Key Points
Geriatric vulnerabilities are frequent among older adults evaluated for CAR-T therapy, enabling risk stratification before therapy.
Older adults with high vulnerability uncovered by GA experienced high toxicity and poor outcomes after CAR-T therapy.
Visual Abstract
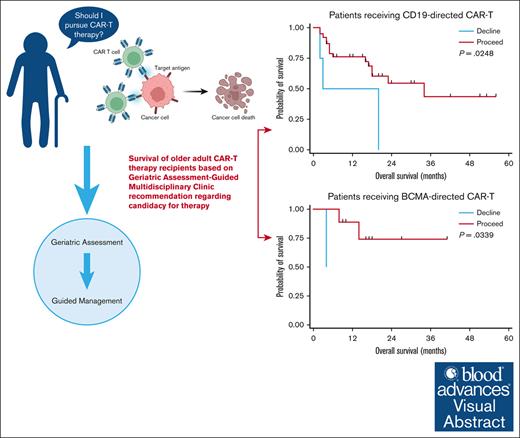
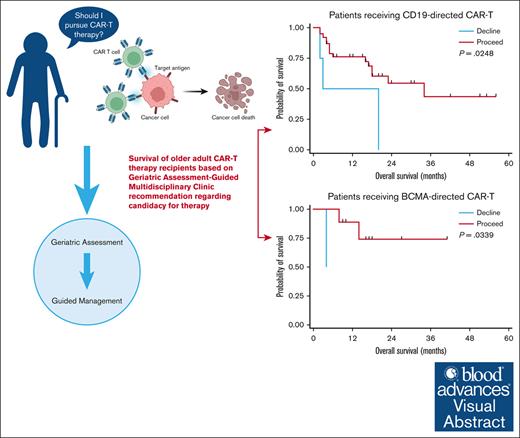
The optimal means of assessing candidacy of older adults (≥65 years) for chimeric antigen receptor T-cell (CAR-T) therapy are unknown. We explored the role of a geriatric assessment (GA)–guided multidisciplinary clinic (GA-MDC) in selecting and optimizing older adults for CAR-T. Sixty-one patients were evaluated in a GA-MDC (median age, 73 years; range, 58-83). A nonbinding recommendation (“proceed” or “decline”) regarding suitability for CAR-T was provided for each patient based on GA results. Fifty-three patients ultimately received CAR-T (proceed, n = 47; decline, n = 6). Among patients who received B-cell maturation antigen (BCMA)–directed (n = 11) and CD19-directed CAR-T (n = 42), the median overall survival (OS) was 14.2 months and 16.6 months, respectively. GA uncovered high rates of geriatric impairment among patients proceeding to CAR-T therapy, with fewer impairments in those recommended “proceed.” Patients recommended “proceed” had shorter median length of stay (17 vs 31 days; P = .05) and lower rates of intensive care unit admission (6% vs 50%; P = .01) than those recommended “decline.” In patients receiving CD19- and BCMA–directed CAR-T therapy, a “proceed” recommendation was associated with superior OS compared with “decline” (median, 16.6 vs 11.4 months [P = .02]; and median, 16.4 vs 4.2 months [P = .03], respectively). When controlling for Karnofsky performance status, C-reactive protein, and lactate dehydrogenase at time of lymphodepletion, the GA-MDC treatment recommendation remained prognostic for OS (hazard ratio, 3.26; P = .04). Patients optimized via the GA-MDC without serious vulnerabilities achieved promising outcomes, whereas patients with high vulnerability experienced high toxicity and poor outcomes after CAR-T therapy.
