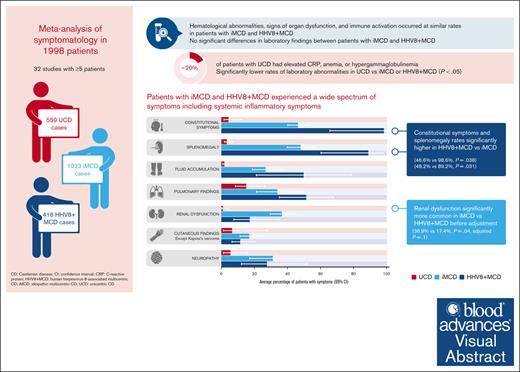
Visual Abstract
Castleman disease (CD) encompasses a spectrum of rare disorders, including unicentric CD (UCD), idiopathic multicentric CD (iMCD), and human herpesvirus 8–associated MCD (HHV8+ MCD). We performed a systematic review of publications reporting ≥5 cases of CD between 1995 and 2021, following preferred reporting items for systematic reviews and meta-analyses guidelines, to describe and compare subtypes. We extracted data on clinical symptoms and laboratory parameters as stated in international consensus diagnostic criteria for iMCD and estimated the frequency of each criterion using meta-analyses. We analyzed 32 studies describing 559 UCD, 1023 iMCD, and 416 HHV8+ MCD cases. Although many symptoms and laboratory abnormalities occurred at similar rates in patients with iMCD and HHV8+ MCD, patients with HHV8+ MCD had significantly higher rates of constitutional symptoms (46.6% vs 98.6%; P = .038) and splenomegaly (48.2% vs 89.2%; P = .031). Renal dysfunction was significantly more common in patients with iMCD than in patients with HHV8+ MCD before adjustment (36.9% vs 17.4%; P = .04; adjusted P = .1). Patients with UCD had lower rates of symptoms and laboratory abnormalities, although these were present in 20% of patients and were particularly pronounced in pediatric UCD. There are many similarities in the symptomatology of iMCD and HHV8+ MCD; many patients experience constitutional symptoms and organ dysfunction. Differences between these subtypes likely reflect differences in pathophysiology and/or comorbidity burdens.
Introduction
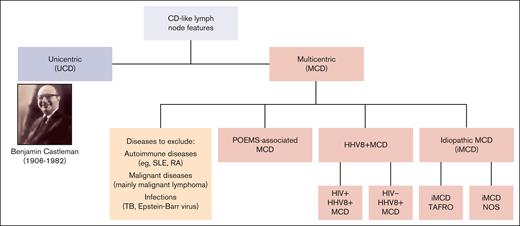
Castleman disease (CD) describes a spectrum of rare disorders with characteristic histopathology and enlarged lymph nodes (Figure 1). Unicentric CD (UCD) mainly occurs as a benign, localized lymphoid hyperplasia, often presenting as a solitary, indolent lymph node swelling. In contrast, multicentric CD (MCD) is potentially life threatening, with multifocal lymphadenopathy, systemic inflammation, and constitutional symptoms. Etiology of idiopathic MCD (iMCD) is unknown; various potential causes for pathological overproduction and dysregulation of proinflammatory cytokines (eg, interleukin-6) have been discussed.1 The clinical picture of iMCD can vary in terms of severity, course, and symptoms. iMCD can present with recurrent episodes of diffuse lymphadenopathy with systemic inflammation, edema, anemia, hypoalbuminemia, and/or multiple organ system dysfunctions, which can be fatal if improperly treated.2 Many MCD cases are caused by uncontrolled infection with human herpesvirus 8 (HHV8+ MCD), a γ-herpesvirus that drives hypercytokinemia via a viral interleukin-6 homologue. Unlike UCD and iMCD, HHV8+ MCD often occurs with concomitant HIV infection, and incidence and prevalence therefore closely follow the trajectory of HIV epidemiology. HHV8+ MCD, whether associated with HIV infection or not, appears to display a more aggressive clinical and biological presentation than iMCD.3
Current classification of CD. NOS, not otherwise specified; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TAFRO, thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly; TB, tuberculosis.
Current classification of CD. NOS, not otherwise specified; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TAFRO, thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly; TB, tuberculosis.
Histopathological features of CD have been well described. They occur over a spectrum from hyperplastic germinal centers with plasmacytosis (plasmacytic/plasma cell histopathology) to atrophic germinal centers with hypervascularity (hyaline vascular/hypervascular histopathology), with a mixed histopathological subtype in between.4 In 2017, international, evidence-based consensus diagnostic criteria for iMCD were established.4 These require the presence of major criteria (lymph node histopathology and multicentric lymphadenopathy) and ≥2 of 11 minor criteria (with ≥1 laboratory abnormality). Diagnostic tests and consensus criteria for iMCD are summarized in Tables 1 and 2. International iMCD management guidelines were published in 20185 and diagnostic and treatment guidelines for UCD in 2020.6 In contrast, there are no published diagnostic criteria for HHV8+ MCD. In the opinion of many experts, evidence of immunohistochemistry for HHV8 latency–associated nuclear antigen, systemic inflammatory symptoms, and detectable HHV8 viremia is sufficient for establishing diagnosis.7
Diagnostic workup for MCD
| Purpose . | Tests . |
|---|---|
| Inflammatory response | CBC, renal function, liver function, CRP, ESR, fibrinogen, immunoglobulins and free light chains, albumin, and ferritin |
| Histopathology | Hypervascular/mixed cellularity/plasmacytic variant |
| Virologic status | HIV serology, HHV8 qPCR (peripheral blood), EBER (lymph node), and LANA-1 (lymph node) |
| Cytokine profile | Interleukin-6 and vascular endothelial growth factor |
| Imaging | CT-PET or CT neck, chest, abdomen, and pelvis |
| Bone marrow evaluation | Monoclonal gammopathy of undetermined significance, myeloma, and reticulin fibrosis |
| Immunology | ANA and rheumatoid factor |
| Organ function | Echocardiogram and pulmonary function (additional organ assessment may be needed in severe cases) |
| Purpose . | Tests . |
|---|---|
| Inflammatory response | CBC, renal function, liver function, CRP, ESR, fibrinogen, immunoglobulins and free light chains, albumin, and ferritin |
| Histopathology | Hypervascular/mixed cellularity/plasmacytic variant |
| Virologic status | HIV serology, HHV8 qPCR (peripheral blood), EBER (lymph node), and LANA-1 (lymph node) |
| Cytokine profile | Interleukin-6 and vascular endothelial growth factor |
| Imaging | CT-PET or CT neck, chest, abdomen, and pelvis |
| Bone marrow evaluation | Monoclonal gammopathy of undetermined significance, myeloma, and reticulin fibrosis |
| Immunology | ANA and rheumatoid factor |
| Organ function | Echocardiogram and pulmonary function (additional organ assessment may be needed in severe cases) |
ANA, antinuclear antibody; CBC, complete blood count; CT, computed tomography; EBER, Epstein-Barr virus–encoded small RNAs; ESR, erythrocyte sedimentation rate; LANA, latency-associated nuclear antigen; PET, positron emission tomography; qPCR, quantitative polymerase chain reaction.
