Key Points
The study shows the safety and long-term efficacy of auto-HCT, even in patients with high-risk cHL who are traditionally considered chemotherapy refractory.
The study shows the long-term efficacy of auto-HCT, even in patients with high-risk cHL who are traditionally considered chemo refractory.
Visual Abstract
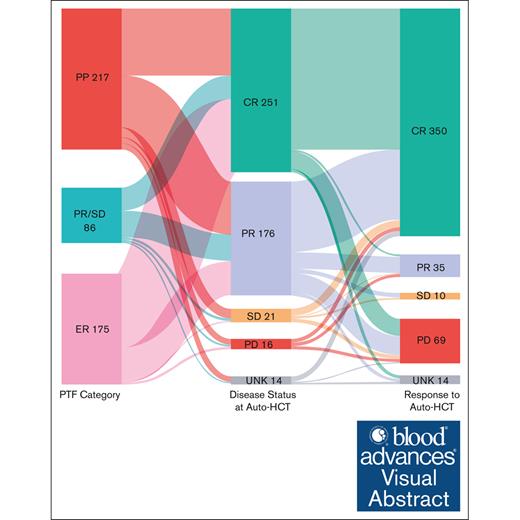
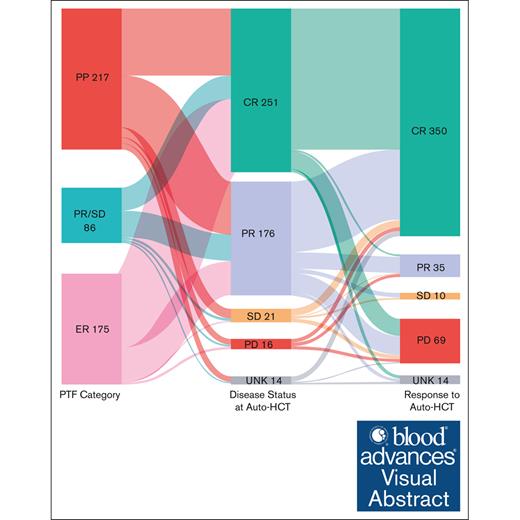
There are limited data assessing the risk scores for primary treatment failure (PTF) in patients with classical Hodgkin lymphoma (cHL; PTF-cHL) undergoing autologous hematopoietic cell transplantation (auto-HCT). ECLIPSE (Evaluation of Classical Hodgkin Lymphoma patients wIth Primary treatment failure and analySis of outcomEs) is a multicenter retrospective cohort of patients with PTF-cHL (aged ≥15 years) diagnosed on or after 1 January 2005, at 15 US medical centers. PTF was defined as 1 of the following patterns of failure: (1) progressive disease by imaging during or within 6 weeks of completion of frontline chemotherapy (primary progression [PP]); (2) partial response (PR) or stable disease (SD) by imaging after completion of frontline treatment (PR/SD); (3) progression of disease by imaging (and confirmed by biopsy) within 12 months of frontline therapy completion after prior documentation of complete response (CR; early relapse [ER]). A total of 478 patients were included in the analysis. Among these, 217 (45%) were PP, 86 (18%) were PR/SD, and 175 (37%) were ER. The 6-month and 1-year cumulative incidence of nonrelapse mortality after auto-HCT were 0.9% and 1.1%, respectively. The median progression-free survival (PFS) and overall survival (OS) after auto-HCT were 4.33 and 10.09 years, respectively. Although those not in CR at the time of auto-HCT were associated with inferior PFS and OS, advanced age and diagnosis before 2011 were associated with inferior OS. This study showcases the safety and long-term efficacy of auto-HCT, even in patients with high-risk disease who are traditionally considered chemotherapy refractory, and will serve as a benchmark for the ongoing transplant vs no transplant trials.
