Key Points
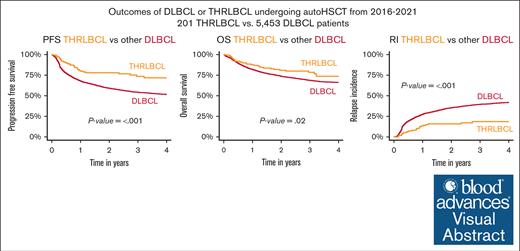
Analysis of 201 patients with R/R THRLBCL vs 5453 with DLBCL undergoing auto-HCT reveals superior PFS, OS, and relapse incidence for THRLBCL.
Auto-HCT for R/R THRLBCL resulted in a 2-year PFS of 78% and 59% in DLBCL, making it attractive for consolidating chemosensitive disease.
Visual Abstract
Although broadly used, consolidative autologous hematopoietic stem cell transplantation (auto-HCT) for relapsed/refractory (R/R) T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) has never been specifically investigated. Here, we have analyzed outcomes of auto-HCT for THRLBCL compared with diffuse large cell B-cell lymphoma not otherwise specified (DLBCL). Eligible for this retrospective registry study were adult patients with R/R THRLBCL and DLBCL, respectively, who underwent a first auto-HCT in a salvage-sensitive disease status as assessed by positron emission tomography–computed tomography between 2016 and 2021 and were registered with the European Society for Blood and Marrow Transplantation database. The primary end point was progression-free survival (PFS) 2 years after transplantation. A total of 201 patients with THRLBCL and 5543 with DLBCL were included. There were no significant differences in terms of disease status at HCT, pretreatment lines, and interval from diagnosis to transplant between the cohorts, but patients with THRLBCL were significantly younger, contained a higher proportion of men, and had a better performance status. Compared with DLBCL, THRLBCL was associated with significantly better 2-year PFS (78% vs 59%; P < .001) and overall survival (OS, 81% vs 74%; P = .02) because of a significantly lower 2-year relapse incidence (16% vs 35%; P < .001). On multivariate analysis, favorable relapse risk (hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.31-0.7) and PFS (HR, 0.58; 95% CI, 0.41-0.82) of patients with THRLBCL remained significant, whereas OS benefits (HR, 0.78; 95% CI, 0.54-1.12) did not. These results were validated in a propensity score–matched analysis. These data prove auto-HCT as an effective treatment option for salvage-sensitive R/R THRLBCL.
Introduction
T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is a rare aggressive lymphoma subtype comprising ∼1% to 5% of all LBCLs.1 Compared with diffuse large B-cell lymphoma not otherwise specified (DLBCL), it affects predominantly younger, male patients, and often presents with an unfavorable International Prognostic Index (IPI) score.2,3 It is characterized by a specific micromilieu with abundant T-cell and histiocyte infiltration and immune-modulating properties, thereby sharing similarities with Hodgkin lymphoma. Indeed, 2% to 30% of patients with THRLBCL actually have a history of nodular lymphocyte–predominant Hodgkin Lymphoma (NLPHL).1,2,4 Second-line treatment of THRLBCL used to follow the same algorithms as for DLBCL. This includes platinum-based salvage regimens with intent to consolidative autologous hematopoietic stem cell transplantation (auto-HCT) for patients refractory to or relapsing after first-line therapy. Although this approach has been considered as standard of care for decades, there is a paucity of data about the efficacy of auto-HCT in relapsed/refractory (R/R) THRLBCL, with only few and collectively uninformative case series published to date.2,4,5
Therefore, we aimed to investigate the outcomes of auto-HCT in patients with R/R THRLBCL compared with DLBCL in a real-world setting.
Patients and methods
Data source
The European Society for Blood and Marrow Transplantation (EBMT) is a voluntary organization comprising >600 institutions mainly from Europe. Accreditation as a member center requires submission of minimal essential data-A form from all consecutive patients to a central registry in which patients may be identified by the diagnosis of underlying disease and type of cellular therapy. Minimal essential data-A data are updated annually. Informed consent for transplantation and data collection was obtained locally according to regulations applicable at the time of auto-HCT. Since 1 January 2003, all member centers have been required to obtain written informed consent before data registration with the EBMT following the current version of the Helsinki Declaration. Since 2020, all centers submitting data to the EBMT need to sign a contract with the EBMT (“Joint Controllership Agreement”), defining data protection rules and duties of EBMT and centers in the data transmission process.
