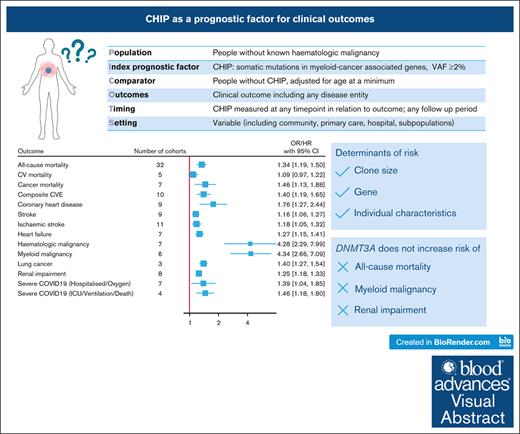
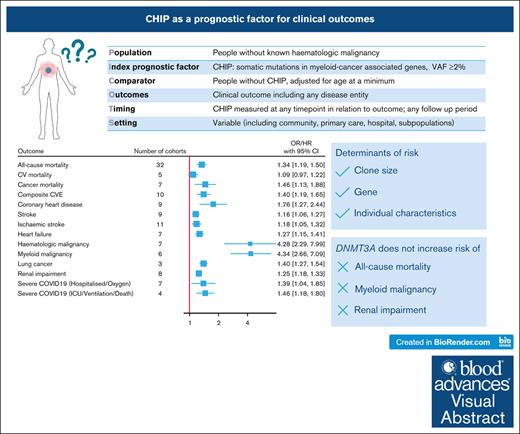
Visual Abstract
With advances in sequencing, individuals with clonal hematopoiesis of indeterminate potential (CHIP) are increasingly being identified, making it essential to understand its prognostic implications. We conducted a systematic review of studies comparing the risk of clinical outcomes in individuals with and without CHIP. We searched MEDLINE and EMBASE and included original research reporting an outcome risk measure in individuals with CHIP, adjusted for the effect of age. From the 3305 studies screened, we included 88 studies with 45 to 470 960 participants. Most studies had a low-to-moderate risk of bias in all domains of the Quality in Prognostic Factor Studies tool. Random-effects meta-analyses were performed for outcomes reported in at least 3 studies. CHIP conferred an increased risk of all-cause mortality (hazard ratio [HR], 1.34; 95% confidence interval, 1.19-1.50), cancer mortality (HR, 1.46; 1.13-1.88), composite cardiovascular events (HR, 1.40; 1.19-1.65), coronary heart disease (HR, 1.76; 1.27-2.44), stroke (HR, 1.16; 1.05-1.28), heart failure (HR, 1.27; 1.15-1.41), hematologic malignancy (HR, 4.28; 2.29-7.98), lung cancer (HR, 1.40; 1.27-1.54), renal impairment (HR, 1.25; 1.18-1.33) and severe COVID-19 (odds ratio [OR], 1.46; 1.18-1.80). CHIP was not associated with cardiovascular mortality (HR, 1.09; 0.97-1.22), except in the subgroup analysis restricted to larger clones (HR, 1.31; 1.12-1.54). Isolated DNMT3A mutations did not increase the risk of myeloid malignancy, all-cause mortality, or renal impairment. The reasons for heterogeneity between studies included differences in definitions and measurements of CHIP and the outcomes, and populations studied. In summary, CHIP is associated with diverse clinical outcomes, with clone size, specific gene, and inherent patient characteristics important mediators of risk.
Introduction
As people age, many acquire mutations in hematopoietic stem cells, resulting in clonal hematopoiesis. Although clonal hematopoiesis is a hallmark of blood cancers, it has long been recognized that large chromosomal alterations, such as loss of Y chromosome1,2 and alterations affecting autosomes,3,4 can occur in people without evidence of hematologic malignancy. Mutation-driven clonal hematopoiesis was first described in 2012 when acquired TET2 mutations were linked to age-associated skewing of X-chromosome inactivation.5 However, it was only through 3 large studies in 2014 that the high prevalence of clonal hematopoiesis characterized by point mutations and small insertion-deletions was appreciated.6-8 The most frequent genes mutated were those known to be involved in myeloid blood cancers; however, only a minority of individuals develop blood cancers, leading to the term clonal hematopoiesis of indeterminate potential (CHIP).9 Methods to detect CHIP include whole genome sequencing, whole exome sequencing, targeted sequencing, and error-corrected sequencing, which differ in their sensitivity and have differing specificity due to variability in gene coverage and criteria for variant calling. CHIP is formally defined by the World Health Organization (WHO) and International Consensus Classification (ICC) as the presence of ≥1 acquired mutations in genes associated with myeloid blood cancers at a variant allele frequency (VAF) of ≥2% and in the absence of a known hematologic disorder or unexplained cytopenia.10,11 Mutations in genes not involved in myeloid cancers also occur, although the clinical significance of these is less well studied and these are not included in the WHO and ICC definition of CHIP.9 The presence of unexplained cytopenias in conjunction with CHIP-like mutations defines the entity of clonal cytopenias of undetermined significance (CCUS).