Adapted from van Rhee et al5
Consensus criteria for idiopathic MCD
| 1. Presence of both major criteria |
| Lymph nodes with typical histology, and |
| lymph nodes enlarged >1 cm in at least 2 stations |
| 2. Presence of at least 2 of 11 minor criteria (including at least 1 laboratory criterion) |
| Clinical symptoms |
| 1. B symptoms (night sweats, fever over 38°C, weight loss, or fatigue) |
| 2. Splenomegaly and/or hepatomegaly |
| 3. Edema, anasarca, ascites, and pleural effusions |
| 4. Lymphocytic interstitial pneumonia |
| 5. “Eruptive” hemangiomatosis |
| Laboratory criteria |
| 1. Elevated CRP (>10 mg/L) or blood sedimentation (>15 mm/h) |
| 2. Anemia (hemoglobin <12.5 g/dL for men and <11.5 g/dL for women) |
| 3. Thrombocytopenia (platelet count, <150 per μL) or thrombocytosis (>400/μL) |
| 4. Hypoalbuminemia (<3.5 g/dL) |
| 5. Renal function impairment (eGFR, <60 mL/min per 1.73m2) or proteinuria (total protein, 150 mg/24 h or 10 mg/100 mL) |
| 6. Polyclonal hypergammaglobulinemia (>1700 mg/dL) |
| 3. Exclusion of other diseases with “Castleman-like” histology |
| Infection-associated diseases: HHV8 (PCR, serology, and/or LANA staining in the lymph node); infectious mononucleosis (EBV, otherwise no exclusion criterion); and lymphadenopathy due to HIV, CMV, toxoplasmosis, and tuberculosis. |
| Defined autoimmune diseases (detected antibodies alone do not exclude iMCD) such as systemic lupus erythematosus, rheumatoid arthritis, adult-onset Still disease, juvenile idiopathic arthritis, and autoimmune lymphoproliferative syndrome. |
| Malignant or lymphoproliferative diseases, diagnosed at the same time (Hodgkin and non-Hodgkin lymphomas, multiple myeloma, plasmacytoma localized primarily in the lymph node, and FDC sarcoma). |
| 1. Presence of both major criteria |
| Lymph nodes with typical histology, and |
| lymph nodes enlarged >1 cm in at least 2 stations |
| 2. Presence of at least 2 of 11 minor criteria (including at least 1 laboratory criterion) |
| Clinical symptoms |
| 1. B symptoms (night sweats, fever over 38°C, weight loss, or fatigue) |
| 2. Splenomegaly and/or hepatomegaly |
| 3. Edema, anasarca, ascites, and pleural effusions |
| 4. Lymphocytic interstitial pneumonia |
| 5. “Eruptive” hemangiomatosis |
| Laboratory criteria |
| 1. Elevated CRP (>10 mg/L) or blood sedimentation (>15 mm/h) |
| 2. Anemia (hemoglobin <12.5 g/dL for men and <11.5 g/dL for women) |
| 3. Thrombocytopenia (platelet count, <150 per μL) or thrombocytosis (>400/μL) |
| 4. Hypoalbuminemia (<3.5 g/dL) |
| 5. Renal function impairment (eGFR, <60 mL/min per 1.73m2) or proteinuria (total protein, 150 mg/24 h or 10 mg/100 mL) |
| 6. Polyclonal hypergammaglobulinemia (>1700 mg/dL) |
| 3. Exclusion of other diseases with “Castleman-like” histology |
| Infection-associated diseases: HHV8 (PCR, serology, and/or LANA staining in the lymph node); infectious mononucleosis (EBV, otherwise no exclusion criterion); and lymphadenopathy due to HIV, CMV, toxoplasmosis, and tuberculosis. |
| Defined autoimmune diseases (detected antibodies alone do not exclude iMCD) such as systemic lupus erythematosus, rheumatoid arthritis, adult-onset Still disease, juvenile idiopathic arthritis, and autoimmune lymphoproliferative syndrome. |
| Malignant or lymphoproliferative diseases, diagnosed at the same time (Hodgkin and non-Hodgkin lymphomas, multiple myeloma, plasmacytoma localized primarily in the lymph node, and FDC sarcoma). |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; FDC, follicular dendritic cell; LANA, latency-associated nuclear antigen; PCR, polymerase chain reaction.
Adapted from Fajgenbaum et al4
To our knowledge, there is no major review summarizing clinical features across all CD subtypes. Given the rarity of all subtypes, information is mainly dispersed in small case series or case reports. It is notable that iMCD diagnostic criteria4 were defined from the existing evidence based on only 244 cases, including 128 cases from a systematic literature review and 79 cases from a clinical trial. Larger data sets are scarce, the largest review of 416 MCD cases did not distinguish between iMCD and HHV8+ MCD,8 and others focused on iMCD only.9 We used meta-analyses to estimate the prevalence of symptoms and laboratory findings for iMCD, HHV8+ MCD, and UCD, as described in the literature, and compared them, via statistical testing, to identify differences between subtypes. Differentiating subtypes is important to ensure timely diagnosis and treatment.
Methods
Search strategy
Following preferred reporting items for systematic reviews and meta-analyses guidelines, we searched PubMed for publications on CD from 1 January 1995 (when HHV8 testing was developed) to 9 February 2021. Searches were restricted to publications on humans reported in English. Search terms used were: (“present∗”[Title] OR “symptom∗”[Title] OR “diagn∗”[Title] OR “clinic∗”[Title]) AND (“castleman disease”[MeSH Terms] OR “castleman”[All Fields]) AND (“disease”[All Fields] OR “castleman disease”[All Fields]) AND (humans[Filter]) AND (english[Filter]) AND (1995:2021[pdat]). We also checked bibliographies of 2 large review articles for additional publications,8,10 and conducted a manual targeted search using Google Scholar and PubMed. The review protocol was not prepublished or registered.