Patient eligibility
Eligibility criteria for this study included the following: diagnosis of R/R THRLBCL and DLBCL, respectively, auto-HCT between 2016 and 2021, first auto-HCT procedure, age of ≥18 years at auto-HCT, and chemosensitive disease status as assessed by positron emission tomography–computed tomography. All patients identified in the EBMT database fulfilling these criteria and having a minimum baseline data set available (age, sex, diagnosis, date of diagnosis, date of auto-HCT, and number of pretreatment lines) represented the study cohort. Classification of DLBCL or THRLBCL was used as reported to the registry. Data cutoff was 12 May 2023.
Definitions
Status at transplantation was defined according to the EBMT definitions: complete response (CR) was defined as the disappearance of tumor masses and disease-related symptoms; partial response was considered when measurable lesions decreased by at least 50%; and relapse was defined as the occurrence of new sites of disease after a CR lasting for 3 months or longer whereas it was considered progression when CR had not been achieved. Monitoring of patients for relapse/progression after transplant was conducted according to individual centers protocols but had to include assessment by positron emission tomography–computed tomography. Relapse or progression was considered to be “chemotherapy sensitive” if at least partial response was achieved after the last course of chemotherapy, otherwise it was considered as “chemotherapy resistant.” Good performance status (PS) was defined as Karnofsky Index score of >80.
Outcome measures
Primary end point was progression-free survival (PFS) 2 years after transplantation, because this was the median follow-up of the cohort as assessed by reverse Kaplan-Meier estimate. Other end points analyzed were nonrelapse mortality (NRM), relapse incidence (RI), and overall survival (OS). OS was defined as the time from transplant to death from any cause. PFS was defined as the time from transplantation until disease relapse/progression or death from any cause, whichever came first. NRM included all causes of death with no prior disease progression/relapse occurring at any time after transplant. RI describes the time from transplantation until disease relapse/progression.
Statistical methods
Patient-, disease-, and transplant-related variables were compared between cohorts using the χ2 test or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. The probabilities of OS and PFS were calculated using the Kaplan-Meier estimate. Cumulative incidences of RI and NRM were calculated accommodating for competing risks.
Patients with THRLBCL and DLBCL, respectively, were matched at a ratio of 1:2. A propensity score with a caliper fixed at 0.2 was used. The variables age, sex, Karnofsky Index, disease status at auto-HCT, HCT comorbidity index, and time interval between diagnosis and transplant were used for matching.
Univariate analyses were made on the matched data using log-rank comparisons for OS and PFS. The Gray test was used for cumulative incidence functions.
Associations among patient, disease, and transplantation-related variables and outcomes of interest were evaluated in a multivariable regression using a Cox proportional hazards model. Covariates with a P < .05 were considered significant. Interactions between the main effect and significant covariates were examined. All tests were 2 sided. The type 1 error rate was fixed at 0.05. Statistical analyses were performed using the R 4.0.2 software package.
Results
Patient characteristics
A total of 5654 patients meeting the eligibility criteria for this study were identified in the EBMT database. Of these, 201 had the diagnosis THRLBCL and 5453 DLBCL (Table 1). Compared with DLBCL, patients with THRLBCL were younger, predominantly male, had better PS, and had less often transformed disease. In contrast, there were no significant differences regarding the time from diagnosis to transplantation, disease status at transplantation, and IPI. In both cohorts, most patients were conditioned with carmustin, etoposid, cytarabin, melphalan (BEAM) or similar regimens. Detailed patient characteristics are presented in Table 1.
