TO THE EDITOR:
Multiple myeloma is associated with significant morbidity and mortality despite recent advances in treatment. Improvements in induction therapy have led to significantly prolonged progression-free survival (PFS) and overall survival (OS).1 Bone disease affects most patients with myeloma, occurring in 90% of patients at any point during their disease course, and is associated with morbidity.2
The use of bone-modifying agents (BMAs) historically improves PFS and OS in multiple myeloma, and incorporation of BMA is considered standard of care for patients with myeloma.3 Numerous BMA options are available and the choice of which to use remains controversial given that there is randomized data supporting both denosumab and zoledronic acid.4 A primary advantage of denosumab is that it can be used in patients with renal failure; however, it is significantly more expensive than zoledronic acid (annual drug cost is ∼$576 for zoledronic acid and $24 528 for denosumab, a difference of almost $24 000.)5
A randomized trial directly comparing zoledronic acid with denosumab previously demonstrated noninferiority of denosumab to zoledronic acid with respect to its primary end point, which was time to first skeletal-related event (hazard ratio [HR], 0.98; 95% confidence interval [CI], 0.85-1.14; P value for noninferiority = .010).6 This trial was not powered for PFS. On an exploratory post hoc analysis of this trial, the median PFS was increased by 10.7 months with denosumab vs zoledronic acid.7 Given that the trial was not formally powered for PFS (despite its large sample size), there is a concern that this may represent a spurious finding. In this real-world study of a large US cohort, we evaluated whether there was any difference in outcomes between those treated with denosumab and bisphosphonates.
The Flatiron Health database is a longitudinal database, comprising deidentified patient-level structured and unstructured data, curated via technology-enabled abstraction.8,9 During the study period, the deidentified data originated from ∼280 US cancer clinics (∼800 sites of care).8 Approval of investigations of these deidentified data by an independent ethics committee/institutional review board was obtained.
This was a new user, active comparator retrospective cohort study of patients receiving bone-modifying treatment with either denosumab or bisphosphonates (zoledronic acid or pamidronate) within 90 days of first-line myeloma therapy in the nationwide Flatiron Health electronic health record-derived deidentified database. We identified all patients initiating first-line treatment for newly diagnosed myeloma in the Flatiron Health research database. To be included, patients had to be aged ≥18 years, have a diagnosis of myeloma confirmed by International Classification of Diseases codes, and be observed for at least 90 days after the date of diagnosis (to allow sufficient time to capture BMA use). Patients who received no cancer-directed therapy were excluded from our study.
Patients were followed from initial diagnosis to 1 August 2023. The primary end point of our study was the difference in real-world PFS and OS between recipients of bisphosphonates and denosumab. Details of the definition and calculation of these outcomes are listed in the supplement.
Propensity score–weighted Cox proportional hazards models were used to calculate adjusted HR and 95% CI for these outcomes. Baseline demographic and clinical characteristics were descriptively assessed in the overall cohort and by stage for each staging system. Continuous variables were reported as median and Q1/Q3 values. Categorical variables were presented as absolute and relative frequencies. The details of the propensity matching we used to adjust differences are described in the supplement.
Among 1515 denosumab users and 1725 bisphosphonate users included in this analysis, 52% of patients were male and the median age was 69 years (interquartile range, 61-76). Characteristics of patients are highlighted in Table 1. Among those treated with bisphosphonates, 1672 patients (97%) received zoledronic acid, 36 (2%) received pamidronate, and 17 (1%) received both.
Compared with bisphosphonate users before weighting, denosumab users were older (mean [standard deviation], 69.4 [10.2] vs 67.4 [10.7] years; P < .001) and more likely to be treated in community oncology practices (82% vs 73%; P < .001). A higher proportion of denosumab users were White (57% vs 55%) and Black patients (18% vs 15%), and a lower proportion of them were Latinx (6% vs 9%) (P = .009). A higher proportion of patients receiving denosumab had an estimated glomerular filtration rate of <60 (26% vs 37%; P < .001).
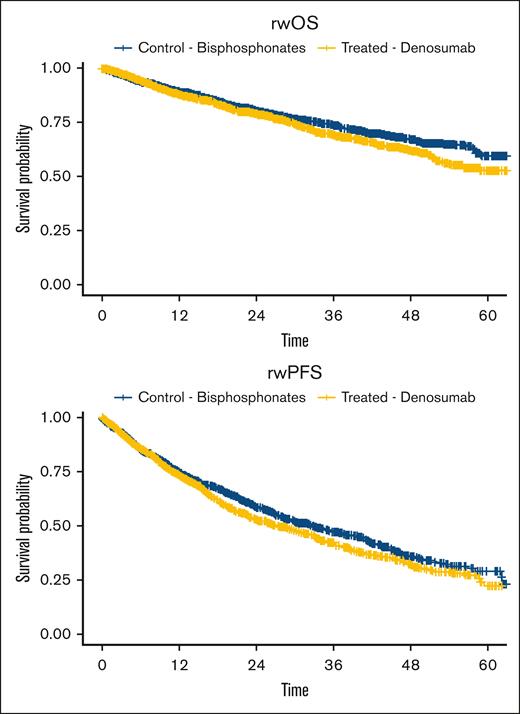
In unweighted models, denosumab was associated with worse PFS (HR, 1.13; 95% CI, 1.01-1.28; P = .04) and OS (HR, 1.18; 95% CI, 1.02-1.36; P = .03) than bisphosphonates. In propensity score–weighted models, denosumab was not associated with a statistically significant difference in PFS (HR, 1.02; 95% CI, 0.91-1.15; P = .7) and OS (HR, 1.00; 95% CI, 0.87-1.16; P > .9) compared with bisphosphonates (Figure 1)
rwOS and rwPFS between patients treated with bisphosphonates vs those treated with denosumab, with time expressed from month since starting treatment. rw, real-world.
