fibrinogen levels, can be used to predict BDUC diagnosis. The training model, trained on 80% of the patients and HCs, included PG parameters that were significantly different in the multivariate analysis between patients with BDUC and HCs without a need for backward elimination (supplemental Table 7).
The final model included fibrinogen, velocity, peak plasmin, EPP, and ST time and displayed a high predictive power to discriminate between patients with BDUC and HCs with a mean AUC of the ROC curve of 0.847. Furthermore, the model generalized well to unseen data in the test set (20% of patients and HC) with an AUC of the ROC curve of 0.856 (Figure 6).
ROC curve analysis (BDUC vs HC). ROC curve analysis for the tested model, including fibrinogen, PG velocity, peak plasmin, EPP, and ST time, in the validation data set (20% of data). FPR, false positive rate; TPR, true positive rate.
ROC curve analysis (BDUC vs HC). ROC curve analysis for the tested model, including fibrinogen, PG velocity, peak plasmin, EPP, and ST time, in the validation data set (20% of data). FPR, false positive rate; TPR, true positive rate.
Discussion
BDUC is a nontrivial bleeding tendency with an undefined etiology10,31 in which alterations in plasma clot formation and fibrinolysis may be an underlying cause for the impaired hemostatic capacity.6,10 In this study, we analyzed PG in patients with BDUC in comparison with age-matched HCs. We observed decreased peak plasmin levels in patients with BDUC, suggesting that the patients had a lower PG potential. Peak plasmin levels correlated with higher plasma clot density and FXIII and inversely with fibrin fiber thickness. However, peak plasmin was not associated with the clot lysis time or parameters of fibrinolysis or the bleeding phenotype. Overall, fibrinogen and a subset of PG parameters yielded excellent AUC scores for differentiating between patients with BDUC and HCs.
The unexpectedly reduced peak plasmin level in patients with BDUCs challenges the conventional expectations because hyperfibrinolysis and thus enhanced PG might have been expected in patients with a bleeding tendency.10 Data on PG in patients with bleeding disorders are generally scarce, and different groups use different parameters to describe the PG potential. Valke et al showed increased peak plasmin in patients with severe hemophilia A when compared with patients with mild hemophilia A or HCs,32 whereas no difference in PG between patients with severe hemophilia and HCs was seen in a study by Matsumoto et al.33 Not surprisingly, the PG potential was very low or undetectable in patients with plasminogen deficiency, whereas patients with homozygous PAI-1 deficiency showed increased peak plasmin levels and EPP, thereby fitting the expected hyperfibrinolytic bleeding disorder.34 However, in other rare bleeding disorders, low EPP was also reported, which is in line with our data.21 It should be noted that, in our study, EPP was dependent on the earlier end of the PG curve in most HCs; nevertheless, it did not differ between patients and HCs who had the same predefined time point when the PG curve ended. Moreover, in afibrinogenemia and prothrombin deficiency, delayed and reduced PG was reported, whereas there was no difference in PG in patients with FX, FVII, or FXIII deficiency.21 Of note, in these rare bleeding disorders, PG was lower in patients with a more severe bleeding phenotype than in those with minor bleeding. In contrast, we did not observe an association between PG and the bleeding phenotype and severity in patients with BDUC. Specifically, there was no difference in any of the PG parameters between patients with normal and those with pathologic Vicenza or ISTH BAT bleeding scores.
These observations of partly reduced PG in bleeding disorders seem to be counterintuitive. We recently identified altered clot structures in patients with BDUC, which showed slower clot formation that produced clots with increased absorbance and faster lysis.6,35,36 Thus, impaired PG in patients with BDUC might reflect impaired conversion of plasminogen to plasmin on abnormally structured fibrin fibers.12 Indeed, reduced plasmin activation has been reported in coarse clots made of thick fibers.37 This hypothesis of lower PG in patients with BDUC that is caused by altered clot structure is supported by our findings of (1) a correlation between the maximum clot absorbance and peak plasmin level and (2) increased fiber thickness, as assessed by confocal microscopy, in clots of patients with BDUC, which correlated negatively with the peak plasmin levels. It should be noted, however, that despite the impaired PG, clots made of thicker fibrin fibers tended to lyse faster, whereas clots made of thin fibers were lysed slower.38-40 Thus, the relative impact of reduced PG vs increased susceptibility of clots to fibrinolysis is difficult to predict.
Several factors mediate clot formation and structure, including the concentrations of thrombin and fibrinogen.29,37,41 Thrombin generation has been reported to be impaired in our patients with BDUC, whereas this was not shown for other patient cohorts of BDUC.6,35 Fibrinogen is also a major determinant of plasma clot characteristics in our patients with BDUC42-44 and we found higher fibrinogen in patients than in HCs in the VIBB.4,7 The differences in PG in this study prevailed after adjustment for fibrinogen, as did the differences in clot structure in our previous study.6 In this study, the incorporation of fibrinogen with PG parameters into the model improved the discriminatory power for patients with BDUC.