Introduction
Classical Hodgkin lymphoma (cHL) is curable in most patients, however, 1% to 5% of patients with early stage disease (and favorable features) and 12% to 25% of patients with advanced disease eventually relapse depending on the type of regimen administered in the first-line setting.1-5 The standard management approach for patients with relapsed or refractory cHL is high-dose chemotherapy and autologous hematopoietic cell transplantation (auto-HCT) in those with chemotherapy-sensitive disease.6-8
Prognostic scores have been built and are widely used in newly diagnosed cHL.9 Similarly, risk factors have been identified that predict poor outcomes after auto-HCT.10-12 However, there is a paucity of data regarding risk scores or prognostic models for patients with primary refractory or high-risk cHL undergoing auto-HCT. In 1 study that evaluated the outcomes of patients with primary refractory cHL, stage IV disease and persistent functional imaging abnormality before auto-HCT were associated with worse outcomes.13 However, the study included patients with cHL diagnosed from 1994 to 2015 and did not account for the therapeutic advances (brentuximab vedotin [BV] and checkpoint inhibitors [CPI]) over the past decade. Furthermore, in the study, only 72% of patients had fluorodeoxyglucose positron emission tomography (FDG-PET) for response assessment. Hence, we sought to evaluate the prognostic factors for patients with high-risk cHL undergoing auto-HCT in the contemporary era.
Patients and methods
Study design
ECLIPSE (Evaluation of Classical Hodgkin Lymphoma patients wIth Primary treatment failure [PTF] and analySis of outcomEs) is a multicenter retrospective cohort study of patients who were aged ≥15 years and diagnosed with cHL between 1 January 2005 and 1 December 2017, at 15 US medical centers. The study was approved by the institutional review boards at all participating sites and was conducted in compliance with the Declaration of Helsinki.
To be eligible for the study, the patients must have received anthracycline-containing chemotherapy regimen in the first-line setting with curative intent and developed PTF. Patients who did not receive auto-HCT were not included in the analysis. Although central pathology review was not performed, the pathology was reviewed by expert hematopathologists at the participating academic centers.
PTF was defined as 1 of the following 3 patterns of failure: progressive disease (PD; by imaging) during or within 6 weeks of completion of frontline chemotherapy (primary progression [PP] cohort); partial response (PR) or stable disease (SD) by imaging at the completion of treatment (PR/SD cohort); or progression of disease by imaging (and confirmed by biopsy) within 12 months of completion of chemotherapy after prior documentation of complete response (CR; early relapse [ER] cohort). Of note, confirmatory biopsies were done in 60% of PP and 70% of PR/SD cohorts, especially in ambiguous cases.
Patients were divided into 2 “eras” based on the year of diagnosis: 2005 to 2010 (era 1) and 2011 to 2017 (era 2). The year 2011 was chosen as the watershed year to coincide with the US Food and Drug Administration approval of BV in the United States because our intent was to see whether there was any improvement in outcomes post-BV approval in the United States. Response before auto-HCT was adjudicated based on Lugano criteria.14
Study end points and definitions
The primary end point was to evaluate the progression-free survival (PFS) in patients with cHL and PTF who underwent auto-HCT. PFS was defined as the time from auto-HCT until lymphoma relapse or progression or death from any cause. Surviving patients without relapse or progression were censored at the last clinical assessment. Secondary end points included the evaluation of nonrelapse mortality (NRM) and overall survival (OS). NRM was defined as death without evidence of lymphoma progression/relapse; relapse or death was considered a competing risk. OS was defined as the time from auto-HCT until death from any cause. Surviving patients were censored at the last follow-up.
Statistical analysis
Demographic and disease characteristics were summarized using medians and ranges for continuous variables and frequencies and percentages for categorical variables. Time-to-event end points including PFS and OS were estimated using the Kaplan-Meier method. NRM was calculated using the cumulative incidence function, treating relapse or death due to disease as competing events. The association between covariates and PFS or OS was assessed through Cox proportional hazards models, and the Fine-Gray model was fit for NRM. Analyses were performed using SAS/STAT software version 15.2, and all statistical tests were 2-sided, with a type-1 error of <.05 indicating statistical significance. All estimates were reported with 95% confidence intervals (CIs).