Patient characteristics: complete cohort
| . | THRLBCL . | DLBCL . | P value . |
|---|---|---|---|
| n = 201 . | n = 5453 . | ||
| Age, y | |||
| Median (range) | 49.3 (19.4-73.7) | 58.5 (18.1-81.2) | <.0001 |
| Interval from diagnosis to HCT | |||
| >12 mo | 133 (66) | 3533 (65) | .7 |
| 0-12 mo | 68 (34) | 1913 (35) | |
| Missing | 0 | 7 | |
| Year of HCT | |||
| Median (range) | 2019 (2016-2021) | 2018 (2016-2021) | .34 |
| Sex | |||
| Female | 40 (20) | 2179 (40) | <.0001 |
| Male | 161 (80) | 3274 (60) | |
| Karnofsky index | |||
| <90 | 35 (19) | 1452 (29) | .004 |
| ≥90 | 149 (81) | 3619 (71) | |
| Missing | 17 | 382 | |
| HCT comorbidity index | |||
| 0 | 126 (71) | 3035 (64) | .12 |
| 1-2 | 29 (16) | 1008 (21) | |
| ≥3 | 22 (12) | 726 (15) | |
| Missing | 24 | 684 | |
| Transformed disease | |||
| No | 180 (92) | 4557 (87) | .03 |
| Yes | 15 (8) | 676 (12) | |
| Missing | 6 | 220 | |
| IPI | |||
| Low risk (0-1) | 20 (16) | 634 (19) | .3 |
| Low-intermediate risk (2) | 39 (31) | 878 (27) | |
| High-intermediate risk (3) | 44 (35) | 1004 (30) | |
| High risk (4 or 5) | 24 (19) | 796 (24) | |
| Missing | 74 | 2141 | |
| No. of lines of therapy | |||
| 2 | 157 (78) | 3977 (73) | .1 |
| ≥3 | 44 (22) | 1476 (27) | |
| Disease status at HCT | |||
| CR | 127 (63) | 3206 (59) | .21 |
| PR | 74 (37) | 2247 (41) | |
| Conditioning regimens | |||
| BEAM | 118 (59) | 2871 (53) | Not done |
| BEAM-like (TEAM, BeEAM, FEAM) | 36 (18) | 1010 (19) | |
| Other (BCNU-, CCNU-, TBI-based, other) | 47 (23) | 1572 (29) |
| . | THRLBCL . | DLBCL . | P value . |
|---|---|---|---|
| n = 201 . | n = 5453 . | ||
| Age, y | |||
| Median (range) | 49.3 (19.4-73.7) | 58.5 (18.1-81.2) | <.0001 |
| Interval from diagnosis to HCT | |||
| >12 mo | 133 (66) | 3533 (65) | .7 |
| 0-12 mo | 68 (34) | 1913 (35) | |
| Missing | 0 | 7 | |
| Year of HCT | |||
| Median (range) | 2019 (2016-2021) | 2018 (2016-2021) | .34 |
| Sex | |||
| Female | 40 (20) | 2179 (40) | <.0001 |
| Male | 161 (80) | 3274 (60) | |
| Karnofsky index | |||
| <90 | 35 (19) | 1452 (29) | .004 |
| ≥90 | 149 (81) | 3619 (71) | |
| Missing | 17 | 382 | |
| HCT comorbidity index | |||
| 0 | 126 (71) | 3035 (64) | .12 |
| 1-2 | 29 (16) | 1008 (21) | |
| ≥3 | 22 (12) | 726 (15) | |
| Missing | 24 | 684 | |
| Transformed disease | |||
| No | 180 (92) | 4557 (87) | .03 |
| Yes | 15 (8) | 676 (12) | |
| Missing | 6 | 220 | |
| IPI | |||
| Low risk (0-1) | 20 (16) | 634 (19) | .3 |
| Low-intermediate risk (2) | 39 (31) | 878 (27) | |
| High-intermediate risk (3) | 44 (35) | 1004 (30) | |
| High risk (4 or 5) | 24 (19) | 796 (24) | |
| Missing | 74 | 2141 | |
| No. of lines of therapy | |||
| 2 | 157 (78) | 3977 (73) | .1 |
| ≥3 | 44 (22) | 1476 (27) | |
| Disease status at HCT | |||
| CR | 127 (63) | 3206 (59) | .21 |
| PR | 74 (37) | 2247 (41) | |
| Conditioning regimens | |||
| BEAM | 118 (59) | 2871 (53) | Not done |
| BEAM-like (TEAM, BeEAM, FEAM) | 36 (18) | 1010 (19) | |
| Other (BCNU-, CCNU-, TBI-based, other) | 47 (23) | 1572 (29) |
B/CCNU, lomustin; BEAM, carmustin, etoposid, cytarabin, melphalan; BeEAM, bendamustin, etoposide, cytarabin, melphalan; FEAM, fotemustib, etoposide, cytarabin, melphalan; PR, partial remission; TBI, total body irradiation; TEAM, thiotepa, etoposide, cytarabin, melphalan.