Selection criteria
After removal of duplicate publications, abstracts were screened by a researcher (Azhaar Ashraf, see “Acknowledgments”) to identify appropriate publications including symptom data for patients with CD. To reduce bias from single case reports of unusual presentations, only those describing clinical data from ≥5 patients were included. Inclusion criteria were: (1) includes clinical data from patients with UCD, HHV8+ MCD, or iMCD; and (2) ≥5 patients. Exclusion criteria were: (1) review articles presenting symptoms from the literature without case reports; (2) describes patients with POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin abnormalities) without iMCD features; (3) no symptom data, or insufficient symptom data reported; (4) clinically selected cohorts focusing on a particular aspect of CD, for example abdominal, thoracic, head, and neck cases (most of these are small studies with patients who are also included in larger cohorts; these were excluded as they may cause a bias regarding the true clinical picture); and (5) randomized controlled trial. Another researcher (C.H.) independently confirmed the screening results. Full-text articles were then independently reviewed by 2 researchers (Azhaar Ashraf and L.G.) for inclusion. Disputes were resolved and results confirmed by a third researcher (C.H.).
We grouped publications into 3 categories: (1) aggregate-level data, publications with cohort-level data on CD symptoms; (2) patient-level data, publications reporting symptom data for each patient; and (3) pediatric data, publications focusing exclusively on pediatric/adolescent patients. To avoid inclusion of the same patient from >1 publication within each of the 3 groups, publications were independently cross-checked by 2 researchers (C.H. and L.G.) by study location, data collection period, and investigators to identify potentially duplicated patients. Where there was a possibility that a patient was included in >1 study, we excluded the study with the fewest patients or the least symptom data.
Data extraction
Three researchers (Azhaar Ashraf, L.G., and Hannah Lewis, see “Acknowledgments”) extracted data into an Excel worksheet, and an author (C.H.) answered queries on symptom classification. C.H. assessed results for potential discrepancies and a statistician (S.L.) noted inconsistent data points; publications were reanalyzed by L.G. to check these data. Descriptive study characteristics were extracted, including country, study center(s), data capture period, CD subtype (UCD, iMCD, or HHV8+ MCD), and patient numbers. For each CD subtype within each study, we extracted data on histology, sex, age, and ethnicity, and for UCD, location of affected lymph node/station.
We recorded the number of patients reporting clinical symptoms and laboratory parameters included in the international consensus diagnostic criteria for iMCD. Many publications reported less-specific clinical symptoms than those in the consensus criteria (eg, “rash”), and often thresholds for laboratory parameters were not reported (eg, “anemia”) or different from the consensus criteria. Therefore, we captured symptoms and laboratory parameters with broader criteria than the definitions in the consensus criteria (supplemental Table 1 shows the data extraction template).
Statistical analysis
Meta-analyses were performed using logistic regression to derive pooled prevalence estimates for each of the clinical symptoms and laboratory parameters, by CD subtype. To account for heterogeneity, random effects meta-analyses were used to estimate mean percentages of patients with each symptom or laboratory parameter. Differences between estimated mean prevalence of symptoms between CD subtypes were tested via a z test of the logit-transformed mean proportions (differences considered statistically significant if P <.05). To account for multiple testing, the false discovery rate (FDR) correction was applied. Results of tests for which there were limited numbers of studies and/or patients should be interpreted with caution. Analyses were conducted in R version 4.1.3 (further details on heterogeneity assessments and exploration of consistency are included in supplemental Methods).
Risk-of-bias assessment
As there was no intervention and the studies were not comparative; the studies were not randomized. Therefore, many of the possible risks of bias were not relevant in this case (such as allocation concealment, unblinding, attrition bias, etc) and the Cochrane risk-of-bias tool for randomized trials, the recommended tool for use in Cochrane reviews, was not applicable. However, consideration has been given to, for example, multiple publication bias (as discussed in “Methods”) for which studies were omitted to prevent duplication of patients. We have considered the potential for (non)reporting bias and any possible impact on, for example, the accuracy or generalizability of the results. Because this review is not of an intervention, this is likely to limit the risk of conflicts of interest and publication bias (whereby “negative” results may be less likely to be reported), as there should be little incentive to not report on the prevalence of observed symptoms/laboratory changes.
Results
Studies analyzed
Titles and abstracts of 428 publications were screened; 122 were further assessed for eligibility. Seventy publications were excluded because of lack of symptom data, potential duplication of patients, or for reasons associated with data quality or risk of bias (Figure 2). In total, 52 studies were included for analysis (Table 3),3,11-45,46-61 including 32 (n = 1998 patients) with aggregated cohort-level data, 15 (n = 335 patients) with patient-level data, and 5 (n = 104 patients) on pediatric patients.
