Key Points
Longitudinal rehabilitation programs may play an important role in the recovery of patients undergoing alloBMT and improve health outcomes.
Lessons learned can help to refine program design and inform future research with the aim of standardizing rehabilitation in this population.
Visual Abstract
Allogeneic blood and marrow transplantation (alloBMT) is a curative treatment for blood cancers associated with various treatment-related adverse events and morbidities for which rehabilitation programs are currently limited. A phase 2 randomized controlled trial (RCT) was conducted to assess the feasibility, acceptability, and impact of CaRE-4-alloBMT, a longitudinal, multidimensional cancer rehabilitation program for patients undergoing alloBMT. The primary outcomes included the feasibility and acceptability of the intervention and the methods. Feasibility was assessed through recruitment, retention, and adherence rates. Acceptability was assessed through qualitative interviews. Secondary clinical outcomes were collected through questionnaires and physiological assessments at 4 time points. A total of 80 participants were recruited and randomized. Recruitment (72%) and retention (70%) rates, along with qualitative findings, support the feasibility of the intervention. Adherence was suboptimal, most notably educational module completion (22.7%). Treatment effect sizes of 0.70 (95% confidence interval [CI], 0.20-1.21; 30-second sit-to-stand test) and 0.46 (95% CI, –0.17 to 1.09; 36-Item Short Form Survey) were observed in favor of the intervention. The results appear promising; however, the findings are limited by missing data owing to attrition. Modifications will be required to refine the program and inform a phase 3 RCT. This trial was registered at www.ClinicalTrials.gov as #NCT04966156.
Introduction
Allogeneic blood and marrow transplantation (alloBMT) is a curative treatment for blood cancers, and its use has increased rapidly over the past decade.1 Ongoing research has helped to optimize the transplant process, which led to improved efficacy, long-term survival, and improve quality of life (QoL).2 Despite this, alloBMT continues to be associated with numerous treatment-related side effects. This includes a high risk for infection, reduced physical functioning, and worsening nutritional status, which are associated with an increased risk for complications, significant treatment-related mortality, and profoundly impaired QoL.2 This suggests that there is an urgent need for longitudinal interventions that focus on prevention and address treatment-related morbidities in this high-need population.3
Cancer rehabilitation has been shown to effectively address patients’ unmet needs and symptoms following treatment4 and is now an essential component of cancer care that focuses on the prevention and management of treatment-related side effects and on optimizing functional status and QoL.5,6 However, rehabilitation programs for alloBMT in transplantation centers are limited and have not been established yet or standardized.7 Studies that investigated the impact of rehabilitation programs for alloBMT are also limited but show some promise.8-12 To date, most interventions have taken a monodimensional approach (ie, exercise) that focused on 1 specific time point across the continuum of acute care (ie, before transplantation,9 were delivered in hospital,10,12,13 or delivered after transplantion14,15). Limitations of the research to date and challenges working with this population are reflected in the heterogeneity of study designs and outcomes, small sample sizes, poor adherence, and high attrition. Moving forward, research that can address these challenges is urgently needed to establish the effectiveness of longitudinal rehabilitation programs for patients who are undergoing alloBMT. In response, we developed an innovative, longitudinal, 6-month multidimensional rehabilitation program for patients who are undergoing alloBMT (CaRE-4-alloBMT). CaRE-4-alloBMT was adapted from an evidence-based program, developed by the Princess Margaret Cancer Rehabilitation and Survivorship program, called CaRE.16,17 CaRE-4-alloBMT is informed by established behavior change theories and harnesses current eHealth technologies to reduce barriers to accessing and providing cancer rehabilitation. CaRE-4-alloBMT uses a person-centered strategy and a multidimensional approach that target physical activity and promote self-management skills related to important relevant issues, such as nutrition and stress management. The purpose of this study was to evaluate the feasibility, acceptability, and impact of CaRE-4-alloBMT.