Outcomes
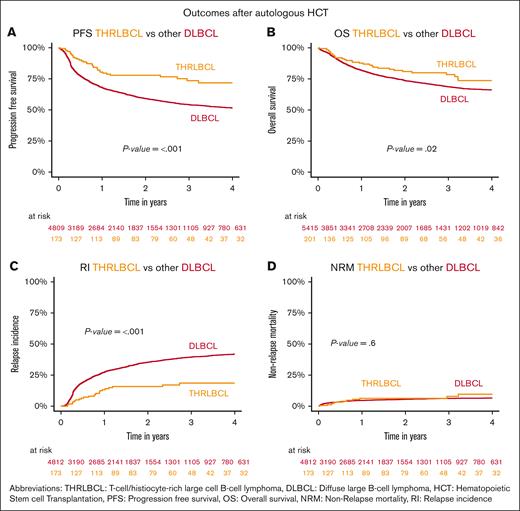
With a median follow-up of 2.1 years (THRLBCL) and 2 years (DLBCL), patients with THRLBCL showed a significantly lower 2-year RI than those with other DLBCL on univariate comparison (16%, 95% confidence interval [CI], 10-23 vs 35%, 95% CI, 34-37; P < .001). This lower RI translated into a significantly superior 2-year PFS (78%, 95% CI, 70-84 vs 59%, 95% CI, 58-61; P ≤ .001) and OS (81%, 95% CI, 73-87 vs 74%, 95% CI, 72-75; P = .02) (Figure 1A-C). A subset analysis suggested that the PFS benefit of THRLBCL over DLBCL relied predominantly on the patient group with a longer time between diagnosis and auto-HCT (supplemental Figure 1E-F). No significant NRM difference was observed (6.3% vs 5.5%; P = .6; Figure 1D). Characteristics of patients with DLBCL undergoing auto-HCT at 0 to 12 or >12 months after diagnosis are provided in supplemental Table 2.
Outcomes of whole THRLBCL and DLBCL cohorts after auto-HCT. Kaplan-Meier curves depicting (A) PFS, (B) OS, (C) RI, and (D) NRM for other patients with DLBCL and THRLBCL within the first 2 years after auto-HCT.
Outcomes of whole THRLBCL and DLBCL cohorts after auto-HCT. Kaplan-Meier curves depicting (A) PFS, (B) OS, (C) RI, and (D) NRM for other patients with DLBCL and THRLBCL within the first 2 years after auto-HCT.
Multivariate adjustment and prognostic factors
On multivariate analysis adjusting for relevant confounders (age, sex disease status, PS, time from diagnosis to auto-HCT, and lines of pretreatment) the lower relapse risk of patients with THRLBCL (hazard ratio [HR], 0.46; 95% CI, 0.31-0.7; P < .001), as well as PFS remained significant (HR, 0.58; 95% CI, 0.41-0.82; P = .002) but OS (HR, 0.78; 95% CI, 0.54-1.12; P = .18) was no longer significant (Figure 2; supplemental Table 1). Other significant factors predicting favorable outcome in all 4 categories were younger age and good PS; for PFS, OS, and RI disease status, lines of previous therapy; and delay from diagnosis to HCT for PFS. In a multivariate analysis restricted to the THRLBCL cohort, the only significant adverse factor for PFS, OS, and NRM was patient age; and female gender as well as previous treatment lines were associated with an increased RI (supplemental Figure 1A-D).
Multivariable analysis of prognostic factors after auto-HCT. HR with 95% CI from multivariable regression using a Cox proportional hazards model of 6 independent variables assessed for prediction of (A) PFS, (B) OS, (C) RI, and (D) NRM within all patients with DLBCL undergoing auto-HCT.