Results
Baseline characteristics
Among the 559 patients with PTF, 478 underwent auto-HCT and were included in the analysis. Among these, 217 patients (45%) were PP, 86 (18%) were PR/SD, and 175 (37%) were ER. FDG-PET was used for response assessment in 94% of patients, whereas computed tomography was used in the remaining 6%. Table 1 shows the baseline characteristics stratified based on the PTF category. The median age was 31 years (range, 15-69 years), with 54% males, 91% with Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, and 61% with advanced stage cHL (61%). The most common cHL subtype was nodular sclerosis (89%), followed by mixed cellularity (9%). The most common first-line treatment was ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) or ABVD/AVD (n = 451 [94%]). None of the patients received BV or CPI in the first-line setting, and only 19% (n = 93) received BV-based salvage therapy at any time point before auto-HCT. Ninety-eight patients (21%) received treatment on a clinical trial, whereas the rest received standard of care/cytotoxic chemotherapy as salvage therapy in the pre–auto-HCT setting. Most of the patients were in CR or PR (92%) at auto-HCT (54% achieving CR and 38% achieving PR). Thirty-nine patients (8%) received maintenance BV after auto-HCT. The median follow-up from the time of auto-HCT among survivors was 34.5 months (range, 0.2-158.5).
Posttransplant salvage therapies
After auto-HCT, 166 patients received first post–auto-HCT salvage therapy; 68 in era 1 and 98 in era 2. In the entire cohort, BV was the most common salvage therapy (n = 56 [34%] including those who received it on clinical trials [which was the case in era 1]), followed by cytotoxic chemotherapy (n = 48 [29%]), clinical trials (n = 27 [16%]), radiation therapy (n = 22 [13%]), and lenalidomide-based therapies (n = 13 [8%]). When evaluating the first post–auto-HCT salvage therapy by era, 34% (n = 23) received BV (on a clinical trial) in era 1, whereas 60% of patients in era 2 received either BV (n = 33 [34%]) or were enrolled on a clinical trial (n = 26 [26%]), which predominantly included BV-based and CPI with or without combination therapies.
NRM
The 6-month and 1-year cumulative incidence of NRM after auto-HCT were 0.9% (95% CI, 0.3-2.1) and 1.1% (95% CI, 0.4-2.5), respectively (supplemental Figure 1A). In the univariable Fine-Gray models, among the variables evaluated for NRM (supplemental Table 1), only the era of diagnosis (<2011) was borderline associated with increased risk for NRM (hazard ratio [HR], 4.30; 95% CI, 0.99-18.77; P = .05; supplemental Figure 1B).