Methods
Study design
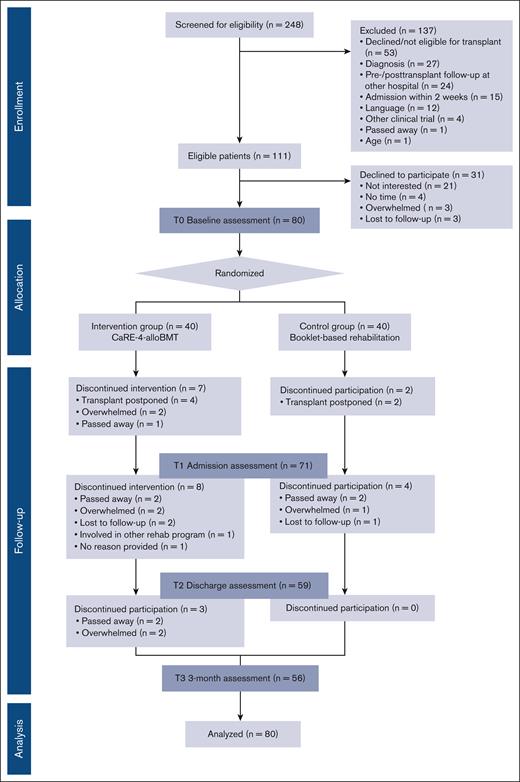
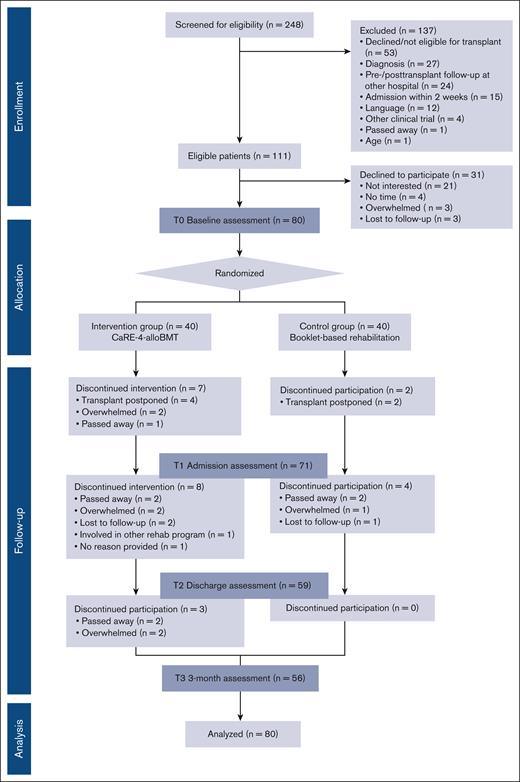
This study was designed as a pragmatic, single-center, 2-arm, phase 2 randomized controlled trial in patients who underwent alloBMT at the Hans Messner Allogeneic BMT Program at the Princess Margaret Cancer Centre, Toronto, Canada. A completed CONSORT18 participant flow diagram is shown in Figure 1. A total of 80 participants were randomized to either receive standard best practice cancer care (CON) or CaRE-4-alloBMT plus best practice cancer care (INT). Assessments were completed at 4 time points, namely at baseline (T0), at alloBMT hospital admission (T1), at discharge (T2), and at 3-months posttransplant discharge (T3). This trial was registered with ClinicalTrials.gov (identifier: NCT04966156) and approved by the University Health Network Research Ethics Board (REB#21-5076). For full methodology details, the protocol has been published.19
Participants
Patients were screened for eligibility based on the following: (1) ≥18 years of age; (2) diagnosed with a hematologic malignancy; (3) completing pre- and posttransplant care at the Princess Margaret Cancer Centre (rather than a hospital closer to their home); (4) able to access online study material; and, (5) able to understand and communicate in English. Participants who did not meet all of the above criteria were excluded. Participants were enrolled in the program at least 2 weeks prior to alloBMT hospital admission.