Multivariable analysis of prognostic factors after auto-HCT. HR with 95% CI from multivariable regression using a Cox proportional hazards model of 6 independent variables assessed for prediction of (A) PFS, (B) OS, (C) RI, and (D) NRM within all patients with DLBCL undergoing auto-HCT.
Matched cohort analysis
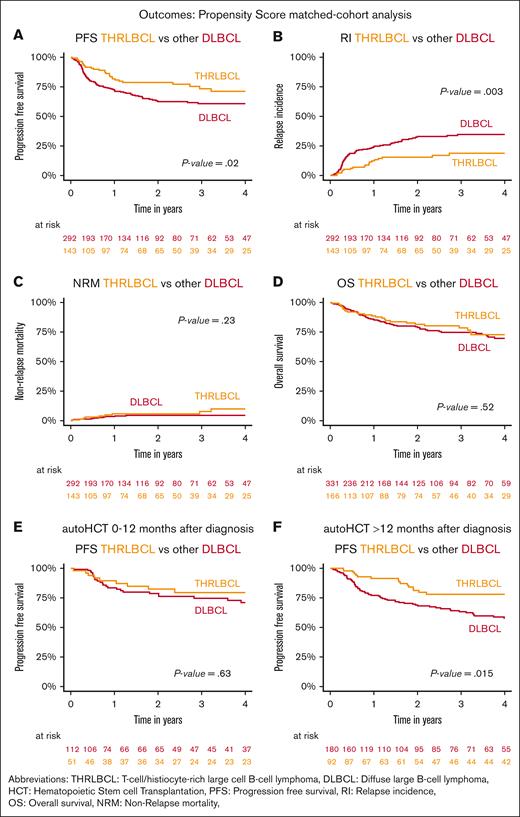
In order to further elucidate the observation of a superior disease control of THRLBCL by auto-HCT compared with DLBCL, we performed a 1:2 analysis based on propensity score–matched cohorts. Using age, sex, PS, disease status, HCT comorbidity index, and time from diagnosis to auto-HCT as matching factors, 166 patients with THRLBCL could be matched with 332 patients with DLBCL. Although also all other characteristics were not significantly different, the matched THRLBCL cohort tended to contain more patients with intermediate IPI scores, and the DLBCL cohort tended to contain more patients who received auto-HCT in CR (supplemental Table 3).
Again, the diagnosis THRLBCL was associated with a significantly reduced RI (16%, 95% CI, 10-23 vs 33%, 95% CI, 27-39; P = .003) and improved PFS (79%, 95% CI, 70-85 vs 63%, 95% CI, 58-68; P = .02) at 2 years after auto-HCT. No significant differences in terms of OS or NRM were observed (Figure 3A-D). The disease control benefit associated with THRLBCL was largely confined to those patients who had an interval of >12 months between diagnosis and auto-HCT (DLBCL 2-year PFS estimate, 71%; 95% CI, 60-80 vs 58%, 95% CI, 49-66) (Figure 3E-F).
Outcomes of propensity score–matched THRLBCL and DLBCL cohorts after auto-HCT. Kaplan-Meier curves of the 2:1 propensity score–matched cohorts of other patients with DLBCL and THRLBCL undergoing auto-HCT depicting (A) PFS, (B) RI, (C) NRM, (D) OS, and (E) PFS of patients undergoing auto-HCT within <12 months or, (F) >12 months after diagnosis.
Outcomes of propensity score–matched THRLBCL and DLBCL cohorts after auto-HCT. Kaplan-Meier curves of the 2:1 propensity score–matched cohorts of other patients with DLBCL and THRLBCL undergoing auto-HCT depicting (A) PFS, (B) RI, (C) NRM, (D) OS, and (E) PFS of patients undergoing auto-HCT within <12 months or, (F) >12 months after diagnosis.
Discussion
Although auto-HCT has been considered as standard of care for R/R THRLBCL for decades, this study is, to our knowledge, the first to analyze autologous transplantation specifically in this orphan entity. The results suggest that safety and efficacy of consolidative auto-HCT in THRLBCL is largely comparable with the benchmark indication DLBCL. Actually, the lower relapse risk and improved PFS observed in our THRLBCL cohort compared with DLBCL even after adjustment for important confounders indicates that disease control provided by auto-HCT may be even superior in this lymphoma subtype. This is an important finding which however needs to be validated in other studies.