A seminal study reported that CHIP is associated with an increased risk of all-cause mortality attributed to cardiovascular (CV) mortality.7 Since then, others have studied the association between CHIP and all-cause mortality and CV outcomes12-31 but their results have been inconsistent. Furthermore, there are an increasing number of studies reporting associations between clonal hematopoiesis and other outcomes, including heart failure (HF),19,22,24,28,32-34 osteoporosis,35 chronic liver disease,36 nonhematological cancers21,28,37 and severe COVID-19 disease.17,38-41 The observation that CHIP increases in prevalence with age and is associated with diseases of aging raises the possibility that CHIP is a passive biomarker of biological age and the association with outcomes is merely correlation rather than an underlying causative effect. However, there is experimental evidence that CHIP may cause a range of nonhematological diseases by promoting the expression of proinflammatory cytokines, such as interleukin-6 (IL-6), IL-1β, and IL-20, which then enhance, for example, atherosclerosis, HF, or osteoclast differentiation to cause osteoporosis.27,35,42,43
Overall, only a minority of individuals with CHIP develop blood cancer during their lifetimes.9 Factors associated with a higher risk of progression include a larger VAF, ≥2 mutations, and mutations in specific genes,7,17,44-48 particularly spliceosome genes (such as SRSF2, SF3B1, and U2AF1), IDH2, JAK2 (particularly for myeloproliferative neoplasms45), and TP53 for therapy-related myeloid neoplasms.49,50 Two risk prediction models (Clonal Hematopoiesis Risk Score44 and MN_Predict45) have recently been developed to predict the absolute risk of myeloid neoplasms in people with CHIP or CCUS. These incorporate genetic features, age, abnormal blood count parameters, and (for MN_Predict45) other biochemistry results. Although specific predictive models have not been validated for other outcomes (such as all-cause mortality or CV disease), clone size, gene, and number of mutations may also influence the risk of nonhematologic outcomes. For example, CV risk is influenced by VAF and genes, with DNMT3A conferring a lower risk than TET2 or spliceosome mutations.7,15,25,27,51
As people with CHIP are increasingly being identified in routine clinical practice, it is important to understand the prognostic implications of CHIP to guide the appropriate monitoring or surveillance of individuals. It is also an important first step in understanding the diverse mechanisms by which CHIP may cause disease, which could be targeted by treatment or preventive strategies.
To better define the role of CHIP as a prognostic factor, we conducted a systematic review to identify outcomes associated with CHIP (Table 1). We hypothesized that CHIP is associated with adverse outcomes and that the magnitude of risk is greater for those with VAF ≥10% or mutations other than DNMT3A mutations.
Methods
This systematic review was performed and reported following the recommendations of the Cochrane Prognosis Methods Group52 and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.53 The review protocol was registered on PROSPERO CRD42022382162. We searched Ovid MEDLINE and EMBASE up to 26 September 2023 with the search terms “Clonal h∗ematopoiesis.mp.” or “clonal hematopoiesis” as a subject heading. The reference lists of the included studies and recent reviews were also examined for additional studies.
Cohort, case-control, cross-sectional, and randomized controlled trials including people with CHIP were considered for inclusion if they reported an age-adjusted risk measure for a clinical outcome. Phenome-wide association studies54 were excluded.
Studies needed to be available as English-language original research papers, including peer-reviewed publications and those reported on preprint servers. CHIP was defined as mutation-driven clonal hematopoiesis in myeloid cancer-associated genes with a VAF ≥2% in people without known hematologic malignancy. Studies were required to report a measure of association (relative risk, odds ratio [OR], or hazard ratio [HR]) adjusted for age, with or without adjustment for additional covariates or sufficient information to calculate an age-adjusted risk measure. Clinical outcomes included any disease entity and mortality. All primary and secondary outcomes reported in the studies were extracted, except for outcomes reported only as part of a phenome-wide association study. Outcome ascertainment could be at any time with respect to the blood sample for CHIP measurement.