Sample size
We conducted a simulation of a range of sample sizes and values of standard deviation for precision of estimate (α = 0.05 and power at 80%) and found that a sample size of 35 to 40 per arm was at the elbow point of the curves. Therefore, a sample size of 80 participants (40 participants per arm) was included in the study, which is considered large enough to examine the feasibility of the study.20
Randomization and blinding
Participants were randomized 1:1 to INT or CON and were stratified based on frailty21 (assessed by alloBMT physician) prior to the T0 assessment. Because of the nature of the intervention, participant and investigator blinding was not possible. Physiological assessors were trained to follow a standardized detailed protocol for outcome assessments.18,22
CaRE-4-alloBMT (intervention)
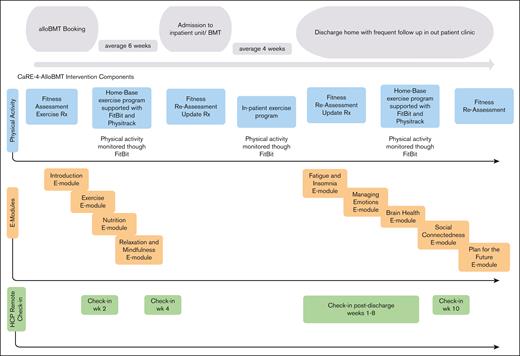
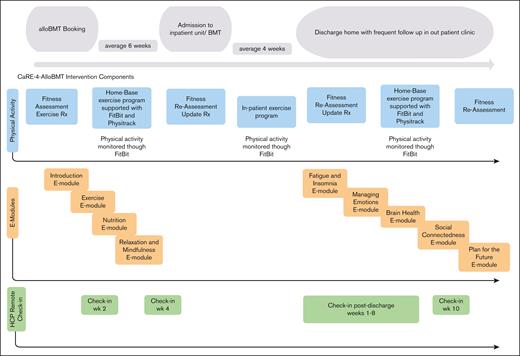
Participants randomized to INT (n = 40) received best practice cancer care plus CaRE-4-alloBMT. The program contained a prehabilitation (pretransplant) phase and a posttransplant phase. Components of the intervention are outlined hereafter (Figure 2) and have been reported in more detail in our protocol paper.18
Individualized progressive exercise prescriptions: a tailored exercise plan23-25 was developed and prescribed by the study registered kinesiologist (RKin). Each program contained cardiovascular, strength, and flexibility training with the goal of reaching at least 90 minutes of moderate intensity aerobic exercise per week. Strength and flexibility training were prescribed 2 to 3 times per week. Exercise programs were discussed and modified at follow-up assessments and during scheduled health coaching appointments with the RKin.
Remote monitoring through wearable technology: participants were provided with a Fitbit Versa 2 for use during the study to track, in real time, physiological data and activity, and to promote behavioral change through participant self-monitoring and awareness. The RKin monitored these metrics and discussed them during their clinical health coaching appointments.16,26,27
e-Learning modules: participants were prescribed 9 e-learning modules to promote self-management skills and symptom management.16,28 Participants were asked to complete the first 4 modules before the transplant and the remaining 5 after the transplant.
Remote clinical support: participants were scheduled for remote clinical health coaching appointments with the RKin at scheduled intervals. Two pretransplant calls were scheduled, at weeks 2 and 4, after enrollment. After the transplant, calls were scheduled weekly for weeks 1 to 8 (after discharge) and then again at week 10 for a total of 11 appointments with each participant. These were offered through Microsoft Teams video or telephone and lasted ∼15 minutes.
Timeline of the intervention components. Rx, prescription. Adapted from Tam et al.19
Timeline of the intervention components. Rx, prescription. Adapted from Tam et al.19
Following participation in the INT program, participants were contacted to complete a qualitative interview to understand participant experience and gain feedback on the program.
Standard of care (control)
Participants randomized to the CON arm (n = 40) received the standard best practice cancer care provided to patients undergoing alloBMT at the Hans Messner Allogeneic Bone Marrow Transplant Centre. This included booklet-based physiotherapy provided to all patients undergoing alloBMT. Patients also underwent a nutritional assessment by a registered dietician during the alloBMT hospital admission where a daily caloric goal was given. Participants enrolled in the CON arm completed all assessments but were not provided with individualized fitness instruction, Fitbits, e-learning modules, or health coaching support.
Study outcomes
Primary outcomes
The primary outcomes of this study were the feasibility of the methods and acceptability of CaRE-4-alloBMT. This included an assessment of the following metrics: (1) recruitment rate; (2) retention rate; (3) adherence to the intervention components, including completion of the e-learning modules, use of the Fitbit, and remote clinical check-in call attendance; (4) acceptability of the intervention and study procedures; and (5) safety of the intervention. The available literature was used to define feasibility indicators a priori. Table 1 summarizes the primary outcome metrics used and the a priori feasibility indicators.
Secondary clinical outcomes
The secondary clinical outcomes were the effects of the INT on physical function (primary) as measured by the subscales of (1) physical functioning and (2) role limitations due to physical health based on the 36-Item Short Form Health Survey (SF-36).29 Other clinical outcomes were the effects of the intervention on self-perceived health status, disability, nutritional status, anxiety, QoL, and cognitive function. Physiological fitness assessments30 were used to measured upper and lower extremity strength and aerobic functional capacity by the RKin. Table 2 summarizes the secondary outcome measures.
Data collection
A database was developed in accordance with the CONSORT18 criteria to track patient eligibility and participant flow throughout the study (Figure 1). Secondary clinical outcomes data were collected at 4 time points. Patient-reported outcome data were stored on REDCap.39 Physiological assessment data were collected by the study RKin and stored in encrypted databases on secure servers.