Of note, with 2-year PFS and RI rates of 59% and 35%, respectively, our DLBCL cohort compares favorably with DLBCL auto-HCT series from earlier periods including those from the EBMT, quite uniformly reporting 2-year PFS and RI rates of ∼50% and 40%, respectively.6-9 Although this improvement might be best explained by better patient selection over time, it stresses that the superiority of the THRLBCL cohort was not because of an unusually poor outcome of our comparator cohort.
An unexpected finding was that an adverse factor for disease control and survival of the whole sample was a longer interval between diagnosis and time of auto-HCT. This appeared to be restricted to the DLBCL part of the population and contradicts earlier observations from EBMT and Center for International Blood and Marrow Transplant Research cohorts, which suggested poorer or similar outcomes of patients with DLBCL after early failure of first-line therapy compared with patients who had failed beyond 12 or 18 months after diagnosis.6,7,10 In the absence of a clear explanation for this observation, it may be speculated that a possible reason for this could be that rapid responders to first salvage partly counterbalance the higher risk associated with early treatment failure.
Although the favorable outcome of auto-HCT in patients with R/R THRLBCL is a novel finding but not a complete surprise, it gains critical practical importance from preliminary reports suggesting that CD19-directed chimeric antigen receptor (CAR) T-cell therapies, which represent the new standard salvage treatment for LBCL in general, appear to be less effective in this orphan entity.4,5,11,12 Thus, auto-HCT can be considered a well-described alternative to CAR T-cell therapy in patients with THRLBCL, at least in those with salvage-sensitive disease, as long as robust evidence for CAR T-cell efficacy in this indication is lacking. Admittedly, the main challenge in the salvage setting of LBCL is to induce a status of sensitive disease as a prerequisite for successful auto-HCT,13-17 and THRLBCL may be not an exception to this rule with auto-HCT rates of ∼50% of patients needing salvage therapy in 1 center.4 However, novel therapeutic principles, such as checkpoint inhibitors, bispecific antibodies, and antibody-drug compounds may increase response rates in the near future. The role of consolidating therapies such as auto-HCT, CAR T cells, or even allogeneic HCT in THRLBCL and other LBCL entities after such novel therapies remains poorly studied but warrants future attention.18-20
A limitation of our study is that the type of posttransplant relapse in transformed lymphomas is not captured by the standard EBMT forms used during the index period, making it impossible to distinguish between NLPHL and THRLBCL relapse in the patients with a background of transformed lymphoma. However, in a previous EBMT analysis we have shown that outcomes of NLPHL autotransplants were largely similar to those observed here for THRLBCL.21 More importantly, only 15 of 201 THRLBCL cases were reported as transformed from NLPHL, altogether suggesting that relapse events of untransformed lymphoma should not critically bias the outcome here. Other limitations of our study are those inherent to transplant registry analyses, that is, retrospective data assessment and data item restrictions, selection bias, and the fact that only patients who actually received transplantation could be considered. In contrast, the uniquely large sample size of our THRLBCL cohort allowing informative matching with DLBCL comparators represents an important strength.
In conclusion, auto-HCT in chemotherapy-sensitive R/R THRLBCL is at least as effective and as safe as in DLBCL, and may represent an attractive alternative for consolidation if other types of cellular therapy should turn out to be unsuitable in this setting.
Authorship
Contribution: S.R. and P.D. initiated the project, designed the figures, and wrote the manuscript; M.N. and H.F. performed statistical analysis and extracted data from the EBMT registry; and M.T.R., W.T., R.S., U.N., N.S., M.A., G.H., M.C., G.K., A.H., J.A.P.-S., A.B., H.G., A.S., N.S., and B.G. provided patient information and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simon Renders, Department of Internal Medicine V, Hematology, Oncology and Rheumatology, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; email: simon.renders@med.uni-heidelberg.de.
References
Author notes
Original data are available on request from the corresponding author, Simon Renders (simon.renders@med.uni-heidelberg.de).
The full-text version of this article contains a data supplement.