Introduction
The use of chimeric antigen receptor T-cell (CAR-T) therapy has demonstrated considerable success in the treatment of an increasing number of relapsed/refractory (R/R) hematologic malignancies.1-5 The malignancies most commonly treated with CAR-T therapy, multiple myeloma (MM) and diffuse large B-cell lymphoma (DLBCL), are diseases of older adults, yet the median age of patients treated on the pivotal clinical trials leading to the approval of these agents skewed substantially younger.4-7 Nationally representative real-world experience mimics this bias, with low uptake of CAR-T therapy in older patients, particularly those aged ≥75 years.8 Concerns in applying CAR-T therapy to this population include higher comorbid burden or health limitations that may predispose to disproportionate toxicity or inferior disease-free longevity. These biases may deprive older adults from potentially effective therapy.9-12
Evidence to support the administration of CAR-T therapy in older patients, largely defined as those aged ≥65 years, is mixed. Some analyses have demonstrated higher rates of neurotoxicity and possibly nonrelapse mortality (NRM) in this population.13-15 Nevertheless, these same analyses demonstrated comparable or even improved response rates and progression-free survival (PFS) in older patients compared with their younger counterparts.13-16 One barrier to increased utilization of CAR-T therapy in older adults may be the lack of clinical tools to reliably predict toxicity and prognosis.17-22 Candidacy for CAR-T therapy is ill-defined, particularly compared with the candidacy for hematopoietic cell transplantation. As treatment paradigms for R/R MM and DLBCL evolve to include CAR-T therapy in earlier lines with simultaneous ongoing concern of product scarcity, refining this definition and developing clinical tools to predict outcomes will facilitate patient and treatment selection. Validated metrics of assessing CAR-T candidacy are emerging, with a particular focus on comorbidities, but the impact that frailty plays on post–CAR-T outcomes is not well described.23
Frailty is an aging-related syndrome of diminished physiologic reserve, resulting in an increased susceptibility to poor health outcomes, and can be identified and quantified by the geriatric assessment (GA). The GA goes beyond age, performance status, and comorbidities to comprehensively assess health domains including physical and functional status, cognition, polypharmacy, nutrition, and social support.24,25 We have previously published the results of our GA–guided multidisciplinary clinic (GA-MDC) before hematopoietic cell transplantation, demonstrating the program’s efficacy in guiding patient selection, intervening on geriatric vulnerabilities, and mitigating NRM.26,27
Herein, we report our experience implementing the GA-MDC in older adults under consideration for CAR-T therapy. We describe baseline geriatric frailties in this population and summarize post–CAR-T therapy outcomes including toxicity, health care utilization, and survival according to baseline results.
Materials and methods
Patient population
The processes and mechanics of the University of Chicago GA-MDC have previously been described.26 Beginning March 2015, our center implemented a programmatic standard requiring patients aged ≥70 years under consideration for CAR-T therapy be seen in the GA-MDC before receiving therapy. This age threshold was chosen based on the higher risk of mortality seen with transplant recipients aged ≥70 years, and the expectation that this age clinically reflected a threshold in which physicians may not offer CAR-T due to concerns about tolerability.9 Patients aged <70 years could be referred at the discretion of the treating physician. The University of Chicago Institutional Review Board granted approval to retrospectively review patient data. The data were maintained in a research electronic data capture clinical database supported by the University of Chicago.
GA-MDC
As described previously, the University of Chicago GA-MDC uses a modified cancer-specific GA.24,28,29 Measures for each domain of the GA, including impairment thresholds, are summarized in Table 1. All patients are aimed to be evaluated in GA-MDC clinic within 2 to 6 weeks before the planned admission for CAR-T. The GA-MDC consists of a single day, serial evaluation by a cellular therapy physician, an advanced practice provider, an infectious disease physician, a physical therapist, a dietician, a pharmacist, and a social worker. Team members provide personalized optimization recommendations to the referring physician and to each patient within each domain of the GA. A focus is placed both on (1) optimizing the patient for the treatment plan (eg, recommendation of a course of physical therapy for impaired physical function; referral to a specialist for optimization of an active comorbidity; dietician consultation to improve caloric intake in patients with history of recent weight loss); and (2) optimizing the treatment plan for the patient (eg, instating delirium precautions or more rigorous monitoring for a patient perceived to be high risk for neurotoxicity based on impaired cognitive testing or prior history of hospital-acquired delirium). Finally, a nonbinding, summary recommendation regarding suitability for CAR-T therapy is provided to the referring physician: “proceed” if the patient should proceed without delay; “defer” if significant optimization is considered necessary before CAR-T therapy; or “decline” if risk of therapy is deemed unacceptable even with health optimization or if necessary conditions are not in place (eg, lack of caregiver). The consensus recommendation is generated based on the perceived risks and benefits of the intervention as guided by results of the GA-MDC. Patients may return to the GA-MDC as needed for re-evaluation; recommendations from the initial GA-MDC visit were used for this analysis. Determining whether patients were successfully optimized was decided by a medical record review (conducted by S.J.Y. and M.T.N.).
Covariates, toxicity, and response assessment
Baseline patient, disease, and treatment details were prospectively collected at the time of GA-MDC evaluation and at the time of leukapheresis. Data regarding toxicity and response assessments were abstracted through medical record review. Short-term outcomes included length of stay (LOS) of index hospitalization; readmission to the hospital within 100 days of receipt of CAR-T therapy; admission to the intensive care unit (ICU) during index hospitalization; incidence and grade of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) according to the American Society for Transplantation and Cellular Therapy Consensus Criteria; need for inpatient or outpatient rehabilitation after discharge; and response to CAR-T therapy according to the Lugano criteria for lymphoma and International Myeloma Working Group Uniform Response criteria for MM.43-46 Date of last follow-up and survival status were also collected. Patients were censored at the time of relapse/progression after CAR-T therapy, or at the time of last follow-up.