Study characteristics
| Data set . | Study . | Country . | No. of centers . | Data collection period . | UCD (n) . | iMCD (n) . | HHV8+ MCD (n) . |
|---|---|---|---|---|---|---|---|
| Aggregate | Alzahrani et al11 | Canada/Saudi Arabia | 2 | January 2005-September 2012 | 12 | ||
| Dong et al12 | China | 1 | November 1977-June 2014 | 62 | 52 | ||
| Lan et al13 | China | 1 | September 2011-September 2017 | 35 | 36 | ||
| Zhang et al14 | China | 4 | January 2001-January 2015 | 121 | 64 | ||
| Zhang et al15 ∗ | China | 1 | June 2015-June 2018 | 25 | |||
| Zhang et al16 | China | 1 | March 2008-June 2019 | 52 | 44 | ||
| Zhao et al17 | China | 1 | January 2010-May 2017 | 65 | 9 | ||
| Boutboul et al18 | France | National study | 1997-2017 | 71 | |||
| Oksenhendler et al3 | France | 1 | 1997-2017 | 27 | 169 | ||
| Hoffmann et al19 | Germany | 11 | January 1998-July 2010 | 52 | |||
| Prakash et al20 | India | 1 | 2002-2014 | 8 | 8 | ||
| Fujimoto et al21 | Japan | 77 | October 2013-October 2016 | 150 | |||
| Iwaki et al22 | Japan | 1 | 1999-2013 | 17 | |||
| Kawabata et al23 | Japan | 1 | January 2005-October 2012 | 21 | |||
| Murakami et al24 † | Japan | National study | June 2005-July 2011 | 342 | |||
| Nishimura et al25 | Japan | 1 | April 2012-June 2018 | 7 | |||
| Volkow-Fernández et al (2020)26 | Mexico | 1 | January 1994-December 2018 | 19 | |||
| Melikyan et al27 | Russia | 1 | 1996-2016 | 6 | |||
| Seo et al28 | South Korea | 1 | January 1990-March 2013 | 28 | |||
| Shin et al29 | South Korea | 1 | May 1981-March 2009 | 43 | 27 | ||
| Gonzalez-Garcia et al30 | Spain | 1 | January 1985-December 2017 | 20 | 23 | ||
| Bower et al31 | United Kingdom | 1 | 2000-2014 | 75 | |||
| Casper et al32 | United States | 2 | 1 January 2000 to 31 December 2009 | 53 | 6 | ||
| Chronowski et al33 | United States | 1 | 1988-1999 | 12 | 9 | ||
| Dispenzieri et al34 | United States | 2 | 30 March 1948 to 18 June 2002 | 53 | 60 | ||
| Herrada et al35 | United States | 1 | March 1977-October 1995 | 8 | |||
| Hill et al36 | United States | 1 | 1997-2012 | 17 | 13 | ||
| Lurain et al37 | United States | National study | 2000-2013 | 19 | |||
| Ramaswami et al38 ‡ | United States | National study | 2011-2018 | 8 | |||
| Uldrick et al39 § | United States | National study | 2004-2009 | 14 | |||
| Uldrick et al40 ‖ | United States | National study | March 2006-September 2010 | 17 | |||
| Venkataraman et al41 | United States | National study | 2004-2010 | 19 | |||
| Pediatric | Parez et al42 | Canada/France | 2 | Not reported | 7 | ||
| Zhang et al43 | China | 1 | January 2001-December 2012 | 9 | 2 | ||
| Borocco et al44 | France | 3 societies | 2016 | 17 | 6 | ||
| Chisholm et al45 | United States | 2 | 1980-2018 | 37 | 2 | ||
| Sopfe et al46 | United States | 1 | 1 January 2005 to 16 May 2017 | 18 | 6 | ||
| Patient-level | Loi et al47 | Australia | 3 | January 1994-December 2001 | 11 | ||
| Dong et al48 | China | 1 | November 1977-July 2008 | 34 | 21 | ||
| Wang et al49 | China | 1 | September 2007-October 2015 | 14 | |||
| Ye et al50 | China | 1 | January 1999-November 2008 | 48 | 4 | ||
| Zhu et al51 | China | 1 | May 2004-October 2012 | 10 | |||
| Dossier et al52 | France | 1 | January 1995-June 2012 | 12 | 18 | ||
| Oksenhendler et al53 ¶ | France/United Kingdom | 1 | 1990-2001 | 60 | |||
| Kojima et al54 | Japan | Not reported | 1992-2007 | 28 | |||
| Sato et al55 | Japan | 1 | Not reported | 6 | |||
| Terasaki et al56 | Japan | 1 | Not reported | 15 | |||
| Gopal et al (2015)57 # | Malawi | 1 | 1 June 2013 to 31 January 2015 | 6 | |||
| Ghosh et al58 | Nepal | 1 | January 2001-March 2008 | 5 | |||
| Kim et al59 | South Korea | 1 | 1989-1998 | 10 | 12 | ||
| Lachmann et al60 | United Kingdom | 1 | Not reported | 5 | |||
| Bowne et al61 | United States | 1 | July 1986-July 1997 | 13 | 3 |
| Data set . | Study . | Country . | No. of centers . | Data collection period . | UCD (n) . | iMCD (n) . | HHV8+ MCD (n) . |
|---|---|---|---|---|---|---|---|
| Aggregate | Alzahrani et al11 | Canada/Saudi Arabia | 2 | January 2005-September 2012 | 12 | ||
| Dong et al12 | China | 1 | November 1977-June 2014 | 62 | 52 | ||
| Lan et al13 | China | 1 | September 2011-September 2017 | 35 | 36 | ||
| Zhang et al14 | China | 4 | January 2001-January 2015 | 121 | 64 | ||
| Zhang et al15 ∗ | China | 1 | June 2015-June 2018 | 25 | |||
| Zhang et al16 | China | 1 | March 2008-June 2019 | 52 | 44 | ||
| Zhao et al17 | China | 1 | January 2010-May 2017 | 65 | 9 | ||
| Boutboul et al18 | France | National study | 1997-2017 | 71 | |||
| Oksenhendler et al3 | France | 1 | 1997-2017 | 27 | 169 | ||
| Hoffmann et al19 | Germany | 11 | January 1998-July 2010 | 52 | |||
| Prakash et al20 | India | 1 | 2002-2014 | 8 | 8 | ||
| Fujimoto et al21 | Japan | 77 | October 2013-October 2016 | 150 | |||
| Iwaki et al22 | Japan | 1 | 1999-2013 | 17 | |||
| Kawabata et al23 | Japan | 1 | January 2005-October 2012 | 21 | |||
| Murakami et al24 † | Japan | National study | June 2005-July 2011 | 342 | |||
| Nishimura et al25 | Japan | 1 | April 2012-June 2018 | 7 | |||
| Volkow-Fernández et al (2020)26 | Mexico | 1 | January 1994-December 2018 | 19 | |||
| Melikyan et al27 | Russia | 1 | 1996-2016 | 6 | |||
| Seo et al28 | South Korea | 1 | January 1990-March 2013 | 28 | |||
| Shin et al29 | South Korea | 1 | May 1981-March 2009 | 43 | 27 | ||
| Gonzalez-Garcia et al30 | Spain | 1 | January 1985-December 2017 | 20 | 23 | ||
| Bower et al31 | United Kingdom | 1 | 2000-2014 | 75 | |||
| Casper et al32 | United States | 2 | 1 January 2000 to 31 December 2009 | 53 | 6 | ||
| Chronowski et al33 | United States | 1 | 1988-1999 | 12 | 9 | ||
| Dispenzieri et al34 | United States | 2 | 30 March 1948 to 18 June 2002 | 53 | 60 | ||
| Herrada et al35 | United States | 1 | March 1977-October 1995 | 8 | |||
| Hill et al36 | United States | 1 | 1997-2012 | 17 | 13 | ||
| Lurain et al37 | United States | National study | 2000-2013 | 19 | |||
| Ramaswami et al38 ‡ | United States | National study | 2011-2018 | 8 | |||