Studies were initially screened for eligibility by review of the title and abstract, followed by a full-text review of potentially eligible studies. The selection of studies, data extraction, and quality assessment were performed by 2 independent reviewers, with conflicts resolved by a third reviewer. Screening was performed using Covidence,55 and data extraction and quality assessment were performed using a combination of Covidence and Microsoft Excel. Data extraction was performed using a standardized data extraction form based on the checklist for critical appraisal and data extraction for systematic reviews of prognostic factor studies.52 Items extracted are listed in the supplemental Methods. Risk measures were extracted separately for individual cohorts and (when reported) for VAF groups (≥2%, 2%-10%, or ≥10%), genes, and the number of mutations. The risk of bias was assessed using a customized Quality in Prognostic Factor Studies tool.52 It was anticipated that there would be clinical heterogeneity in the study population, CHIP testing methods, and outcome definitions, and we quantified statistical heterogeneity by calculating I2. Where the same outcome was reported in at least 10 studies or cohorts, we assessed for small study effects by visual inspection of contour-enhanced funnel plots.
Similar outcomes were grouped together and random-effects meta-analyses using restricted maximum likelihood estimation were performed for outcomes reported in at least 3 studies, with OR/HR considered equivalent measures of risk. Where participants or cohorts overlapped between studies, we included only the estimate from the largest study or the 1 with the smallest standard error if the 2 studies reported a similar sample size. Preplanned subgroup analyses were conducted if at least 3 cohorts from 2 studies reported different subgroups of VAF (2%-10% and/or ≥10%) or genes (DNMT3A, TET2, ASXL1, TP53, and JAK2). There were insufficient studies to perform subgroup analyses of other genes or the number of mutations. We also considered exploratory subgroup analyses (e.g, based on study cohort, sequencing method, study design, and risk measure [OR or HR]) to explore the causes of heterogeneity, in which there were sufficient studies to allow this. The results of the meta-analyses are displayed as forest plots, and the contributing data (including extracted data from overlapping cohorts that were not included in the meta-analysis) are available in the supplemental Tables. All analyses were performed using Stata v18.56 Please refer to the supplemental Methods for additional explanations of the methodology.
Results
Search results
After excluding duplicates, 3305 studies were identified in the search: 3053 were excluded by title and abstract screening, 252 studies underwent full-text review, and from these data were extracted from 88 studies (supplemental Figure 1). Risk factors for CHIP (i.e. CHIP was the dependent variable in the analyses) are not reported here. The characteristics of the included studies are shown in supplemental Table 1. They included case-control, cohort, and cross-sectional studies, with study sizes ranging from 45 to 470 960 participants. Studies were published from 2014 onwards, and 63 of the 88 studies (72%) were published in or after 2022. The median duration of follow-up ranged from 30 days to 18 years. Outcomes that were reported in at least 3 studies and therefore qualified for meta-analysis were related to mortality (all-cause mortality, CV mortality, and cancer mortality), cancers (hematologic malignancy, myeloid malignancy, and lung cancer), CV disease (composite cardiovascular events [CVE], coronary heart disease [CHD], HF, stroke, and ischemic stroke), severe COVID-19 disease and renal impairment. supplemental Table 2 indicates where there were sufficient studies to perform subgroup analyses based on VAF or genes for each outcome.
Quality of evidence
The quality assessment using the Quality in Prognostic Factor Studies tool is summarized in supplemental Figure 2. Most studies had at least 1 domain with a moderate rating but only 14 of the 88 studies had a high rating for ≥1 domains. Almost all studies had at least a moderate risk of bias in the confounding domain, as these were all observational studies with confounding mainly being controlled for through multivariable adjustment in statistical analyses. Study attrition bias is generally poorly reported in cohort studies, resulting in most studies having at least a moderate risk of bias in this domain. All studies used objective methods to detect the presence of CHIP and were consequently judged to have a low risk of bias in this domain; however, it should be noted that there is no clear gold standard method for detecting CHIP.
Funnel plots for all-cause mortality, composite CVE, CHD, stroke, and ischemic stroke are shown in supplemental Figure 3. Funnel plot asymmetry, raising suspicion of publication bias or small study effects, was observed in CHD.