Qualitative data were collected from semistructured interviews with a random subsample of 15 INT participants (target) or until saturation was achieved. Interviews contained questions on the program content, structure, and delivery. Transcripts and recordings from the qualitative interviews (conducted via Microsoft Teams) were saved in secure servers at the University Health Network.
Data analysis
Primary feasibility and acceptability data
Study feasibility was evaluated using descriptive statistics (Table 1) and included (1) recruitment and eligibility rates; (2) attrition rate; (3) adherence to the intervention; (4) capture of outcome data, complete and missing, at each time point; and (5) safety of the intervention. Based on the available literature, feasibility rates were determined a priori (Table 1).
Qualitative interviews were conducted to gather feedback, inform program acceptability, and steer future intervention refinement. Qualitative interview recordings were transcribed verbatim and cleaned for formatting. The transcripts were analyzed using Braun and Clarke’s 6 phases of thematic analysis.40 The analysis was primarily deductive with a number of categories that were pre-selected to align with the main program components, program benefits, barriers to participation, and areas for improvement. Additional codes were derived inductively by the first author (S.T.) following initial coding. Qualitative themes, analytical notes, and example quotations were derived following discussions with the research team.
Secondary clinical data
Results
Patient baseline information
A total of 80 participants were enrolled from September 2021 to August 2022 and randomized to INT (n = 40) or CON (n = 40). All 80 participants completed the T0 baseline assessment. A total of 56 participants completed the T3 time point (Figure 1). Patient demographic data are presented in Table 3.
Transplant practices and the characteristics of participants who underwent alloBMT (n = 71) are presented in supplemental Table 1.
Primary feasibility outcomes
The recruitment rate of eligible participants was 72% with reasons for declining documented (Figure 1. CONSORT diagram). The overall retention rate was 70% (n = 56) with a total of 24 participants dropping out before T3. The time points of the dropouts by group are found in the CONSORT diagram. Feasibility was also measured by adherence to the following intervention components: (1) Fitbit usage (81.2%), (2) e-learning module completion (22.7%), and (3) clinical support appointment attendance (62.1%). Safety was measured as any adverse events experienced as a consequence of the intervention. No adverse events were reported. Acceptability was assessed through qualitative interviews with a random subsample of INT participants (n = 13). The qualitative findings are discussed in the next section.
Primary acceptability finding
A total of 13 qualitative interviews were conducted with a random subsample of INT participants. Themes addressed both the feasibility of the methods and the acceptability of the CaRE-4-alloBMT program (Table 4).
Four main themes were identified related to participant views on the feasibility of the program design, and these centered around satisfaction and areas for improvement. Participants appreciated the flexibility of the program, especially tailoring the intensity to accommodate their transplant recovery process. Participants also found the program easy to understand, accessible, and organized. Areas of improvement included extending the length of the program after the transplant and tailoring the questionnaires to the specific stages of the transplant process.
In terms of acceptability, all participants interviewed were very satisfied with the program and found it helpful. Notably, the Fitbit motivated participants to get up and move, and the health coaching appointments held participants accountable to the program. Participants’ views on the e-learning modules were split. Some participants found that although the e-learning modules were a burden to complete, certain modules were helpful in recovery. Barriers to participation played a large role in adhering to the program. This included the lack of time before the transplant, and the symptom barriers and unpredictable recovery after the transplant, resulting in a poor physical and mental state.