| Uldrick et al39 § | United States | National study | 2004-2009 | 14 | |||
| Uldrick et al40 ‖ | United States | National study | March 2006-September 2010 | 17 | |||
| Venkataraman et al41 | United States | National study | 2004-2010 | 19 | |||
| Pediatric | Parez et al42 | Canada/France | 2 | Not reported | 7 | ||
| Zhang et al43 | China | 1 | January 2001-December 2012 | 9 | 2 | ||
| Borocco et al44 | France | 3 societies | 2016 | 17 | 6 | ||
| Chisholm et al45 | United States | 2 | 1980-2018 | 37 | 2 | ||
| Sopfe et al46 | United States | 1 | 1 January 2005 to 16 May 2017 | 18 | 6 | ||
| Patient-level | Loi et al47 | Australia | 3 | January 1994-December 2001 | 11 | ||
| Dong et al48 | China | 1 | November 1977-July 2008 | 34 | 21 | ||
| Wang et al49 | China | 1 | September 2007-October 2015 | 14 | |||
| Ye et al50 | China | 1 | January 1999-November 2008 | 48 | 4 | ||
| Zhu et al51 | China | 1 | May 2004-October 2012 | 10 | |||
| Dossier et al52 | France | 1 | January 1995-June 2012 | 12 | 18 | ||
| Oksenhendler et al53 ¶ | France/United Kingdom | 1 | 1990-2001 | 60 | |||
| Kojima et al54 | Japan | Not reported | 1992-2007 | 28 | |||
| Sato et al55 | Japan | 1 | Not reported | 6 | |||
| Terasaki et al56 | Japan | 1 | Not reported | 15 | |||
| Gopal et al (2015)57 # | Malawi | 1 | 1 June 2013 to 31 January 2015 | 6 | |||
| Ghosh et al58 | Nepal | 1 | January 2001-March 2008 | 5 | |||
| Kim et al59 | South Korea | 1 | 1989-1998 | 10 | 12 | ||
| Lachmann et al60 | United Kingdom | 1 | Not reported | 5 | |||
| Bowne et al61 | United States | 1 | July 1986-July 1997 | 13 | 3 |
Most publications described retrospective studies, except for 7 prospective studies marked by respective footnote symbols in the table.
Single-center, single-arm, phase 2 study
Prospective observational study
Open-label, single-center pilot study
Prospective pilot study
Prospective study
Prospective cohort study
Prospective longitudinal cohort study
Table 3 shows study characteristics for publications analyzed. The aggregated cohort-level data set contains data for most symptoms and characteristics for each CD subtype across 1998 patients, making it the focus of this manuscript. The smaller patient-level data set (which contains potentially overlapping patients with the aggregated data, and therefore cannot be combined) describes symptoms and laboratory findings for individual patients. Analysis of these data confirms findings from the aggregated data set (supplemental Table 2).
Demographics and disease characteristics
Table 4 shows demographics of 1998 patients in the cohort-level data set. There was a predominance of males with HHV8+ MCD (88.5%) and iMCD (59.1%), whereas women appeared slightly more often affected with UCD (53.7%). Patients with UCD tended to be younger than patients with iMCD and HHV8+ MCD. Most patients with iMCD (91.8%) and UCD (84.7%) were of Asian ethnicity in this study. Only 58 patients (6.4%) with iMCD were White. In contrast, almost all patients with HHV8+ MCD (93.7%) were White or Black/African American. The higher proportion of males vs females with iMCD was consistent in pediatric patients, and in Asian and non-Asian groups.
Patient demographics and disease characteristics: an aggregate data set
| Characteristic . | UCD . | iMCD . | HHV8+ MCD . |
|---|---|---|---|
| Sex, n (%)∗ | |||
| Female | 239 (53.7) | 377 (40.9) | 48 (11.6) |
| Male | 206 (46.3) | 545 (59.1) | 368 (88.5) |
| Not reported | 114 | 101 | 0 |
| Age (y)† | |||
| Average mean age (no. of studies, n) | 33.6 (2, 73) | 47.4 (4, 412) | 42.8 (5, 91) |
| Average median age (no. of studies, n) | 35.4 (7, 372) | 47.5 (14, 792) | 43.6 (9, 337) |
| Range (no. of studies, n) | 4-77 (6, 188) | 3-84 (13, 619) | 23-69 (7, 208) |
| Studies not reporting age data (n) | 3 | 3 | 0 |
| Ethnicity, n (%)∗ | |||
| African/African-American/Black | 17 (3.7) | 10 (1.1) | 106 (42.1) |
| Asian | 387 (84.7) | 835 (91.8) | 4 (1.6) |
| White | 52 (11.4) | 58 (6.4) | 130 (51.6) |
| Others | 1 (0.22) | 7 (0.8) | 12 (4.8) |
| Not reported | 102 | 113 | 164 |
| Histology, n (%)∗ | |||
| Hyaline vascular | 430 (88.3) | 145 (16.4) | 3 (12.0) |
| Plasma cell | 47 (9.7) | 586 (66.2) | 13 (52.0) |
| Mixed | 10 (2.1) | 154 (17.4) | 9 (36.0) |
| Not reported | 72 | 138 | 391 |
| UCD manifest location, n (%)∗ | |||
| Abdominal | 89 (34.4) | ||
| Axilla | 11 (4.3) | ||
| Head and neck | 57 (22.0) | ||
| Inguinal/pelvis | 9 (3.5) | ||
| Other locations | 6 (2.3) | ||
| Thoracic | 87 (33.6) | ||
| Not reported | 300 |
| Characteristic . | UCD . | iMCD . | HHV8+ MCD . |
|---|---|---|---|
| Sex, n (%)∗ | |||
| Female | 239 (53.7) | 377 (40.9) | 48 (11.6) |
| Male | 206 (46.3) | 545 (59.1) | 368 (88.5) |
| Not reported | 114 | 101 | 0 |
| Age (y)† | |||
| Average mean age (no. of studies, n) | 33.6 (2, 73) | 47.4 (4, 412) | 42.8 (5, 91) |
| Average median age (no. of studies, n) | 35.4 (7, 372) | 47.5 (14, 792) | 43.6 (9, 337) |
| Range (no. of studies, n) | 4-77 (6, 188) | 3-84 (13, 619) | 23-69 (7, 208) |
| Studies not reporting age data (n) | 3 | 3 | 0 |
| Ethnicity, n (%)∗ | |||
| African/African-American/Black | 17 (3.7) | 10 (1.1) | 106 (42.1) |
| Asian | 387 (84.7) | 835 (91.8) | 4 (1.6) |
| White | 52 (11.4) | 58 (6.4) | 130 (51.6) |
| Others | 1 (0.22) | 7 (0.8) | 12 (4.8) |
| Not reported | 102 | 113 | 164 |
| Histology, n (%)∗ | |||
| Hyaline vascular | 430 (88.3) | 145 (16.4) | 3 (12.0) |
| Plasma cell | 47 (9.7) | 586 (66.2) | 13 (52.0) |
| Mixed | 10 (2.1) | 154 (17.4) | 9 (36.0) |
| Not reported | 72 | 138 | 391 |
| UCD manifest location, n (%)∗ | |||
| Abdominal | 89 (34.4) | ||
| Axilla | 11 (4.3) | ||
| Head and neck | 57 (22.0) | ||
| Inguinal/pelvis | 9 (3.5) | ||
| Other locations | 6 (2.3) | ||
| Thoracic | 87 (33.6) | ||
| Not reported | 300 |
Percentages are reported for the percentage of patients for whom the characteristic has been reported (ie, not including patients with missing or unknown data for that characteristic). Percentages may not total 100 because of rounding errors.