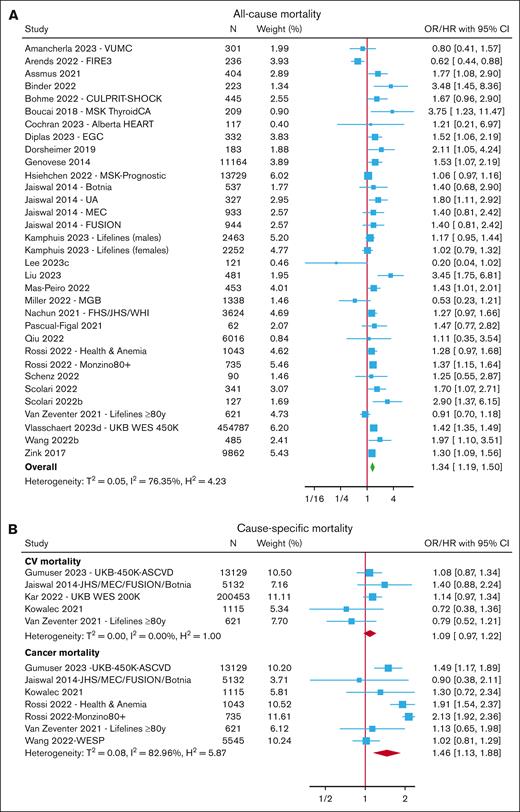
Mortality
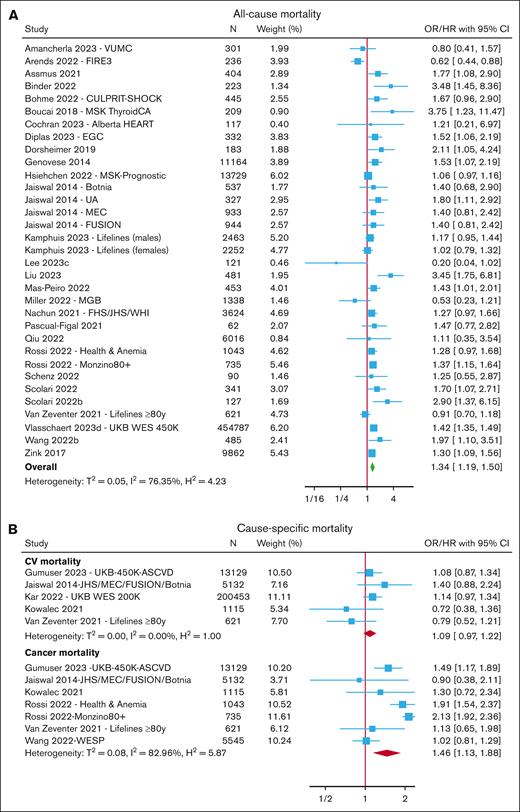
After excluding overlapping cohorts, meta-analyses were performed for all-cause mortality (reported in 32 cohorts across 28 studies),6,7,13,15,17,19-21,24,28,32,57-81 CV mortality7,15,28,63,74 (reported in 5 cohorts), and cancer mortality7,15,37,63,67,74 (reported in 7 cohorts) (Figure 1; supplemental Tables 3-4).
Risk of mortality with CHIP. Forest plots of studies reporting the risk of all-cause mortality (A) and cause-specific mortality (B). Boxes are drawn proportional to the weight of the individual study in the meta-analysis, and horizontal lines represent 95% CI values. The summary estimate is shown by the diamond, and the width of this corresponds to the 95% CI. The vertical line indicates an OR/HR of 1 (ie, no association). The horizontal axis is plotted on a log scale; therefore, the CIs are symmetrical. N represents the total number of participants in the study.
Risk of mortality with CHIP. Forest plots of studies reporting the risk of all-cause mortality (A) and cause-specific mortality (B). Boxes are drawn proportional to the weight of the individual study in the meta-analysis, and horizontal lines represent 95% CI values. The summary estimate is shown by the diamond, and the width of this corresponds to the 95% CI. The vertical line indicates an OR/HR of 1 (ie, no association). The horizontal axis is plotted on a log scale; therefore, the CIs are symmetrical. N represents the total number of participants in the study.
Overall, the presence of CHIP was associated with an increased risk of all-cause mortality (OR/HR, 1.34; 95% confidence interval [CI], 1.19-1.50) and cancer mortality (OR/HR, 1.46; 95% CI, 1.13-1.88) but not CV mortality (HR, 1.09; 95% CI, 0.97-1.22). In subgroup analyses (supplemental Figure 4; supplemental Tables 3-4), CHIP with a VAF ≥10% was associated with an increased risk of both all-cause mortality (OR/HR, 1.35; 95% CI, 1.26-1.47) and CV mortality (HR, 1.31; 95% CI, 1.12-1.54). Meta-analysis of gene-specific risk estimates from 6 cohorts indicated an increased risk of all-cause mortality with TET2-CHIP (HR, 2.01; 95% CI, 1.09-3.70) but not with DNMT3A-CHIP (HR, 1.48; 95% CI, 0.88-2.51).
There was significant heterogeneity among studies reporting all-cause mortality (I2 = 76%) and participants included in these studies derived from a variety of cohorts, including population-based cohorts and those selected for a specific characteristic, such as older age or presence of disease for example, COVID-19, cancer or CV disease. Given a large number of studies, we were able to further explore this heterogeneity by performing subgroup analyses of studies grouped by cohort (supplemental Figure 5). Lower heterogeneity was seen in the CV subgroup (I2 = 8%) and population-based subgroup (I2 = 37%). The greatest risk of all-cause mortality was seen in cohorts with underlying CV disease (HR, 1.69; 95% CI, 1.41-2.02), followed by population-based cohorts (HR, 1.34; 95% CI, 1.19-1.50). There was no increase in all-cause mortality in studies limited to those with COVID-19, cancer, or older individuals, and these subgroups also showed greater heterogeneity (I2 = 70%-93%). There was little difference in the risk estimates between subgroups based on the sequencing method (data not shown).