Secondary clinical outcomes
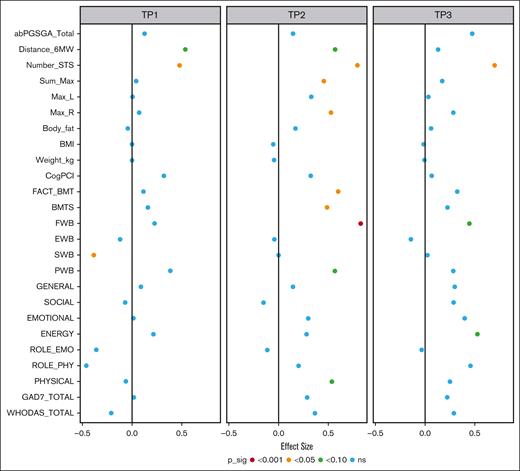
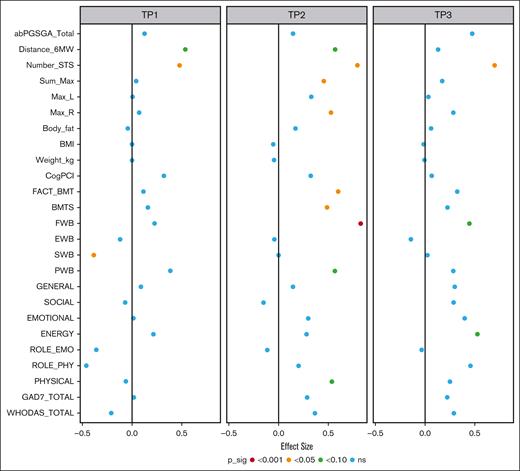
Physical functioning (primary outcome) was measured on the SF-36 using the subscales of physical functioning and role limitations due to physical health. Treatment effects exceed the minimum clinically important differences (MCIDs) reported at 3 to 5 points for the SF-36.45 For the physical functioning subscale, moderate effects were seen at T2 (effect size [ES], 0.54; 95% CI, –0.05 to 1.13), and treatment effects of 14.1 points were observed. Small effects were seen at T3 (ES, 0.25; 95% CI, –0.37 to 0.88), and treatment effects reach 6.64 points. The subscale of role limitations due to physical health problems showed an increase in effect size from T2 (ES, 0.20; 95% CI, –0.40 to 0.80) to T3 (ES, 0.46; 95% CI, –0.17 to 1.09). Treatment effects at these time points also exceeded the MCIDs at 8.13 (T2) and 18.5 (T3). The physiological data suggested similar trends. Large effect sizes were observed as measured by the 30-second sit-to-stand test (30s-STS) at both T2 (ES, 0.79; 95% CI, 0.31-1.28) and T3 (ES, 0.70; 95% CI, 0.20-1.21), and treatment effects exceeded the MCIDs.46 A summary of the treatment effects and effect sizes is shown in Figure 3. Table 5 reports all effect sizes and treatment effects.
Summary of the treatment effect and effect sizes. This plot summarizes the estimated effect sizes for each outcome at admission, discharge, and follow-up. The color of the point corresponds to the level of statistical significance of the treatment effect with no controls for multiple testing. Positive effect sizes indicate that, given equal baseline values, we would expect a better outcome value in the intervention group. ns, not significant.
Summary of the treatment effect and effect sizes. This plot summarizes the estimated effect sizes for each outcome at admission, discharge, and follow-up. The color of the point corresponds to the level of statistical significance of the treatment effect with no controls for multiple testing. Positive effect sizes indicate that, given equal baseline values, we would expect a better outcome value in the intervention group. ns, not significant.
A difference was observed between groups at baseline (lower in INT) in many outcomes. Greater positive effects were reported at T2 (discharge) than at T3 (3-month follow-up) in many outcomes favoring the INT group. Changes over time suggest a more stable transplant journey for INT vs CON (supplemental Figure 2).
Discussion
This phase 2 randomized controlled trial generated valuable information regarding the CaRE-4-alloBMT intervention and study design. The results suggested that the intervention, its delivery, and method of evaluation are acceptable and safe. Recruitment (72%) was high when compared with published exercise interventions in this population,9-12,47 indicating high interest in this type of program. Overall, retention (70%) met the a priori indicator of feasibility; however, significantly higher attrition was found in the INT arm. A total of 54% of participants dropped out because of nonavoidable reasons, including having their transplant canceled (25%) or passing away (29%). Because of the longitudinal nature of the intervention and its commencement before alloBMT admission, it was expected that a proportion of participants may experience disease progression that led to a canceled transplant or mortality before the transplant, which was confirmed in the present study. The varying disease status, frailty, and high mortality as a consequence of alloBMT also explain the proportion of participants that had passed away before the end of the study. These proportions should be incorporated in the sample size calculations for future planning. Replacement of participants who do not end up receiving a transplant could also be considered. Avoidable reasons for dropout make up the remaining 46%. Of these, many participants (both INT and CON) noted feeling too overwhelmed by the treatment and symptom burdens to continue in the study. This may explain poor adherence to the modules and suggests that there is a need to modify the intervention to better suit the needs and preferences of this population. Previous studies have demonstrated the feasibility of exercise rehabilitation programs for people undergoing alloBMT; however, the findings regarding adherence and retention are still conflicting in the literature.10,15,48 It is important to consider that this study was conducted from September 2021 to February 2023. This was during the time of the COVID-19 pandemic, and this may have had an impact on the study outcomes, including worsening participant’s disease status, QoL, and overall well-being, and on the retention and adherence to the program.49-51
Overall, participants in the INT arm found the intervention to be acceptable and found that the Fitbit and health coaching appointments were crucial in motivating exercise (increased daily step count) and increasing accountability to exercising. This has been supported in published literature.27 However, adherence to e-learning modules was low; potential reasons for this were discussed in the qualitative interviews, notably the lack of relevance of certain modules to participants’ recovery processes and the limited period for completion. This may be improved by prescribing specific modules that address the needs of the individual participant and lengthening the follow-up period of the program, which would allow more time to complete the modules. This approach is supported by evidence that suggests the importance of tailoring and building in flexibility in alloBMT education, specifically the timing, content, and format of education.52 Incorporating education curriculum within health coaching appointments could also be explored.