One study reporting HHV8+ MCD data, and 1 reporting iMCD data provided age at onset, which has been included in the summary information.
Plasmacytic histopathological subtype was most common for iMCD and HHV8+ MCD (66.2% and 52.0% when known, respectively), whereas hyaline vascular was most common for UCD (88.3% when known; Table 4). UCD location was reported for 316 of 559 patients. When known, approximately one-third of UCD cases were thoracic, one-third abdominal, and 22.0% in the head/neck region (Table 4).
Laboratory findings
Laboratory findings were similar in patients with iMCD and HHV8+ MCD (Figure 3). Rates of elevated C-reactive protein (CRP) were 92.4% (95% confidence interval [CI], 64.5-98.8) in patients with iMCD and 89.6% (95% CI, 67.4-97.3) in patients with HHV8+ MCD. Similarly, hypergammaglobulinemia was present in most patients with iMCD and HHV8+ MCD (96.8% [95% CI, 96.7-96.9] and 100% [95% CI, 47.8-100], respectively). Anemia and hypoalbuminemia were present in 89.4% (95% CI, 59.8-98.0) and 60.8% (95% CI, 40.3-78.1) of patients with iMCD, and 74.9% (95% CI, 56.2-87.4) and 77.4% (95% CI, 60.8-88.3) of patients with HHV8+ MCD. Thrombocytopenia was present in fewer patients: 17.3% (95% CI, 7.0-36.9) and 37.3% (95% CI, 22.1-55.6) of patients with iMCD and HHV8+ MCD, respectively. There were no significant differences in laboratory findings between patients with iMCD and HHV8+ MCD. Patients with UCD had significantly lower rates of laboratory abnormalities vs those with iMCD or HHV8+ MCD (P < .05; Figure 3). However, elevated CRP, anemia, and hypergammaglobulinemia occurred in almost 20% of patients.
Average percentage of laboratory findings. Aggregate cohort-level data showing average percentage of laboratory findings in patients with UCD, iMCD, or HHV8+ MCD (95% CIs) identified using a random effect meta-analysis model. ns, not significant.
Average percentage of laboratory findings. Aggregate cohort-level data showing average percentage of laboratory findings in patients with UCD, iMCD, or HHV8+ MCD (95% CIs) identified using a random effect meta-analysis model. ns, not significant.
Clinical presentation of CD
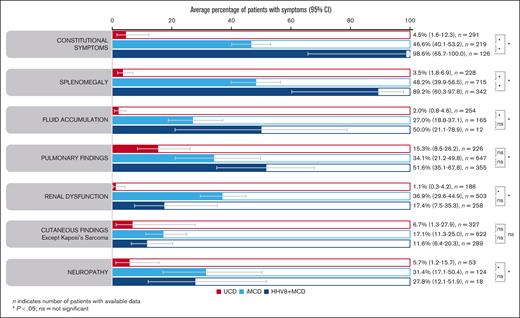
Enlarged lymph nodes were explicitly reported for almost all patients with iMCD (98.2% [95% CI, 96.8-99.0]) and HHV8+ MCD (99.8%; 95% CI, 92.5-100). Constitutional symptoms were present in significantly more patients with HHV8+ MCD (98.6% [95% CI, 65.7-100]) than with iMCD (46.6% [95% CI, 40.1-53.2]; P = .038; Figure 4). This was reflected in the significantly higher rate of fever reported in 317 patients with HHV8+ MCD (96.2% [95% CI, 76.4-99.5]) vs 710 with iMCD (48.4% [95% CI, 36.1-60.9]; P = .007). Splenomegaly was also present in significantly more patients with HHV8+ MCD (89.2% [95% CI, 60.3-97.8]) than with iMCD (48.2% [95% CI, 39.9-56.5]; P = .031; Figure 4). Similarly, hepatomegaly was observed in significantly more patients with HHV8+ MCD (74.1% [95% CI, 35.9-93.6; n = 31]) than with iMCD (27.7% [95% CI, 23.5-32.3 ;n = 390]; P = .038).
Average percentage of symptoms. Aggregate cohort-level data showing average percentage of symptoms in patients with UCD, iMCD, or HHV8+ MCD (95% CIs) identified using a random effects meta-analysis model. ns, not significant.
Average percentage of symptoms. Aggregate cohort-level data showing average percentage of symptoms in patients with UCD, iMCD, or HHV8+ MCD (95% CIs) identified using a random effects meta-analysis model. ns, not significant.