The counterintuitive reduction in peak plasmin in patients with BDUC is consistent with the findings of paradoxically increased levels of the fibrinolysis inhibitors TAFI and alpha2-antiplasmin in our BDUC patient cohort7,10 and, partly, also in other bleeding cohorts.7,15,16,19 Although alpha2-antiplasmin acts as a direct inhibitor of plasmin, TAFI might interfere with PG by removing carboxy-terminal lysines, the plasminogen activation sites, from fibrin.45 TAFI is activated by thrombin and TM, and both TAFI and TM delay PG kinetics in an obesity mouse model.13 The increase in TAFI levels in patients with BDUCs may lead to less C-terminal lysines45 and therefore to reduced PG. However, no association between peak plasmin levels and alpha2-antiplasmin or TAFI was identified in our study. In contrast, we observed a positive correlation between the peak plasmin level and FXIII.46 This correlation may not necessarily imply a causal relationship, because previous analyses in patients with FXIII deficiency showed no alteration in PG.21 In line with this, in mice, FXIII-deficient plasma or the presence of inhibitors against activated FXIII did not demonstrate alterations in PG.13
Our study is based on a large, well-characterized cohort of all available BDUC samples; however, the study has limitations. One such limitation is that the calibrated, automated PG assay did not account for endogenous tPA or active PAI-113 and therefore we could not assess abnormal functions for these key proteins in the fibrinolytic pathway. In addition, the observed increase in EPP may have been influenced by the assay design, particularly the addition of tPA before the initiation of PG, however, the same amount of tPA was added to the BDUC and HC samples at the same time. Further studies are needed to understand the underlying mechanisms, including the potential differences in fibrinogen or fibrin binding or the kinetics of the conversion of plasminogen to plasmin in patients with BDUC. Furthermore, the interplay between PG dynamics and the structure of plasma clots must be interpreted with caution. It should be noted that although the TTP for PG was approximately 6 minutes in patients with BDUC, the clots analyzed via confocal microscopy were incubated for 3 hours. Despite this discrepancy, we incorporated confocal microscopy data because it provided sufficient resolution to evaluate fibrin density and fiber diameter in clots of both patients with BDUC and HCs. It is important to note, however, that we measured clot structure in only a subset of samples. These samples were randomly selected to mitigate selection bias and are deemed representative of the broader cohort. Overall, our findings underscore the complexity of BDUC and highlight the need for future and more comprehensive approaches, possibly using multiomics and advanced computational methods, to validate the findings and to fully elucidate the underlying mechanisms in BDUC.
In summary, we have identified a disrupted PG kinetic profile in patients with BDUC that might be linked to altered clot structure. Our regression model, which incorporated key PG parameters and fibrinogen, showed an excellent AUC in ROC analysis for discriminating between patients with BDUC and HCs. These results emphasize the importance of evaluating coagulant and fibrinolytic pathways and clot structure to fully understand the hemostatic and fibrinolytic potential in BDUC. Additional research is necessary to identify the plasma factors that play a role in impaired clot formation and PG dynamics in this patient population.
Acknowledgments
Funding for this project was provided by an European Hematology Association Research Mobility Grant awarded by the European Hematology Association and the Prof Heimburger Award from CSL Behring. The Vienna Bleeding Biobank was supported by an unrestricted grant from CSL Behring, the Medical-scientific fund of the Mayor of the federal capital Vienna (grant 20023), and the Anniversary Fund of the Austrian National Bank (grant 18500). D.M. received the Physician Pathway Scholarship of the Medical University of Vienna for protected research time. A.S.W. received funding from the National Institutes of Health (grant R01HL126974). S.E.R. received funding from the American Heart Association postdoctoral award (23POST1019349).
Authorship
Contribution: D.M., S.E.R., I.P., A.S.W., and J.G. designed the study; D.M., C.A., and I.P. recruited patients; C.d.M. performed confocal microscopy; D.M. and S.E.R. performed the laboratory measurements; B.d.L. provided materials for the plasmin generation analysis; H.H. coordinated biobanking; D.M. and M.C.K. performed the statistical analyses; D.M., S.E.R., I.P., A.S.W., and J.G. analyzed the data; D.M., S.E.R., I.P., A.S.W., and J.G. interpreted the data; D.M., S.E.R., A.S.W., and J.G. wrote the manuscript; and all authors reviewed, edited, and approved the manuscript in its final form.
Conflict-of-interest disclosure: D.M. reports receiving honoraria for advisory board meetings and lectures from CSL Behring. C.A. reports receiving honoraria from Bayer, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda for lectures and/or participation in advisory board meetings. I.P. reports receiving honoraria from Bayer, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda for lectures and advisory board meetings. A.S.W. reports receiving honoraria and/or participating in advisory board meetings for Takeda. J.G. reports receiving honoraria for lectures and advisory board meetings and receiving research funding for the Medical University of Vienna from CSL Behring, Novartis, Amgen, Sobi, and Takeda. B.d.L. reports being employed by Synapse Research Institute and being a member of the Stago Diagnostic group that produces calibrated automated thrombography for thrombin generation measurements in plasma. The remaining authors declare no competing financial interests.
Correspondence: Johanna Gebhart, Division of Hematology and Hemostaseology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; email: johanna.gebhart@meduniwien.ac.at.
References
Author notes
Original data are available on request from the corresponding author, Johanna Gebhart (johanna.gebhart@meduniwien.ac.at).
The full-text version of this article contains a data supplement.