Statistical analyses
Comparisons of baseline variables (Table 2) were done between (1) receipt of CAR-T and (2) GA-MDC recommendation decision using Fisher exact tests for categorical measures and Wilcoxon tests for continuous measures. Overall survival (OS) and PFS estimates were calculated using Kaplan-Meier methods. The log-rank statistic was used to compare the differences in OS and PFS between GA-MDC recommendation groups. Additional subgroup survival and comparisons were done by CAR-T therapy product and by GA-MDC component measures. In multivariable analyses for OS, we controlled for physician–reported Karnofsky performance status, C-reactive protein (CRP), and lactate dehydrogenase (LDH) at the time of lymphodepleting therapy because these markers have been associated with negative clinical and functional outcomes after CAR-T therapy in some patient cohorts.47,41 All analyses were performed using SAS statistical software (version 9.4, Cary NC).
The University of Chicago Institutional Review Board granted approval to retrospectively review patient data.
Results
Patients
From December 2017 to April 2022, 61 patients with a median age of 73 years (range, 58-83) were evaluated in the GA-MDC before CAR-T therapy. Fifty-eight patients aged ≥70 years received CAR-T therapy at our institution during this time, 50 of whom attended the GA-MDC (86%). The reason for missed evaluations could not be identified through medical record review. All patients received CAR-T therapy inpatient.
The most common disease indications for referral were R/R DLBCL (n = 36) and MM (n = 14) (Table 2). The median number of prior lines of therapy was 2 (range, 2-3) among patients with DLBCL, and 6 (range, 4-11) among patients with MM. At the time of GA-MDC evaluation, 22 of 36 patients (67%) with DLBCL had high-risk disease as demonstrated by an International Prognostic Index score of ≥3, and 10 of 14 patients (71%) with MM had Revised International Staging System stage ≥2 disease.
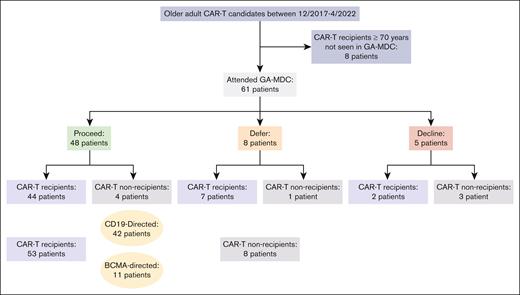
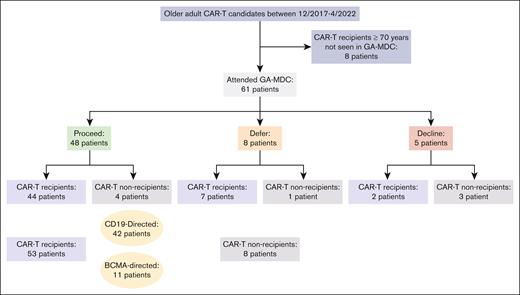
Among the 61 patients evaluated in the GA-MDC, 48 were recommended to proceed with CAR-T therapy (“proceed”), 44 of whom received CAR-T therapy (Figure 1). Eight patients were recommended for deferral until further optimization (“defer”), 7 of whom received CAR-T therapy. Among these 7 patients, 2 had successfully completed optimization recommendations that constituted the initial barrier. Five patients were recommended against proceeding with CAR-T therapy (“decline”), 2 of whom received CAR-T therapy against MDC recommendation.
Consolidated Standards of Reporting Trials (CONSORT) diagram of patients studied. Figure includes all older adults who received CAR-T at the University of Chicago between December 2017 and April 2022, most of whom were evaluated in the GA-MDC. The GA-MDC recommendation is that given at the conclusion of the GA-MDC assessment (“proceed,” “defer,” and “decline”).
Consolidated Standards of Reporting Trials (CONSORT) diagram of patients studied. Figure includes all older adults who received CAR-T at the University of Chicago between December 2017 and April 2022, most of whom were evaluated in the GA-MDC. The GA-MDC recommendation is that given at the conclusion of the GA-MDC assessment (“proceed,” “defer,” and “decline”).