Fluid accumulation, pulmonary findings, and cutaneous findings were less frequently seen but were found at similar ranges in patients with iMCD and HHV8+ MCD (Figure 4). Renal dysfunction was significantly more common in iMCD (36.9% of 503 patients) than HHV8+ MCD (17.4% of 258 patients; P = .04; not significant after FDR correction, P = .085; Figure 4). The higher rates of renal dysfunction in patients with iMCD vs HHV8+ MCD were also apparent in the patient-level data set (45.3% of 97 patients with iMCD vs 11.8% of 18 with HHV8+ MCD). Interestingly, neuropathy was reported in 31.4% (95% CI, 17.1-50.4) and 27.8% (95% CI, 12.1-51.9) of patients with iMCD and HHV8+ MCD, respectively. Except for cutaneous findings, patients with UCD had significantly lower rates of symptoms than patients with iMCD or HHV8+ MCD (Figure 4); however, pulmonary findings were observed in 15.3% (95% CI, 8.5-26.2) of cases. These findings (from aggregated cohort-level data) were also observed in the patient-level data set (supplemental Table 2).
It is noteworthy that there was considerable between-patient variability in clinical presentation of each CD subtype, as shown by the large range of symptoms reported between studies of the same subtype (supplemental Figure 1). For example, rates of pulmonary findings ranged from 14.0% to 88.9% in iMCD studies, and from 21.1% to 85.7% in HHV8+ MCD studies. Rates of anemia ranged from 50.0% to 100% in iMCD studies, and from 48.1% to 94.7% in HHV8+ MCD studies.
Pediatric presentation (UCD)
In publications exclusively describing pediatric CD, insufficient iMCD cases were available to draw conclusions about iMCD presentation in children and adolescents (n = 16; Table 3). No cases of HHV8+ MCD were reported. Characteristics of 88 pediatric/adolescent patients with UCD are shown in supplemental Table 3. Average median age was 11.8 years (range, 1.3-22). Sex distribution was even (50% female). Ethnicity was only reported for 27 patients. UCD location was reported for most patients (98%); more than half (53.5%) were head and neck manifestations, 24.4% were abdominal, and 16.3% were thoracic.
Symptoms and laboratory findings in pediatric UCD cases are shown in supplemental Table 4. Of note, pediatric patients with UCD had more signs of systemic inflammation than adult patients with UCD; 45.1% (95% CI, 20.9-71.8; n = 50) of pediatric patients with UCD had elevated CRP (vs 19.7% of adults [95% CI, 11.2-30.9]). Constitutional symptoms were reported in 44.4% (95% CI, 21.5-69.2; n = 18) of patients, significantly higher than the rate in adult patients with UCD patients (4.5% [95% CI, 1.6-12.3]; P = .0005).
Discussion
Our review provides a comprehensive analysis of the clinical features of CD subtypes. We found many similarities between patients with iMCD and HHV8+ MCD, most had enlarged lymph nodes and signs of inflammation. Hematological abnormalities (eg, anemia), as well as signs of organ dysfunction (eg, hypoalbuminemia) and immune activation (eg, hypergammaglobulinemia), were common and found at similar ranges in both groups. Thrombocytopenia, fluid accumulation, and pulmonary and cutaneous findings were less frequent, and at similar ranges in both groups. Neuropathy was reported in a larger proportion of patients than typically recognized (∼30% for iMCD and HHV8+ MCD).
Some differences between subtypes were apparent. Patients with iMCD had lower rates of constitutional symptoms, fever, and splenomegaly than patients with HHV8+ MCD. Although this likely reflects heterogeneity of pathomechanisms, constitutional symptoms, cytopenia, and hepatosplenomegaly occur in untreated HIV infection, therefore the lower rate of constitutional symptoms in iMCD vs HHV8+ MCD may reflect our study timeframe, which encapsulates a period with high rates of HIV infection and AIDS-related deaths.62 In a French study comparing 27 iMCD cases with 169 HHV8+ MCD cases over 20 years, HHV8+ MCD displayed a more aggressive clinical and biological presentation than iMCD.3 However, iMCD should not be considered benign: with 5-year mortality rates of 23% to 49%,15 patients may develop organ failure, and MCD sometimes progresses to non-Hodgkin lymphoma.63
Interestingly, renal dysfunction was more frequently found in patients with iMCD vs UCD and HHV8+ MCD (nonsignificant after FDR correction). Similarly, in an analysis of 271 patients with iMCD from a US health care claims database, renal dysfunction was reported in a third of patients with iMCD.64 Many patients with iMCD may present with TAFRO (thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly) syndrome, an iMCD variant displaying symptoms including renal insufficiency.65,66 Because many studies were performed before the introduction of the TAFRO definition, there were insufficient data in this study to distinguish patients with iMCD-TAFRO from those without TAFRO.
We confirmed findings from previous reviews suggesting that systemic symptoms may be present in UCD.8 Although UCD is typically thought of as an asymptomatic disease with solitary lymphadenopathy, intermediate cases may exist, namely UCD with inflammatory symptoms and MCD with only a few stations affected.6 In our analysis, inflammation signs (eg, elevated CRP) occurred in almost 20% of patients with UCD; and anemia, hypergammaglobulinemia, and pulmonary findings were seen in 15% to 20% of cases. Whether these cases represent a distinct entity remains to be elucidated. The relatively high rate of systemic inflammation in pediatric UCD may suggest differences in etiology between UCD age groups. We also confirmed that sex distribution shows a predominance of males in HHV8+ MCD and iMCD, whereas women appear slightly more often affected with UCD.8,64 These findings were apparent in pediatric patients and across ethnicities.