CV disease
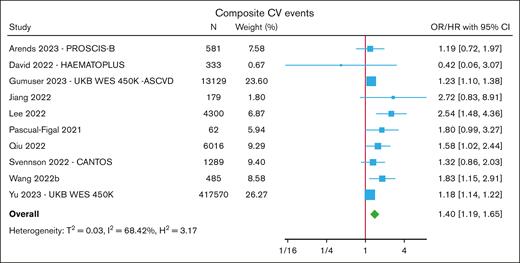
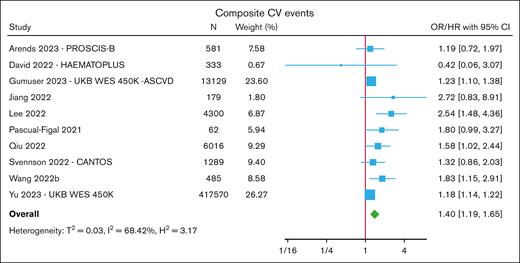
The most frequently reported CV outcome was a composite end point12-25,31 of either all-cause or CV mortality combined with ≥1 myocardial infarctions, coronary artery disease (most commonly defined as undergoing coronary revascularization procedures), angina, stroke, HF hospitalization, or peripheral arterial disease. In a meta-analysis of 10 cohorts, CHIP was associated with an increased risk of composite CVE (HR, 1.40; 95% CI, 1.19-1.65) (Figure 2; supplemental Table 5). In subgroup analyses, the overall risk was numerically similar when restricted to cohorts reporting CHIP with VAF ≥10% (HR, 1.23; 95% CI, 1.07-1.41) and between DNMT3A-CHIP (HR, 1.63; 95% CI, 1.08-2.46) and TET2-CHIP (HR, 1.58; 95% CI, 1.21-2.08) (supplemental Figure 6).
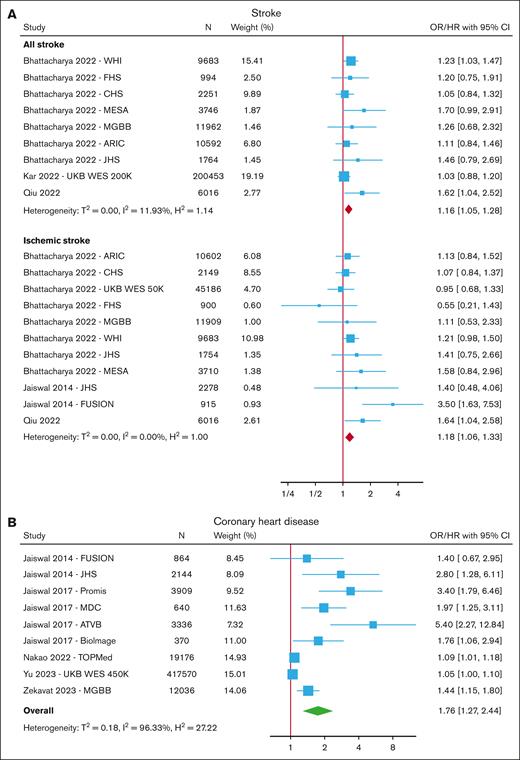
CHD (defined as myocardial infarction and/or coronary revascularization with or without chronic ischemic heart disease or angina) was also increased in those with CHIP (OR/HR, 1.76; 95% CI, 1.27-2.44) in a meta-analysis of 9 cohorts (Figure 3; supplemental Table 6).7,13,21,25,27-30,73,82 There was significant heterogeneity between studies (I2 = 96%), with the highest risk estimates seen in case-control studies (ATVB (Atherosclerosis, Thrombosis, and Vascular Biology Italian Study Group) and PROMIS (The Pakistan Risk of Myocardial Infarction Study) cohorts) that reported OR, and more modest risk estimates in more recent large cohort studies. In subgroup analyses (supplemental Figure 7) restricted by VAF, the increased risk was only significant for CHIP with VAF ≥10% (OR/HR, 1.44; 95% CI, 1.02-2.04) but not for those with VAF 2% to 10% (OR/HR, 1.25; 95% CI, 0.91-1.72). In meta-analyses restricted by gene, there was a trend toward an association between CHD and mutations in DNMT3A, TET2, ASXL1, and JAK2, but none of these reached statistical significance (supplemental Figure 7).