Among the 36 patients with DLBCL evaluated in the GA-MDC, 28 patients were recommended “proceed,” all of whom received CAR-T therapy at a median of 29 days (range, 8-74) from GA-MDC evaluation. Six patients were recommended “defer,” 5 of whom received CAR-T therapy at a median of 33 days from GA-MDC evaluation (range, 14-119). Two patients were recommended “decline,” and neither received CAR-T therapy. Among the 14 patients with MM evaluated in the GA-MDC, 10 were recommended “proceed,” 8 of whom received CAR-T therapy at a median of 24 days (range, 10-45) from GA-MDC evaluation. One patient was recommended “defer” and went onto receive CAR-T therapy 19 days from the date of GA-MDC evaluation. Three patients were recommended “decline,” 2 of whom received CAR-T therapy.
In those 13 patients recommended “defer” or “decline,” the GA-MDC decision was guided by ≥1 of following findings: deficits in physical function and/or nutrition meeting criteria for frailty (7 patients) and severe protein-calorie malnutrition (6 patients); mild cognitive impairment warranting further diagnostic investigation (5 patients); lack of caregiver support (2 patients); uncontrolled depression (1 patient); active and/or decompensated comorbidities warranting further diagnostic investigation or evaluation by a specialist (6 patients); and uncontrolled infection (1 patient). Eleven of these 13 patients were found to have deficits in multiple domains.
In sum, 53 patients received CAR-T therapy after evaluation in the GA-MDC. Among these, 42 patients received CD19-directed products and 11 patients received B-cell maturation antigen (BCMA)–directed products. The CD19-directed products used were axicabtagene ciloleucel (9 patients), lisocabtagene maraleucel (15 patients), tisagenlecleucel (9 patients), brexucabtagene autoleucel (4 patients), and investigational products (5 patients). The BCMA-directed products used were idecabtagene vicleucel (1 patient), ciltacabtagene autoleucel (5 patients), and investigational products (5 patients). The median time from GA-MDC evaluation to CAR-Ttherapy infusion was 25 days (interquartile range, 19-33). Among the 8 patients not receiving CAR-T therapy, 6 enrolled onto hospice care, and 2 went on to receive salvage chemotherapy.
Disease characteristics including LDH at the time of lymphodepleting therapy, Revised International Staging System stage (MM only), International Prognostic Index scores (DLBCL only), and number of prior lines of systemic therapy received did not significantly differ between CAR-T therapy recipients vs nonrecipients or by GA-MDC recommendation (Table 2).
GA results
Data on baseline GA findings are summarized in Table 3. Missing data across the GA metrics were accounted for, in part, by the different iterations of GA used by the GA-MDC over the years. There were high rates of quantifiable geriatric impairments in patients evaluated by GA-MDC, including among those ultimately recommended for CAR-T therapy. Among the subgroup of patients deemed fit to proceed immediately to CAR-T therapy (ie, those recommended “proceed,” n = 48), 51% walked <400 meters on the 6-minute walk test, a threshold that is considered impaired. Furthermore, 51% were deemed frail based on grip strength, and 37% scored ≤26 on the Montreal Cognition Assessment (MOCA), a threshold consistent with mild dementia or worse. Results were similar when analyzing the cohort of 53 patients who ultimately received CAR-T therapy, who represent a largely, but not fully, overlapping group.
Patients recommended “defer”/“decline” (n = 13) had still higher rates of impairment in several GA measures than patients recommended for CAR-T therapy. In the “timed up and go” test, impairments were found in 45% of patients recommended “defer”/“decline” vs 8% among those recommended “proceed” (P = .01). In the 6-minute walk test, impairments were found in 92% of patients recommended “defer”/“decline” vs 51% recommended “proceed” (P = .01). In grip strength, impairments were found in 85% vs 51% of patients recommended “defer”/“decline” and “proceed,” respectively (P = .03).
Considering patients with MM (n = 14) had endured more lines of therapy by the time of GA-MDC assessment than patients with DLBCL (n = 36), we compared the rates of geriatric vulnerabilities between the 2 populations. Patients with MM had numerically higher rates of frailties within the physical function (timed up and go, 33.3% vs 13.3%; 4-meter walk, 42.9% vs 20.6%; P = .19 and P = .16, respectively) and functional status domains (Medical Outcomes Study physical health, 100% vs 68.4%; P = .15) without reaching statistical significance.