PFS
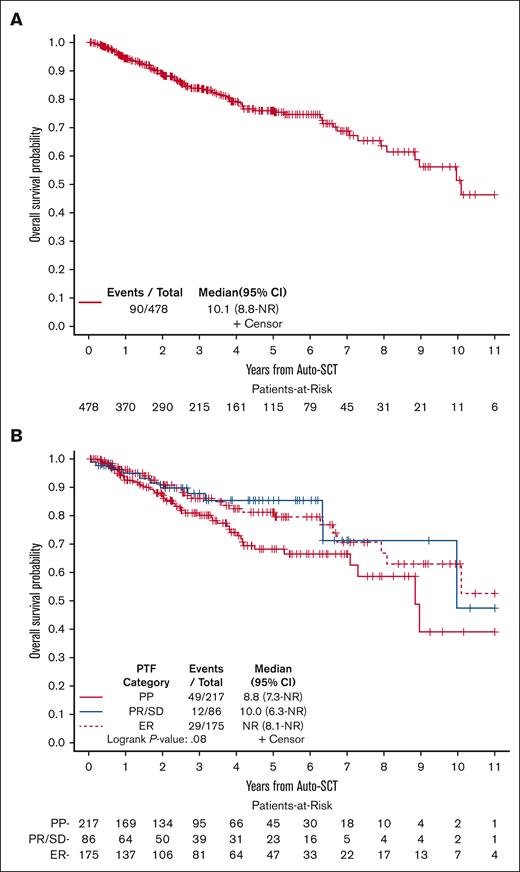
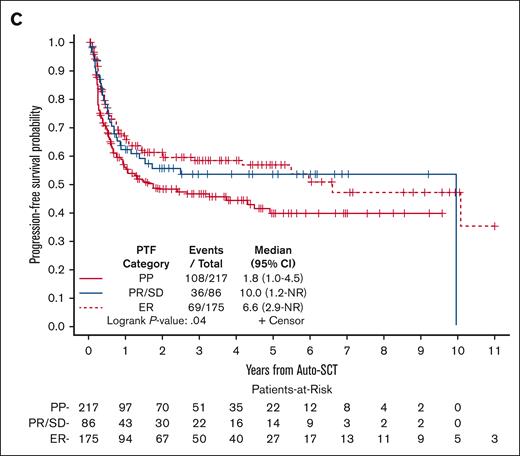
The median PFS after auto-HCT was 4.33 years (95% CI, 1.77-10.09). The 2- and 5-year PFS rates were 54% (95% CI, 49-59) and 49% (95% CI, 43-54), respectively (Figure 1A). The 2- and 5-year PFS rates for patients who were in CR at the time of auto-HCT were 64% (95% CI, 57-70) and 59% (95% CI, 51-65), respectively, and for those in PR were 48% (95% CI, 40-55) and 43% (95% CI, 34-51), respectively (Figure 1B). Based on the category of PTF, the 2- and 5-year PFS rates were 48% and 40% for PP, 56% and 54% for PR/SD, and 60% and 57% for ER, respectively (Figure 1C).
In the univariable Cox models, among the variables evaluated for PFS (supplemental Table 2), disease status at auto-HCT and PTF category were associated with PFS. Compared with those in CR at auto-HCT, patients who were in PR (HR, 1.59; 95% CI, 1.18-2.13; P = .002), SD (HR, 2.36; 95% CI, 1.35-4.16; P = .003), or PD (HR, 2.90; 95% CI, 1.54-5.48; P = .001) had significantly inferior PFS. Patients with ER had better PFS (HR, 0.68; 95% CI, 0.50-0.93; P = .01) than PP patients. However, in the multivariable model, the only variable that remained statistically significant was disease status at auto-HCT (supplemental Table 2).
OS
The median OS after auto-HCT was 10.09 years (95% CI, 8.84 to not reached). The 2- and 5-year OS after auto-HCT were 89% (95% CI, 85-92) and 76% (95% CI, 71-81), respectively, (Figure 2A). Based on the category of PTF, the 2- and 5-year OS were 87% and 68% for PP, 90% and 85% for PR/SD, and 91% and 81% for ER, respectively (Figure 2B).
OS for patients with PTF undergoing auto-HCT. (A) All patients. (B) Based on the category of PTF. NR, not reached.
OS for patients with PTF undergoing auto-HCT. (A) All patients. (B) Based on the category of PTF. NR, not reached.
Among the variables evaluated for OS in the univariable Cox models (supplemental Table 3), age (in 5-year increments), less than CR (PR, SD, or PD) at auto-HCT, presence of B symptoms, and those diagnosed in era 1 were associated with significantly inferior OS. After adjusting for these variables in the multivariable analysis, variables that remained significantly associated with inferior OS included age (for every 5-year increase, HR, 1.16; 95% CI, 1.07-1.25; P < .001), those diagnosed in era 1 (compared with era 2, HR, 1.96; 95% CI, 1.21-3.19; P = .007), and disease status at auto-HCT (<CR), wherein patients who were in PR (HR, 1.70; 95% CI, 1.06-2.71; P = .03), SD (HR, 2.23; 95% CI, 0.92-5.37; P = .07), or PD (HR, 5.92; 95% CI, 2.40-15.59; P < .001) had inferior OS compared with those in CR (supplemental Table 3).