Both the risk of all strokes (defined as ischemic and hemorrhagic stroke, or unspecified) and ischemic stroke were increased in those with CHIP (HR, 1.16; 95% CI, 1.05-1.28 for all strokes and HR, 1.18; 1.06-1.33 for ischemic stroke) (Figure 3; supplemental Table 7).7,13,20,21,26,28 A similar risk estimate for all strokes was seen in the subgroup analysis restricted to those with VAF ≥10% (HR, 1.19; 95% CI, 1.07-1.33) (supplemental Figure 8).
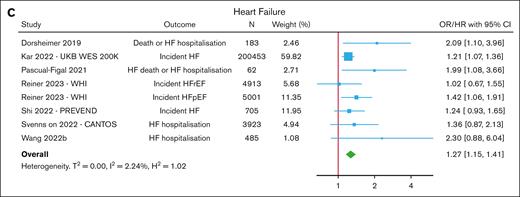
Seven studies reported on the association between CHIP and HF,19,22,24,28,32-34 defined as either a clinical diagnosis of HF (unspecified or specified as with preserved or reduced ejection fraction) or hospitalization for HF with or without death. In a meta-analysis of these studies, CHIP was associated with an increased risk of HF (OR/HR, 1.27; 95% CI, 1.15-1.41) (Figure 3; supplemental Table 8). Meta-analyses of gene-specific risk estimates indicated trends toward an association between DNMT3A and TET2, but neither of these reached statistical significance (supplemental Figure 9).
Malignancy
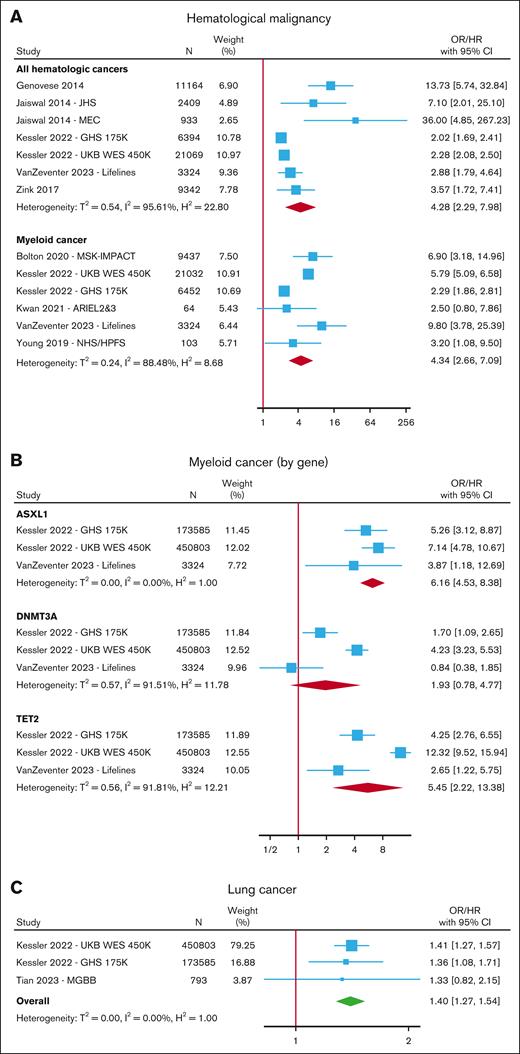
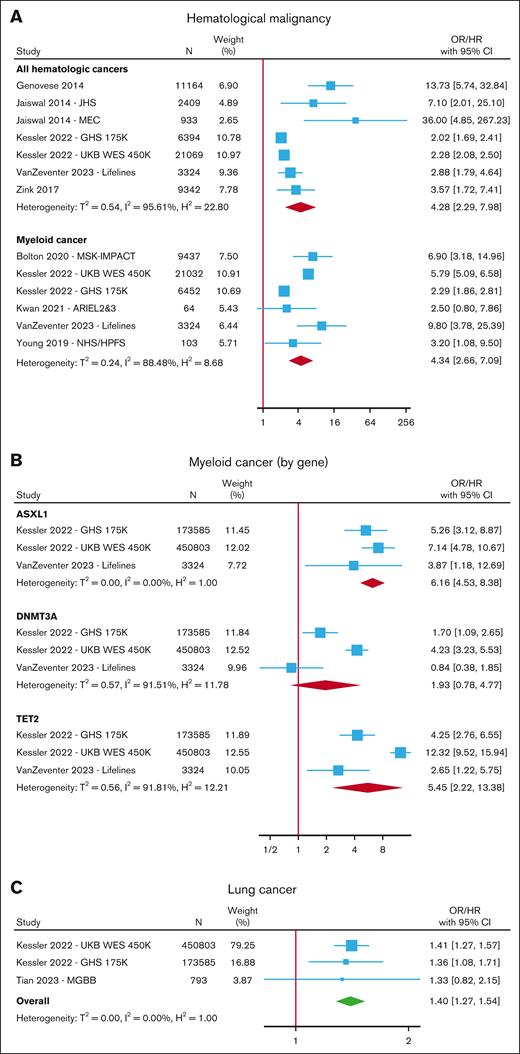
CHIP was associated with an increased risk of hematologic malignancy6,7,15,17,21,28,30,46,49,73,75,78,81,83-86 in a meta-analysis of 7 cohorts (HR, 4.28; 95% CI, 2.29-7.98) (Figure 4; supplemental Table 9), with a trend toward higher risk for those with VAF ≥10% (HR, 8.46; 95% CI, 2.34-30.67) (supplemental Figure 10). In a meta-analysis of 6 cohorts, CHIP was associated with an increased risk of myeloid malignancy (OR/HR, 4.34; 95% CI, 2.66-7.09) (Figure 4A), and in subgroup analyses, the risk was higher for TET2 (HR, 5.45; 95% CI, 2.22-13.38) and ASXL1 (HR, 6.16; 95% CI, 4.53-8.38) mutations compared with DNMT3A, in which the results failed to reach statistical significance (HR, 1.93; 95% CI, 0.78-4.77) (Figure 4B; supplemental Figure 11).