Toxicity, NRM, and survival
The median duration of follow-up among patients treated with CAR-T therapy (from the date of CAR-T infusion) was 16.3 months (range, 1.6-56.2). The median duration of follow-up among those not treated with CAR-T therapy (from the date of GA-MDC evaluation) was 3.9 months (range, 0-42.3). Rates of grade ≥2 CRS were 18.2% and 35.7% in the BCMA-directed (n = 11) and CD19-directed groups (n = 42), respectively. Rates of grade ≥2 ICANS rates were 9.1% and 38.1% in the BCMA-directed and CD19-directed groups, respectively. No patient died within 30 days of receipt of CAR-T therapy; 3 patients died within 100 days of infusion (causes of death attributable to cardiogenic shock in 1 patient, progression of disease in 1 patient, and neurologic deterioration considered unrelated to ICANS in 1 patient). The 1-year and 2-year NRM rates were 7.5% (4/53) and 11.3% (6/53), respectively. Of the 6 NRM deaths that occurred within 2 years of CAR-T therapy infusion, 5 were among those receiving CD19-directed CAR-T therapy. One additional nonrelapse-related death occurred beyond 2 years.
These deaths were attributed to cardiogenic shock (1 patient), COVID pneumonia (3 patients), bacteremia (1 patient), and neurologic deterioration considered unrelated to ICANS (2 patients). Of the latter 2 patients, 1 patient was admitted 599 days after CAR-T infusion with generalized weakness, dysphagia, muscle contractures, and encephalopathy. Brain imaging and lumbar puncture studies did not reveal evidence of disease relapse or explanation for the clinical findings. Notably, this patient had a history of central nervous system involvement of high-grade B-cell lymphoma and had experienced grade 4 ICANS during his admission for CAR-T. The patient was transitioned to comfort care in light of progressive symptoms. The second patient developed progressive encephalopathy 10 days after CAR-T infusion, which did not improve with ICANS therapy, and was eventually found to have BK encephalitis. Neurologic symptoms did not improve with effective treatment of the infection, and the patient was transitioned to comfort care. Her preadmission cognitive testing had been consistent with mild cognitive impairment.
Among patients who received BCMA-directed and CD19–directed CAR-T therapy, the median PFS was 10 months (range, 2.0-26.6) and 9.9 months (range, 0.9-56.0), respectively. The median OS was 14.2 months (range, 4.2-41.5) for BCMA–directed CAR-T therapy and 16.6 months (range, 1.6-56.2) for CD19-directed CAR-T therapy.
All 8 patients who did not receive CAR-T therapy were deceased by the end of the follow-up period. The median OS among this group was 3.9 months (range 1.7-41.5). Causes of death in this group were disease progression (5 patients), infection (1 patient), and second malignancy (1 patient). One patient was lost to follow-up. In this group of 8 patients, 4 had been recommended “proceed” by the GA-MDC, whereas 4 were recommended “defer”/“decline.” Reasons for not proceeding to CAR-T among the 4 patients recommended “proceed” were progression of disease (n = 2), infection (n = 1), and new multifocal brain lesions of unknown etiology rendering the patient ineligible to receive CAR-T therapy on protocol (n = 1).
Prognostication of GA measures for CAR-T outcomes
All GA measures were assessed for their univariate prognostic significance for the following outcomes: need for ICU stay during CAR-T therapy admission, LOS during CAR-T therapy admission, need for inpatient or outpatient rehabilitation after discharge, readmission rate, onset and severity of CRS and ICANS, response rate, PFS, and OS. Associations with statistical significance are listed in Table 4. Patients with CRP >3 at the time of GA-MDC had a median survival of 8.6 months vs 17.3 months in those with normal CRP (P = .03). Patients with impaired 6-minute walk at the time of GA-MDC evaluation were more likely to both require discharge to rehabilitation facility (30% of patients with impairment vs 4% of patients without impairment; P = .05) and readmission within 100 days (44% of patients with impairment vs 13% of patients without impairment; P = .05). Notably, cognitive dysfunction as measured by MOCA was not associated with onset or severity of CRS or ICANS.
Post–CAR-T morbidity and mortality according to dynamic GA-MDC recommendations
We next incorporated successful optimization after GA-MDC to reassign treatment recommendation; this revised recommendation will be referred to as the “dynamic” GA-MDC recommendation. Thus, deferred or declined patients successfully completing optimization recommendations that constituted the initial barrier were reclassified as “proceed” for the dynamic GA-MDC recommendation to better track with clinical practice. All others were classified as “decline.” Patients were considered successfully optimized if recommendations made by the GA-MDC providers were carried out. In some cases, this was a matter of diagnostic workup or referral to a specialist (eg, hepatology evaluation in a patient with cirrhosis). In cases in which frailty or malnutrition constituted the initial barrier, patients were considered successfully optimized if there was demonstrable improvement in these deficits upon re-evaluation.