Cause of death
Among the 90 patients who were deceased, information on the cause of death was available in 83 patients. Disease progression was the most common cause of death (n = 57), whereas infection was the most common non–disease-related cause of death (n = 11), followed by organ failure (n = 4) and others (n = 11). When we looked across the eras, 40 patients died of disease progression and 17 patients due to nondisease–related causes in era 1, whereas 17 patients died due to disease progression and 9 due to nondisease–related causes in the era 2.
Discussion
In this study evaluating the outcomes of patients with cHL and PTF who underwent auto-HCT, we found that the disease status at auto-HCT (PR, SD, or PD) was independently associated with significantly inferior PFS and OS. Other factors that were associated with significantly inferior OS in the multivariable analysis included age (in 5-year increments) and era of diagnosis (diagnosed in era 1).
In the study by Brockelmann et al,15 5 significant risk factors were identified in the multivariable analysis, including stage IV disease, time to relapse ≤3 months, ECOG performance status ≥1, bulk ≥5 cm, and less than PR (on computed tomography) to salvage chemotherapy, that were prognostic of survival in patients with relapsed or refractory cHL undergoing auto-HCT. However, the study did not use FDG-PET–based disease assessment. Several studies subsequently showed the prognostic relevance of FDG-PET–based CR before auto-HCT16-25 including in primary refractory patients. However, none of these studies accounted for the other high-risk cHL categories such as PR/SD or ER cohorts or era of diagnosis. We found that the disease status at auto-HCT (less than CR) was associated with inferior survival (both PFS and OS) in our study after adjusting for other high-risk factors, which is in line with the prior studies.
Patients with cHL diagnosed in era 1 had significantly inferior OS compared with those in era 2, which is likely due to the availability and increased use of newer salvage therapies after 2011. For instance, 34% received BV (on a clinical trial) compared with 60% who received novel therapies (BV, BV-based, or CPI with or without combination therapies) either as a standard of care or in the trial setting in era 2. In our study, we found that advanced age was associated with increased risk of mortality, which is likely related to nondisease–related reasons as evidenced by the observation that 48% of patients aged >45 years died due to nondisease–related etiology compared with 26% in those who were aged ≤45 years.
In our study, we found that patients undergoing auto-HCT had a low rate of NRM (1.1% at 1 year) and demonstrated long-term efficacy as evidenced by the median PFS and OS of 4.33 years and 10.09 years, respectively. This is important to note especially considering that these are all high-risk patients with cHL. We believe that these results could serve as a benchmark for ongoing randomized controlled trials evaluating the need and role of auto-HCT consolidation in relapsed or refractory cHL (ClinicalTrials.gov identifier: NCT03618550). Furthermore, in countries that do not have access to CPI-based salvage regimens, the results of this study support the use of auto-HCT in this high-risk disease group.
With the incorporation of BV and CPI into earlier lines of therapy,26,27 the outcomes of patients with cHL have significantly improved, including those achieving CR before auto-HCT. However, despite the advances, a subset of patients progress on both BV and CPI-based therapies (double refractory)28 and portend poor outcomes. Auto-HCT remains an option for relapsed or refractory cHL including double refractory patients (if they are able to achieve chemosensitivity), although allogeneic HCT might be more appropriate in the double refractory group.29
The study is subjected to the inherent limitations of a retrospective study design, including the nonuniform selection of treatment regimens in the first-line and relapse settings. The study only included patients who underwent auto-HCT (infused cells rather than an intent to transplant). All patients in the study received cytotoxic-based chemotherapy regimens in the first line with no BV or CPI-based regimens. Additionally, only 19% of patients received BV-based salvage therapy, and 1% received CPI in the salvage setting at any point before auto-HCT, which is a major limitation of the study because these agents are currently used in the salvage setting before auto-HCT in the United States. Nevertheless, for patients with high-risk disease as defined in this study, in most parts of the world (outside the United States), the standard of care is still auto-HCT. Incorporation of BV or CPI into salvage regimens is expected to improve disease status before auto-HCT; however, patients with progression/failure after frontline regimens that contain BV or CPI may be worse off than patients in our series.