We revealed some differences vs previous reviews. Compared with a literature review of 128 iMCD cases (1995-2015),9 we found markedly lower rates of symptoms in iMCD; Liu et al reported fluid accumulation in 74% of patients (vs 27% in our analysis), organomegaly in 78% (vs 48%), renal dysfunction in 71% (vs 37%), and thrombocytopenia in 44% (vs 17%).9 This may be because of publication bias toward atypical and severe cases as, in contrast to our analysis (limited to studies with ≥5 cases), many individual case reports were included. In contrast, a retrospective study of 180 patients with HHV8− MCD, mainly from China, published rates of symptoms similar to ours.67 We also observed marked differences between studies analyzing the same subtypes. Another Chinese meta-analysis (1634 patients with CD) identified 139 patients with asymptomatic iMCD.68 Our study yielded no asymptomatic patients, but symptoms may not have been extensively reported in the studies analyzed. Recently, Mukherjee et al mapped an inventory of 27 symptoms categorized by organ system in patients with iMCD and evaluated symptom impact in 11 domains of daily living. Their findings suggest that symptom burden in iMCD may be more extensive than previously thought.69
It should be noted that most iMCD (and UCD) cases came from China and Japan (driven by more publications from these regions), whereas most HHV8+ MCD data were from the United States and Europe (driven by more publications from these regions). The disproportionate number of cases from certain regions of the world is an important limitation of our work. Although the impact of ethnicity on clinical presentation of CD is unknown, it is possible that CD presentation in Asian patients may differ from those in other geographic locations, limiting the generalizability of our results. It is notable that rates of fluid accumulation and low platelets were remarkably low (<10%) in the largest study from Japan.24 In fact, the authors noted differences in symptoms such as pulmonary involvement, secondary amyloidosis, electrocardiogram abnormalities, and rates of HIV and HHV8 infection between Japanese patients with MCD and those of other countries. Moreover, rates of thrombocytopenia were higher in patients with iMCD outside Japan, approaching those of HHV8+ MCD. We know that a substantial portion of patients with iMCD in Japan have TAFRO syndrome and thus thrombocytopenia, so this observation may be a result of publication bias. Publication bias can arise when a majority of studies included in a meta-analysis are from a specific region. Funding sources, and cultural and political factors, can influence publication decisions.70 Therefore factors such as local research priorities, diagnostic practices, methodology (eg, study design preference, clinical reporting standards), or local health care practices could influence publication of results. The limited geographical and ethnic diversity of patients included in our analysis is important to consider because our data may underrepresent certain racial or ethnic groups for whom CD may present differently to the predominantly Asian population in our analysis. Further publication of CD symptomatology in diverse populations is essential to continue to improve our understanding, and diagnosis, of patients with this rare disease. Ongoing enrollment of patients from around the world, particularly from the United States and other English-speaking countries, in the Advancing Castleman Care with an Electronic Longitudinal Registry, E-Repository, and Treatment/Effectiveness Research Natural History Registry will help to fill in these gaps.
Given the rarity of CD, the number of studies available for inclusion in the meta-analyses was sometimes small, affecting precision of the results. This work has limitations because of uncontrolled data collection. Symptoms and laboratory parameters were not systematically collected, and few studies followed consensus guidelines for iMCD.4 Because we collected CD diagnosis/classification determined by study investigators, we were unable to exclude misdiagnosed cases. There were likely cases included that would not meet the histopathological definition of iMCD or UCD. Furthermore, many publications did not report HHV8 status and very few described patients with POEMS.
Evidence of statistical heterogeneity was identified in many of the meta-analyses and this was incorporated via random effects. Heterogeneity may be due to a combination of clinical (variability between patients and outcomes) and/or methodological diversity between studies (processes, data collection tools, etc). It was not possible to formally explore causes of heterogeneity because there were insufficient studies to reliably investigate this. Therefore, it is not possible to explore the extent to which differences in estimates between subtypes may be due to clinical or methodological diversity. In addition to the aggregated data, we evaluated and compared studies with individual patient-level data (supplemental Material). This served as an internal control; analysis of patient-level data largely confirmed findings from the aggregated data, and differences between iMCD and HHV8+ MCD remained consistent in all evaluations. Although the patient-level data provide support for our findings from the aggregated data, they are also subject to the same limitations as the aggregated data set, namely uncontrolled data collection, heterogeneity, and geographical limitations. Although these limitations could impact the generalizability of our results, our study adds value to existing evidence by providing information that can help distinguish the presentation of CD subtypes and determine a correct diagnosis.
This review found many similarities between iMCD and HHV8+ MCD, but also some differences regarding symptoms and laboratory findings, likely reflecting heterogeneity of underlying pathological mechanisms and comorbidities. Our description of the clinical presentation of CD complements diagnosis and management guidelines for iMCD and UCD and provides a detailed description of the clinical presentation of HHV8+ MCD. We hope this will help physicians better identify and diagnose these rare diseases. Additionally, our work highlights inconsistent reporting of symptoms and laboratory parameters in the literature. To further our collective understanding of these diseases, diagnostic criteria4 should be applied to describe CD symptoms and laboratory abnormalities when reporting case presentations or studies.
Acknowledgments
The authors thank Azhaar Ashraf and Hannah Lewis of TVF Communications for their support with performing the literature review and data extraction. The authors thank Lynsey McColl of Select Statistical Services for insightful discussions and support with the statistical analyses and their interpretation.
This study was funded by Recordati Pharmaceuticals Ltd. D.C.F. acknowledges the National Institutes of Health (grant R01HL141408), the US Food and Drug Administration (grant R01FD007632), and the Castleman Disease Collaborative Network for research funding support.
Authorship
Contribution: Recordati Pharmaceuticals Ltd conceived and designed the analysis in collaboration with C.H., had full access to the data, revised the manuscript critically for important intellectual content, and approved the final version of the manuscript; L.G. performed the literature search, data collection, and data extraction; S.L. performed statistics; C.H. and K.K. conceived and designed the analysis; C.H. assessed and verified data and wrote the manuscript with substantial contributions and support from L.G. and S.L.; D.C.F. and E.O. critically evaluated the methodology, analysis, and data interpretation; and all authors had full access to the data, revised the manuscript critically for important intellectual content, and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.H., E.O., and D.C.F. have received consulting fees from Recordati Pharmaceuticals. S.L. has received payment from TVF Communications for statistical analysis. L.G. has received payment from TVF Communications for medical writing. C.H. and E.O. have received honoraria from Recordati Pharmaceuticals. E.O. has participated in an advisory board for Recordati Pharmaceuticals. D.C.F. has received institution payments from Recordati Pharmaceuticals. K.K. is an employee of Recordati Pharmaceutical.
Correspondence: Christian Hoffmann, Infektionsmedizinisches Centrum Hamburg (Stadtmitte), Glockengießerwall 1, 20095 Hamburg, Germany; email: hoffmann@ich-hamburg.de.
References
Author notes
The data that support the findings of this study are available upon reasonable request from the corresponding author, Christian Hoffmann (hoffmann@ich-hamburg.de).
The full-text version of this article contains a data supplement.