Risk of cancer with CHIP. Forest plots of studies reporting the risk of hematological malignancy (A) separated into all hematologic cancers and myeloid cancer, (B) myeloid cancer in gene-specific subgroups, and (C) lung cancer.
Risk of cancer with CHIP. Forest plots of studies reporting the risk of hematological malignancy (A) separated into all hematologic cancers and myeloid cancer, (B) myeloid cancer in gene-specific subgroups, and (C) lung cancer.
A few studies reported on the risk of various solid organ malignancies in individuals with CHIP, but only incident lung cancer was reported in at least 3 studies comprising 3 nonoverlapping cohorts.17,28,87 In a meta-analysis of these 3 cohorts, the risk of lung cancer increased in those with CHIP (HR, 1.40; 95% CI, 1.27-1.54 for any CHIP and HR, 1.61; 95% CI, 1.42-1.83 for CHIP with VAF ≥10%) (Figure 4C; supplemental Figure 12; supplemental Table 10). All of these studies adjusted for smoking in their analyses.
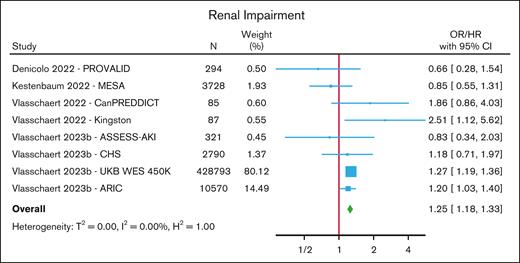
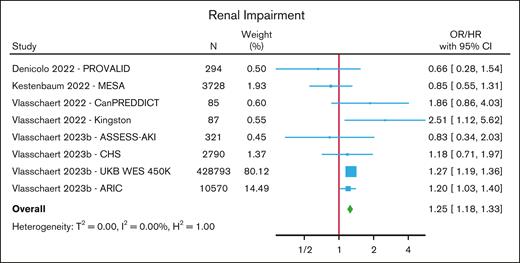
Renal impairment
One nested case-control and 3 cohort studies (comprising 8 cohorts) investigated the association between CHIP and renal impairment,88-91 with renal impairment being defined as a diagnosis of acute kidney injury, a prespecified decline in estimated glomerular filtration rate on serial measurements, renal replacement therapy, or a composite end point of various measures of renal impairment (estimated glomerular filtration rate decline, renal replacement therapy, or macroalbuminuria) with death from renal disease. In a meta-analysis of these studies, CHIP was associated with an increased risk of renal impairment (HR/OR, 1.25; 95% CI, 1.18-1.33) (Figure 5; supplemental Table 11). An elevated risk was seen for those with non-DNMT3A mutations (HR/OR, 1.40; 95% CI, 1.19-1.64) but not for those with DNMT3A mutations (HR, 1.04; 95% CI, 0.84-1.29) (supplemental Figure 13).