In conclusion, in this large multicenter cohort study, we report the outcomes of patients with cHL and PTF undergoing auto-HCT and show the factors prognostic of survival for this high-risk disease group. This study will serve as a benchmark for the ongoing transplant vs no transplant trials and showcases the safety and long-term efficacy of auto-HCT, even in patients with high-risk disease who are traditionally considered chemotherapy refractory.
Authorship
Contribution: N.E., L.J.C., and A.C.X. contributed to conception and design; N.E. and Y.H. performed data analysis; N.E. contributed to manuscript writing, including first draft preparation; and all authors contributed to interpretation and collection and assembly of data, provided critical and insightful comments, and provided the final approval of the manuscript.
Conflict-of-interest disclosure: N.E. reports research funding from BeiGene and Eli Lilly; speakers' bureau fees from BeiGene and Genentech; advisory board fees from ADC Therapeutics, Ipsen, and Lilly; and consultancy fees from Novartis. M.H. reports research support/funding from Takeda Pharmaceutical Company, ADC Therapeutics, Spectrum Pharmaceuticals, and Astellas Pharma; consultancy fees from ADC Therapeutics, Omeros, CRISPR Therapeutics, Bristol Myers Squibb (BMS), Kite, AbbVie, Caribou, Genmab, and CRISPR; speakers' bureau fees from ADC Therapeutics, AstraZeneca, BeiGene, and Kite; and data monitoring committee fees from Genentech, Myeloid Therapeutics, and CRISPR Therapeutics. F.J.H.-I. reports advisory board fees from Incyte, Epizyme/Ipsen, BMS, BioGene, Amgen, AbbVie, Kite, Gilead, Collecter, Dava Oncology, Novartis, and ADC Therapeutics. S.K.B. reports advisory board/consulting fees from Affimed, Acrotech, Daiichi Sankyo, Kyowa Kirin, and Seagen, and data and safety monitoring committee fees from Janssen. R.K. reports advisory board fees from BMS, Gilead Sciences/Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, Epizyme, Genentech/Roche, EUSA Pharma, and Calithera; grants/research support from BMS, Takeda, BeiGene, Gilead Sciences/Kite, and Calithera; and speakers' bureau fees from AstraZeneca, BeiGene, and MorphoSys. J.B.C. reports consultant/adviser fees from AstraZeneca, AbbVie, BeiGene, Janssen, Loxo/Lilly, Kite/Gilead, and ADC Therapeutics, and research funding from Leukemia and Lymphoma Society, Genentech, AstraZeneca, Novartis, Loxo/Lilly, and BMS/Celgene. J.S. reports research funding from Adaptive Biotechnologies, AstraZeneca, BMS, Incyte, Kite, Merck, Pharmacyclics, Seagen, and TG Therapeutics, and consulting fees from Adaptive Biotechnologies, AstraZeneca, Atara Biotherapeutics, BMS, CRISPR Therapeutics, Pharmacyclics, and Seagen. The remaining authors declare no competing financial interests.
Correspondence: Narendranath Epperla, Division of Hematology and Hematologic Malignancies, Department of Medicine, Huntsman Cancer Institute, The University of Utah, 2000 Circle of Hope Dr, Salt Lake City, UT 84112; email: naren.epperla@hci.utah.edu.
References
Author notes
Data are available on request from the corresponding author, Narendranath Epperla (naren.epperla@hci.utah.edu).
The full-text version of this article contains a data supplement.