rwOS and rwPFS between patients treated with bisphosphonates vs those treated with denosumab, with time expressed from month since starting treatment. rw, real-world.
Sensitivity analyses performed to assess the robustness of these findings to adjustment for international staging system, eastern cooperative oncology group score, and estimated glomerular filtration rate; previous treatment with triplet therapy; the use of a time-varying covariate for autologous stem cell transplant; and censoring at denosumab exposure among patients initially treated with bisphosphonates showed similar effects (approximately 11.7% of patients used denosumab during follow-up) (supplemental Table 5 onward). Results of sensitivity analysis were consistent with the finding of a lack of difference in outcomes observed in primary analysis.
In this large retrospective cohort study of patients with myeloma receiving either denosumab or bisphosphonates, we found no difference in PFS and OS, in contrast to an exploratory post hoc analysis of a previous trial that had suggested a PFS benefit of 10.7 months among those using denosumab.7 However, our study did not evaluate skeletal events as an outcome, and some residual confounding may persist.
We found differences in characteristics between recipients of bisphosphonates and denosumab. In particular, we found that patients receiving denosumab were more likely to be treated at community centers and were 2 years older (69 vs 67 years) and have renal insufficiency. Although we adjusted for these variables and found no differences in outcomes in a multivariate model, it may be that unmeasured confounders may still exist and remain unadjusted for a common issue in retrospective observational research.10 We also were unable to analyze real-world dosing schemas of these drugs, which may affect costs, given that both denosumab and zoledronic acid may be dosed at various frequencies (monthly, every 3 months or 6 months, etc). Denosumab was more likely to be given in the community and to older patients, which could lead to unmeasured differences in factors such as receipt of intensive therapies and clinical trials, although the rates of transplant were similarly low between the 2 arms.
In this aforementioned randomized trial, even though receipt of myeloma therapies was balanced between the denosumab and the zoledronic acid arms, the PFS difference is likely spurious. This is caused by several reasons: PFS as an end point is inherently vulnerable to censoring,11 and even an inert drug can lead to a benefit in PFS as a result.12 Although we are unable to comment on what degree censoring affected results of the randomized trial comparing denosumab with zoledronic acid, it must be noted that OS is inherently less susceptible to censoring11 and that OS was not different between denosumab and zoledronic acid.7 This trial was not powered for PFS, and as a result, the differences in PFS can be caused by chance alone. The finding that denosumab affects PFS is also suspicious given that as a single agent (in smoldering myeloma) it had no disease responses, indicating that it lacks antimyeloma activity.13
In conclusion, our large cohort study demonstrates no difference in either PFS or OS between zoledronic acid and denosumab in patients with myeloma, although residual confounding may have affected our results. Given cost considerations, the use of zoledronic acid represents a valuable cost-saving opportunity for health care systems, although ideally a future randomized trial powered for PFS should be conducted to address this question.
Contribution: G.S.C. and M.S.A. conceived the idea and analyzed the data; J.S.G., B.C.-H.C. and R.R. contributed analytic tools and analyzed the data; G.R.M. wrote the first draft of the manuscript and analyzed the data; K.S., D.S., and P.R.P. critically reviewed the manuscript and assisted in its writing; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: G.R.M. reports royalties from MashupMD for writing (the institution has received funding from Janssen for site principal investigator role). G.S.C., J.S.G., M.S.A., and R.R. report employment with Flatiron at the time of writing; and equity holding in Roche, a publicly traded company, at the time of writing. D.S. reports research funding from Gilead; consultancy and research funding from Arcell, Pfizer, Bioline, Sanofi, Janssen, and GlaxoSmithKline; consultancy for Bristol Myers Squibb; and AbbVie; research funding from Amgen, Cantex, and Rochex. P.R.P. reports employment with Exelixis. The remaining authors declare no competing financial interests.
Correspondence: Ghulam Rehman Mohyuddin, Huntsman Cancer Institute, University of Utah, 1950 Circle of Hope Dr, Salt Lake City, UT 84103; email: g.mohyuddin@hci.utah.edu.
References
Author notes
The full-text version of this article contains a data supplement.