TO THE EDITOR:
Brexucabtagene autoleucel (brexu-cel) is a second-generation autologous chimeric antigen receptor (CAR) T cell incorporating the CD28 costimulatory domain and targeting the CD19 antigen. Brexu-cel was recently approved for adult patients with relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (BCP-ALL), based on the results of the ZUMA-3 phase 2 study. After a median follow-up of 39 months, brexu-cel showed a complete remission (CR)/CR with incomplete hematologic recovery rate of 71% among the 55 treated patients, with a median overall survival (OS) of 26 months.1 An updated analysis reported a median OS of 47 months after 3 years of follow-up in responders.2 Cytokine release syndrome (CRS) occurred in 89% of patients, with 24% experiencing severe CRS (grade ≥3). Neurological events immune effector cell–associated neurotoxicity syndrome (ICANS) occurred in 60% of patients, with 25% experiencing severe events.
In addition to brexu-cel, other CAR T-cell therapies have been investigated in adult BCP-ALL. Tisagenlecleucel (tisa-cel) approved for children and young adults aged ≤25 years, demonstrated an 82% overall response rate and a 3-year OS rate of 63% in the ELIANA study.3 Obecabtagene autoleucel, investigated in the FELIX study, showed a 78% CR/CR with incomplete hematologic recovery rate and a 59% 1-year OS rate, along with a favorable safety profile.4
Although numerous studies have investigated the determinants of the efficacy and safety of CAR T cells in children and adult BCP-ALL, limited data have been so far published for patients treated with brexu-cel outside the ZUMA-3 study. In this real-world study, we aimed at investigating the outcome of adult patients treated with brexu-cel in the early access program (EAP) in France.
Eligibility for inclusion in the French EAP required patients to be aged ≥18 years with CD19+ BCP-ALL in morphological relapse, meeting the following criteria: first relapse if the first remission lasted ≤12 months, R/R disease after ≥2 lines of systemic therapy, or relapse after allogeneic hematopoietic stem cell transplant (allo-HSCT). For the latter, patients must have been at least 100 days after allo-HSCT and off immunosuppressive medications for at least 4 weeks at the time of leukapheresis (LKP). The study included 80 patients who underwent LKP with the intent to manufacture brexu-cel, with efficacy and safety analyses conducted on the 67 who eventually received infusion. Data collection was cut off in June 2023. All participants were prospectively enrolled in the Dispositif d'Enregistrement et de Suivi des CAR-T (DESCAR-T) registry (ClinicalTrials.gov identifier: NCT04328298), which has been gathering real-world data on patients treated with CAR T cells in France since July 2018. Written informed consent was obtained from all patients enrolled. This study has institutional review board approval.
Between May 2019 and February 2023, a total of 80 adults with R/R BCP-ALL from 22 French centers underwent LKP for brexu-cel manufacturing. Six patients (7.5%) required a second LKP. Of these 80 patients, 13 (16.3%) did not receive infusion due to various reasons: 9 due to death, 1 because of disease progression, 2 due to manufacturing failures, and 1 for other reasons. The median time from LKP to infusion was 37 days (interquartile range [IQR], 35-49). Patient characteristics for the 67 who received infusion are detailed in Table 1. The cohort comprised 35 males and 32 females, with a median age of 44 years (IQR, 34-54); only 2 patients were aged <26 years. A Philadelphia chromosome was detected in 20 of 67 patients (30%). Patients were extensively pretreated, having received a median of 3 treatment lines (IQR, 1-8). Specific prior treatments included blinatumomab (BLIN) in 63% of patients, inotuzumab ozogamicin in 18%, and allo-HSCT in 70%. Before apheresis, 10 of 67 patients (15%) had active central nervous system (CNS) disease (CNS-2/3). After bridging therapy and before lymphodepletion (LD), the median bone marrow (BM) blast infiltration was 2% (IQR, 0%-18.5%), with 23% of patients having >25% blasts. Extramedullary disease was present in 7 patients (10.4%). Patients who did not received infusions were significantly older and had fewer previous transplants (Table 1). Their outcome was particularly dismal compared with patients who received infusion, with a median OS from LKP of 1.2 months (95% confidence interval [CI], 0.8-1.6; supplemental Figure 1). Bridging strategies (supplemental Table 1) involved immunotherapy in 8 of 67 patients (12%), with inotuzumab ozogamicin used in 63% (5/8) and BLIN in 38% (3/8).