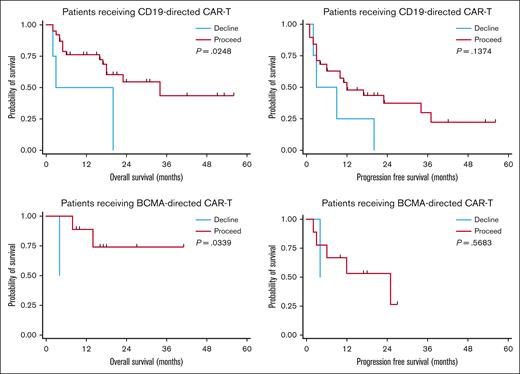
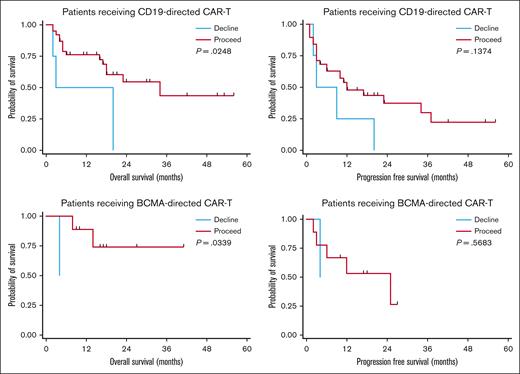
Using these definitions, 47 patients were recommended “proceed” and 6 were recommended “decline.” There was no difference in response rates or PFS in the BCMA–directed or CD19–directed treatment groups by dynamic GA-MDC recommendation. In patients evaluated by the GA-MDC who went on to receive CAR-T therapy with CD19-directed therapy (n = 42), those recommended “proceed” by this dynamic classification experienced longer OS than those recommended “decline” (median OS, 16.6 months [95% confidence interval [CI], 7.2-19.8] vs 11.4 months [95% CI, 1.7-20.0], respectively; P = .02; Figure 2). Similarly, a survival difference was detected among patients who received BCMA–directed CAR-T therapy based on dynamic GA-MDC recommendation (“proceed” median OS, 16.4 months [95% CI, 9.2-26.6]; “decline” median OS, 4.2 months [95% CI, 127 days to 129 days]; P = .03). In multivariable analyses, we controlled for MD–reported Karnofsky performance status, CRP, and LDH at the time of lymphodepleting chemotherapy for the entire cohort who received CAR-T therapy (n = 53). Dynamic GA-MDC treatment recommendation remained prognostic for OS (HR, 3.26; 95% CI, 1.16-8.08; P = .04). The association held significance in the CD19-directed subgroup (HR, 3.07; 95% CI, 1.28-10.18; P = .02) but was inestimable in the BCMA-directed subgroup.
Survival based on dynamic GA-MDC recommendation. PFS and OS among patients receiving CD19-directed or BCMA–directed CAR-T therapy stratified by GA-MDC dynamic recommendation (“proceed” vs “decline”). Deferred or declined patients successfully completing optimization recommendations that constituted the initial barrier were reclassified as proceed for the dynamic GA-MDC recommendation. All others were classified as declined.
Survival based on dynamic GA-MDC recommendation. PFS and OS among patients receiving CD19-directed or BCMA–directed CAR-T therapy stratified by GA-MDC dynamic recommendation (“proceed” vs “decline”). Deferred or declined patients successfully completing optimization recommendations that constituted the initial barrier were reclassified as proceed for the dynamic GA-MDC recommendation. All others were classified as declined.
Rates of grade ≥2 CRS and ICANS did not statistically differ by dynamic GA-MDC treatment recommendation (Table 5). Among all patients who received CAR-T therapy, those recommended “proceed” by the dynamic classification had shorter LOS (median, 17.0 vs 30.5 days; P = .05) and were less likely to require home or inpatient rehabilitation services after discharge (10.6% vs 66.7%; P = .01) than those recommended “decline.” Among patients recommended “proceed,” 3 of 47 (6.4%) were admitted to the ICU during CAR-T therapy admission, compared with 3 of 6 patients (50%) who were recommended “decline.”
Finally, we descriptively assessed the survival of the 4 patients who were recommended against CAR-T therapy and did not receive it and the 6 patients who were recommended against CAR-T therapy but did receive it. In the former category, patients experienced survival of 52 days (cause of death, septic shock), 62 days (cause of death, progression of disease), and 161 days (cause of death, different malignancy) from the time of GA-MDC evaluation. One patient enrolled in hospice shortly after GA-MDC evaluation and was subsequently lost to follow-up. These 4 patients experienced a short mean OS of 93 days, with 2 deaths unlikely to have been prevented by further treatment (septic shock in 1 patient and solid malignancy that was rapidly progressive in another). In the latter category, the mean OS was 296 days (range, 52-605) from the time of CAR-T infusion. Notably, 5 of these patients experienced NRM, with 3 of these events occurring within 4 months of CAR-T infusion (due to cardiac toxicity in 1 patient, COVID pneumonia in 1 patient, and neurotoxicity in 1 patient).