Clinical outcomes
The study groups were not balanced at baseline with the INT group preforming poorer on a number of outcomes. The difference in scores were likely because of random chance and could be addressed with a larger sample size. Although we stratified participants based on frailty scores in an effort to help balance the groups, this was not successful. It may be that the Older Americans Resources and Services Instrumental Activities of Daily Living (OARS IADL) scale21 that was used in this study to assess frailty was not sufficient. Moving forward, the use of frailty scoring could be improved by including related physiological outcomes (eg, grip strength test and the timed Up and Go test).53
The preliminary estimates of the effect of the intervention were encouraging and suggests that the CaRE-4-alloBMT program prevents deterioration in physical functioning, especially at the transplant discharge time point. Results of the treatment effects were greater than the reported MCIDs, suggesting clinically relevant benefits of the intervention, although these results would need to be confirmed in a larger phase 3 trial.
Study limitations
This study was not without limitations. This study was conducted in a single, comprehensive, tertiary-care center located in an urban setting, which limits its generalizability. With an 80-participant sample size, we acknowledge that our results cannot be definitive. Analysis of the outcomes has shown baseline imbalances between the groups in some measures. This may introduce bias in the intervention effect estimates, although statistical adjustments were made to account for baseline differences. Rapid attrition and missingness in the data also introduce bias in the estimates obtained in this study. The approach taken to account for this included using a mixed effects model and all observed data. No imputation was performed; hence, survivorship bias may be present as a consequence. Selection bias may also be present among participants who volunteered to participate in the study and who completed the qualitative interviews. This may limit the validity of the results. The measurement of adherence and fidelity was limited to the use of the tools provided and not the actual completion of the exercise prescribed. This could be enhanced in future studies to better understand the mediational role of exercise on the outcome variables. Finally, because of the nature of the intervention, funding, and staffing, it was not possible to conduct this as a double-blind study. Therefore, performance biases of the participants cannot be ruled out. Although assessors were trained to follow a standardized detailed protocol,54 confirmation biases cannot be ruled out. Moving forward, these biases can be further mitigated by using objective outcome measures (ie, 30s-STS) and blinding the fitness assessment outcome assessor.
Once these pilot findings are confirmed through larger-scale studies, clinicians may have additional evidence to implement longitudinal, multidimensional rehabilitation programs in transplant centers. Programs such as CaRE-4-alloBMT, grounded in self-management and behavioral change theory, play an important role in the rehabilitation of patients undergoing alloBMT and may lead to improved health outcomes. Lessons learned from this feasibility study can help to refine the program design and inform future research with the aim of standardizing rehabilitation in this high-need population.
Acknowledgments
The authors thank all members of the Cancer Rehabilitation and Survivorship and the Hans Messner Allogeneic Blood and Marrow Transplantation Programs at the Princess Margaret Cancer Centre for their critical discussion of this study.
The authors thank the generous support of the Butterfield Drew Chair in Cancer Survivorship and the Princess Margaret Cancer Foundation for funding this study.
Authorship
Contribution: All authors contributed to the study design; S.T., P.L., D.H., and J.M.J. performed the research; L.A. and E.G.A. analyzed the data; S.T. and J.M.J. designed and drafted the manuscript; all authors contributed equally to the revisions and finalization of this study; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer Michelle Jones, Cancer Rehabilitation and Survivorship Program, Princess Margaret Cancer Centre Toronto, 200 Elizabeth St, PMB-B-045, Toronto, ON, M5G 2C4 Canada; email: jennifer.jones@uhn.ca.
References
Author notes
The protocol for the current study has been published and can be accessed at https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0285420. The data set is available once institutional review board approval and data sharing agreements are in place.
The full-text version of this article contains a data supplement.