Patient characteristics and early outcome
| . | Infused (n = 67) . | Noninfused (n = 13) . | P value . |
|---|---|---|---|
| Characteristics | |||
| Age, median (range), y | 44 (22-69) | 58 (32-70) | .014 |
| ECOG-PS, n (%) | |||
| 0 | 17/67 (30.4) | 2/13 (15.4) | .42∗ |
| 1 | 31/67 (55.4) | 8/13 (61.5) | |
| 2+ | 8/67 (14.2) | 3/13 (23.1) | |
| Baseline | |||
| CNS involvement | 7/67 (10.4) | 0/13 (0) | .59 |
| Ph+ ALL | 20/67 (29.9) | 2/13 (15.4) | .33 |
| Prior lines, n (%) | |||
| 1 | 5/67 (7.5) | 3/13 (23.1) | .55† |
| 2 | 27/67 (40.3) | 5/13 (38.5) | |
| 3+ | 35/67 (52.2) | 5/13 (38.5) | |
| Prior therapy, n (%) | |||
| Allo-HSCT | 47/67 (70.2) | 4/13 (30.8) | .011 |
| Inotuzumab | 12/67 (17.9) | 4/13 (30.8) | .28 |
| BLIN | 42/67 (62.7) | 9/13 (69.2) | .76 |
| Before CAR T cell | |||
| CNS involvement | 10/67 (14.9) | N/A | |
| BM blasts ≥25% | 14/64 (22) | N/A | |
| Safety and early outcome | |||
| CRS, n (%); grade 3+ | 54/67 (80.6); 4/54 (7.4) | N/A | |
| ICANS, n (%); grade 3+ | 32/67 (47.8); 6/32 (18.8) | N/A | |
| CR | 52/67 (77.6) | N/A | |
| MRD-negative CR | 41/52 (78.8) | N/A |
| . | Infused (n = 67) . | Noninfused (n = 13) . | P value . |
|---|---|---|---|
| Characteristics | |||
| Age, median (range), y | 44 (22-69) | 58 (32-70) | .014 |
| ECOG-PS, n (%) | |||
| 0 | 17/67 (30.4) | 2/13 (15.4) | .42∗ |
| 1 | 31/67 (55.4) | 8/13 (61.5) | |
| 2+ | 8/67 (14.2) | 3/13 (23.1) | |
| Baseline | |||
| CNS involvement | 7/67 (10.4) | 0/13 (0) | .59 |
| Ph+ ALL | 20/67 (29.9) | 2/13 (15.4) | .33 |
| Prior lines, n (%) | |||
| 1 | 5/67 (7.5) | 3/13 (23.1) | .55† |
| 2 | 27/67 (40.3) | 5/13 (38.5) | |
| 3+ | 35/67 (52.2) | 5/13 (38.5) | |
| Prior therapy, n (%) | |||
| Allo-HSCT | 47/67 (70.2) | 4/13 (30.8) | .011 |
| Inotuzumab | 12/67 (17.9) | 4/13 (30.8) | .28 |
| BLIN | 42/67 (62.7) | 9/13 (69.2) | .76 |
| Before CAR T cell | |||
| CNS involvement | 10/67 (14.9) | N/A | |
| BM blasts ≥25% | 14/64 (22) | N/A | |
| Safety and early outcome | |||
| CRS, n (%); grade 3+ | 54/67 (80.6); 4/54 (7.4) | N/A | |
| ICANS, n (%); grade 3+ | 32/67 (47.8); 6/32 (18.8) | N/A | |
| CR | 52/67 (77.6) | N/A | |
| MRD-negative CR | 41/52 (78.8) | N/A |
ECOG-PS, Eastern Cooperative Oncology Group performance status; MRD, minimal residual disease; N/A, nonapplicable; Ph, Philadelphia chromosome.
ECOG 0/1 vs 2+.
1/2 lines vs 3+.
CRS and ICANS were observed in 54 of 67 patients (81%; with 7% experiencing grade ≥3) and 32 of 67 patients (48%; with 19% experiencing grade ≥3), respectively. One patient died of ICANS grade 5. To manage CRS, 37 patients (55%) were treated with tocilizumab, of whom 2 also received siltuximab. Additionally, anakinra was administered to 6 patients (9%), including 3 who did not respond to tocilizumab, and 31 patients (46%) received steroids. Sixteen patients required intensive care unit admission, with a median stay of 5 days (range, 1-21). Furthermore, 24 severe (grade 3+) infectious complications were reported in 19 patients (28%).
A CR was achieved in 52 of 67 patients (78%), of whom 41 of 52 (79%) were minimal residual disease negative. An older age (odds ratio [OR], 1.08; 95% CI, 1.02-1.15; P = .009), a prior allo-HSCT (OR, 8.4; 95% CI, 2.34-30.1; P = .001), and a lower pre-LD BM blast infiltration (OR, 0.97; 95% CI, 0.95-0.99; P = .011) were significantly associated with a higher likelihood of achieving a CR.