Discussion
We report on our experience with a GA-MDC in evaluating older adults under consideration for CAR-T therapy. We found that GA-MDC treatment recommendation regarding patient suitability for CAR-T therapy, derived from the results of the GA, was associated with survival after CAR-T therapy even when controlling for certain traditional prognostic factors. Additionally, GA-MDC treatment recommendation was associated with duration of hospitalization, need for ICU admission, and need for rehabilitation upon discharge.
Our findings add to the growing body of literature supporting the role of GA in evaluating and optimizing older adults pursuing cellular therapy. Similar to other reports, we noted high rates of geriatric vulnerabilities in older adults receiving CAR-T therapy, which facilitated the GA-MDC risk stratification.22,48 Zhang et al found an abbreviated GA used to categorize patients as fit vs unfit/frail was predictive of survival in 31 R/R patients with DLBCL receiving CD19-directed CAR-T therapy.22 In contrast to our findings, the survival advantage in the study by Zhang et al was mediated by higher response rates seen in fit patients; intriguingly, these patients experienced higher CAR-T expansion within 30 days after infusion than patients deemed unfit/frail. Recently, Rejeski et al reported that patients with increased abdominal fat and muscle mass experienced the highest response rates after CD19-directed CAR-T therapy, highlighting the capacity of adipose tissue to modulate antitumor immune responses. Such studies offer some biological basis for the detrimental impact of frailty including impaired nutritional status on outcomes in cellular therapy recipients.47,49
In this study, the survival difference we detected between fit vs frail patients was due to a high rate of NRM in frail CAR-Ttherapy recipients rather than differences in response rates or duration. Of 53 recipients, 7 suffered deaths unrelated to disease progression. Five of these patients had been recommended to defer CAR-T therapy until significant health optimization could be achieved; recommendations that were not implemented due to time constraints. Three of these 5 patients were admitted to the ICU during their admission for CAR-T therapy.
It may be argued that the GA-MDC, by recommending against CAR-T therapy in certain patients, is withholding potentially life-saving therapy from patients who might benefit despite their frailty. To address this concern, we evaluated the outcomes of patients who were recommended against CAR-T therapy and did not receive it, descriptively comparing them against outcomes of those who were recommended against CAR-T therapy but received it. The patients in the former group experienced a short mean survival of ∼3 months, with almost half of them passing away from causes unlikely to have been prevented by disease-directed therapy. Among those patients in the latter group, mean survival was longer at 296 days after CAR-T (range, 52-607), indicating there were indeed patients who derived durable benefit. However, 5 of the 6 patients in this group experienced NRM, with 3 of these events occurring early after CAR-T infusion.
These data highlight 2 key points in evaluating older and particularly frail or comorbid patients for CAR-T therapy: first, shared decision-making is essential in determining whether incurring a high risk of toxicity for potential therapeutic benefit is within a patient’s goals of care; second, examining health-related quality of life longitudinally in CAR-T therapy recipients as well as in those who ultimately defer therapy are necessary data to support informed decision-making by patients and physicians alike.50
Identifying predictors of ICANS is of particular interest in older adults, given their apparent increased susceptibility to this toxicity.13,14,51 We found functional impairment to be associated with ICANS development. Lin et al prospectively assessed the impact of a geriatric consultation in 48 patients aged ≥65 years with DLBCL undergoing CD19-directed CAR-T therapy and found that polypharmacy and mobility impairment were associated with ICANS development.48 Interestingly, in neither study was baseline cognitive impairment, as assessed by MOCA, associated with neurotoxicity.
We note that no individual GA variable was predictive of survival in our analysis, whereas the sum of these variables used to generate a GA-MDC treatment recommendation was predictive. This highlights the value of a multidisciplinary approach to health assessment when evaluating older adults for cellular therapy. Lin et al48 found that the intervention of geriatrics consultation had no impact on survival in older recipients of CAR-T therapy. Notably, the implementation of recommendations from the geriatrics consultation were not tracked or accounted for in that study. Our study analyzed the difference in outcomes in patients who were and were not optimized as recommended after evaluation in the GA-MDC, which likely accounts for the difference in findings.