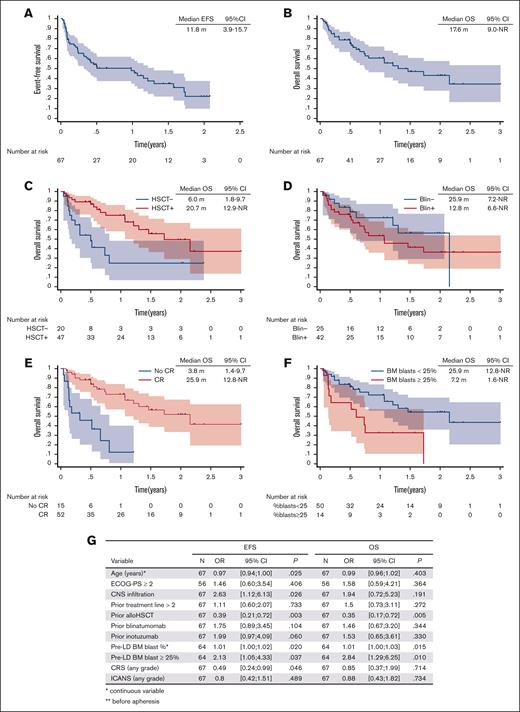
The median follow-up duration for this study was 18.0 months (95% CI, 12.4-23.9). Eight patients were successfully bridged to allo-HSCT while in continuous CR, with 4 undergoing a second allo-HSCT. Among the 52 responders, 19 (37%) experienced relapse. At relapse, a loss of CD19 expression was observed in 25% (4/16) of the evaluated patients. The median relapse-free survival, event-free survival (EFS), and OS were 13.6 months (95% CI, 5.3-19.7), 11.8 months (95% CI, 3.9-15.7), and 17.6 months (95% CI, 9.0 to not reached [NR]), respectively. For patients who achieved a CR, the median OS was 25.9 months (95% CI, 12.8 to NR).
A significantly shorter EFS was observed in younger patients, in patients with CNS infiltration before apheresis, in those without prior allo-HSCT, and in those with higher pre-LD BM blast infiltration (Figure 1). Similarly, a shorter OS was noted in patients without prior allo-HSCT and those with a higher tumor burden (Figure 1). Notably, although the median interval between allo-HSCT and brexu-cel therapy was 15.0 months, patients who received brexu-cel <1 year after allo-HSCT had a significantly shorter OS than those treated longer after their transplant (hazard ratio, 0.34; 95% CI, 0.12-0.91; P = .031). Prior HSCT was associated with a reduced risk of CAR T-cell failure, with no impact on treatment-related mortality (supplemental Figure 2).
Outcome after brexu-cel infusion. Kaplan-Meier curve of survival probability and 95% confidence interval for EFS (A) and OS (B) in the infused cohort. OS according to prior HSCT (C), prior BLIN (D), response to brexu-cel (E), and pre-LD tumor burden (E). (G) Univariate analysis for EFS and OS. ECOG-PS, Eastern Cooperative Oncology Group performance status; NR, not reached.
Outcome after brexu-cel infusion. Kaplan-Meier curve of survival probability and 95% confidence interval for EFS (A) and OS (B) in the infused cohort. OS according to prior HSCT (C), prior BLIN (D), response to brexu-cel (E), and pre-LD tumor burden (E). (G) Univariate analysis for EFS and OS. ECOG-PS, Eastern Cooperative Oncology Group performance status; NR, not reached.
Our analysis of the DESCAR-T registry confirms the efficacy and safety of brexu-cel for adult patients with R/R BCP-ALL. Similar to tisa-cel,5,6 real-world data indicate an improvement in safety compared with initial pivotal studies, likely due to a clinical learning curve and variations in patient profiles not seen in early trials. In our cohort, tumor burden before LD emerged as a critical prognostic factor. This factor had been identified in real-world studies of other CD19-targeting CAR T-cell therapies and in the ZUMA-3 study.5,7 Whether more intensive bridging therapy improves patient outcomes by better controlling tumor mass remains an open question. Unlike other studies, we did not observe an impact from prior exposure to BLIN in our cohort, which could be due to the limited size of our cohort or a selection bias based on CD19 expression that we did not track.2,5,7 Interestingly, we observed a beneficial effect of a prior allo-HSCT, especially if performed more than a year ago, mirroring prior observation that the interval between transplant and tisa-cel therapy correlates with EFS and OS.8 Unlike the ZUMA-3 study, which excluded patients with neurological involvement, our study included such patients from the French EAP. Intriguingly, our data suggest that these patients may have a limited benefit from brexu-cel, a finding not reported with tisa-cel that requires confirmation through larger cohorts.9
In conclusion, this multicenter, real-world study highlights the utility and acceptable safety profile of brexu-cel, particularly benefiting patients with low tumor burden or those with prior allo-HSCT, especially if performed >1 year ago. Further studies involving a broader patient base and longer follow-up periods are crucial to fully delineate clinical outcomes and optimize therapeutic strategies for all patient subsets.
Contribution: F.R. and N.B. designed the research, analyzed the data, and wrote the manuscript; F.R., D.B., T.M., S.F., A.H., E.B., S.M., L.G., P.C., M.L., S.N., M.B., I.L., A.F., V.C., C.S., N.M., C.C.-L., M.J., A.B., A.T.B., E.G., G.R.-G., T.L., and N.B. managed the patients and provided clinical data; V.L. is the Group for Research on Adult Acute Lymphoblastic Leukemia network coordinator; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: N.B. received honoraria and research funding from Novartis. F.R. received honoraria from Kite/Gilead. E.B. received honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Nicolas Boissel, Hematology Adolescents and Young Adults, Saint-Louis Hospital, 1 Ave Claude Vellefaux, 75010 Paris, France; email: nicolas.boissel@aphp.fr.
References
Author notes
Data are available on request from the corresponding author, Nicolas Boissel (nicolas.boissel@aphp.fr).
The full-text version of this article contains a data supplement.