Limitations of our work include missing data from the baseline GA assessment due to different iterations of the GA used during the study period, the retrospective nature of the analysis, and the small sample size particularly in regard to patients receiving BCMA-directed therapy and patients recommended against CAR-T therapy. Nonetheless, we still detected a significant survival difference based on MDC–generated treatment recommendation driven by a strikingly high rate of NRM among patients who were inadequately optimized after the MDC evaluation and/or were recommended against treatment. Data on some known prognostic factors including comorbidities were not used due to being incomplete or not available for this cohort (eg, pulmonary function testing not routinely done). Certain comorbidities, particularly if decompensated, undoubtedly played into the decision to defer therapy.23,47 Additionally, this study examines outcomes in patients receiving various types of CAR-T products including investigational and commercial agents; the purpose of our study, however, was not to comment on or compare the efficacy of these different products but to assess the utility of the GA-MDC in older CAR-T therapy recipients more broadly and regardless of product received. Finally, it must be acknowledged that there was some degree of subjectivity in how the results of the GA were synthesized to generate a treatment recommendation. The intent of the assessment is to arm clinicians with objective data regarding patient fitness across health domains to enable more informed decision-making on a patient-by-patient basis, rather than to set absolute thresholds for treatment.
At our center, most patients eligible for GA-MDC evaluation underwent GA (53 of 61 patients). We believe the GA-MDC framework may be broadly applicable because all the personnel are routinely engaged in evaluation and management of CAR-T therapy patients at our institution (eg, physical therapist, CAR-T therapy physician, dietician, social worker, and pharmacist). Less resource intensive models of GA with management warrant investigation in this setting. Guidelines now exist proposing all patients with cancer aged ≥65 years undergo a practical GA with management approach.52 Given the high rates of frailty seen in older adults newly diagnosed with DLBCL and MM, we believe the GA-MDC will continue to play an important role in patient selection even as approvals for CAR-T therapy expand to include earlier use.53,54
The GA-MDC enables identification of older patients susceptible to poor outcomes after CAR-T therapy. Future investigation should prioritize the development of strategies to mitigate the high rates of post–CAR-T toxicities and excess NRM seen in vulnerable patients. Considerations include whether such patients may be better served by alternative therapies such as bispecific antibodies or by treatment delays using bridging therapy to allow for time to address deficits uncovered by the GA. The GA-MDC could also be explored as a means of identifying patients at low risk for major complications and thus suitable for outpatient administration of CAR-T therapy, to limit resource utilization and costs associated with the therapy.
In summary, our findings suggest that comprehensive health assessment in older adults who are candidates for CAR-T therapy allows for effective risk stratification. GA-MDC optimized patients without serious vulnerabilities achieved promising outcomes; in contrast, patients with high vulnerability experienced high toxicity and poor outcomes.
Authorship
Contribution: S.J.Y. wrote the manuscript, aided in data collection, and interpreted data; J.F.C. performed analyses and assisted in manuscript writing; A.A., M.R.B., B.A.D., S.K., P.A.R., J.K., and A.J. edited the manuscript; K.K., M.M., A.A., and S.J. assisted in data collection; and M.T.N. designed the study, interpreted data, assisted in data collection, and assisted in manuscript writing.
Conflict-of-interest disclosure: A.A. serves on the advisory board of AstraZeneca and Magenta Therapeutics, and serves as a consultant to AbbVie. B.A.D. serves as a consultant for Janssen and Cota, Inc, and is an independent reviewer for clinical trial for Bristol Myers Squibb (BMS). A.J. reports consulting and serving on the advisory boards with honoraria for AbbVie, Amgen, BMS, Gracell, GSK, Janssen, and Sanofi. P.A.R. has served as a consultant and/or advisory board member for AbbVie, Novartis, BMS, ADC Therapeutics, Kite/Gilead, Sana Biotechnology, Nektar Therapeutics, Nurix Therapeutics, Intellia Therapeutics, CVS Caremark, Genmab, BeiGene, Janssen, and Pharmacyclics; received honoraria from Novartis; and research support from BMS, Kite Pharma, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, AstraZeneca, Genentech, and Tessa Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Mariam T. Nawas, University of Chicago Comprehensive Cancer Center, 5841 S. Maryland Ave, Chicago, IL 60637; email: nawasm@uchicagomedicine.org.
References
Author notes
Presented in abstract form at the 2023 Tandem Meetings | Transplantation and Cellular Therapy Meetings of American Society of Transplantation and Cellular Therapy (ASTCT) and Center for International Blood and Marrow Transplant Research (CIBMTR), Orlando, FL, 17 February 2023.
Original data are available upon reasonable request from the corresponding author, Mariam T. Nawas (nawasm@uchicagomedicine.org).