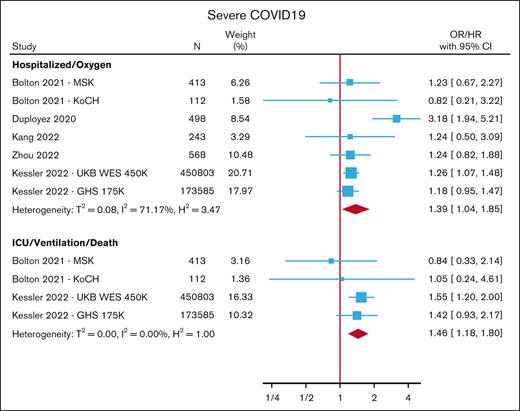
COVID-19
Five studies (comprising 7 cohorts) assessed the association between CHIP and COVID-19 disease severity.17,38-41 One of these studies included 2 population-based cohorts, and the remainder (1 case-control and 3 cohort studies) exclusively included COVID-19-positive individuals. The outcomes reported include ventilation, hospitalization, and severe COVID-19. However, severe COVID-19 was defined variably across studies and could encompass the need for supplemental oxygen, hospitalization, ventilation, and/or death from COVID-19. For the meta-analysis, we grouped study results based on outcome definitions into those pertaining to 1) the need for supplemental oxygen or hospitalization; or 2) ventilation or death. CHIP carriers were at an increased risk of having severe COVID-19 disease measured by both supplemental oxygen/hospitalization (OR, 1.39; 95% CI, 1.04-1.85) and ventilation/death (OR, 1.46; 95% CI, 1.18-1.80) (Figure 6; supplemental Table 12).
Other results
Other outcomes not reported in the meta-analyses (because they were not reported in at least 3 studies) are listed in supplemental Table 13. These include other CV outcomes (peripheral vascular disease,30 cardiac allograft vasculopathy after heart transplant,57 coronary microvascular dysfunction,92 atrial fibrillation,28 and other arrhythmias,93 and others15,76,94,95), incident solid organ cancers,21,28,37 chronic obstructive pulmonary disease,74,96 gout,51 Alzheimer disease,97 liver disease,36 osteoporosis,35 type 2 diabetes,98 infections,99,100 immune-mediated conditions (including Behcet,101 anti-neutrophil cytoplasm antibody-associated vasculitis,102 and giant cell arteritis103), and functional scores.104
Discussion
In this systematic review and meta-analysis of 88 studies, we confirmed that CHIP is associated with an increased risk of myeloid malignancies, all-cause mortality, and CV disease. In addition, we found that CHIP was associated with an increased risk of cancer mortality, stroke, HF, lung cancer, renal impairment, and severe COVID-19 disease. We found clone size to be an important determinant of the risk of CV mortality, CHD, and blood cancer, but not all-cause mortality, composite CVE, stroke, or lung cancer. Although this may indicate that clone size is a less important determinant of risk for these outcomes, it should be noted that there were insufficient studies to look specifically at VAF 2% to 10% for these outcomes, and large studies using whole exome or whole genome sequencing methods generally had high proportions of people with VAF ≥10% in the unselected VAF group. This association was gene-specific for some outcomes. In Particular, DNMT3A mutations did not significantly increase the risk of all-cause mortality, myeloid malignancy, or renal impairment. In the meta-analyses, we did not find differential effects between DNMT3A and TET2 mutations on CV outcomes. There are insufficient data to comment on the gene-specific risks of other outcomes. There are also insufficient data to evaluate outcomes in individuals with multiple DNMT3A mutations, concurrent mutations in DNMT3A and other genes, or specific variants. The risk of all-cause mortality also varied by the population studied.
Our review highlights the lack of a standardized approach to define CHIP in studies investigating associations. Recent WHO and ICC definitions have restricted the genes and VAF criteria to be considered for CHIP, and proposed guidelines for the curation of CHIP variants have recently been published.81 Nevertheless, there were many different methods reported in the included studies, with no clear gold standard. Different methods have different sensitivities, which are likely to affect the associations, especially those that are dependent on the clone size. There is also variability in the genomic regions covered, specific variants included, and criteria for variant calling, which can affect the reported measures of associations in the same cohort.81 In addition to heterogeneity in the method of CHIP testing, we also observed heterogeneity in the populations studied. We only had sufficient studies to perform exploratory subgroup analysis based on population for the outcome of all-cause mortality, and we observed a numerically higher HR for studies involving individuals with underlying CV disease than for population-based studies. This suggests that the adverse risk conferred by CHIP may depend on interactions with other predisposing factors. Consistent with this hypothesis, the reported mediators between CHIP and the risk of adverse CV outcomes include diet quality,12 low-density lipoprotein levels,18 and the presence of a concurrent IL-6-receptor polymorphism.13,81 Similarly, biochemical results, such as cholesterol and cystatin C levels, have been included in a validated model for predicting the risk of hematologic malignancy in people with CHIP/CCUS.45 The risk of hematologic malignancy with CHIP may also be mediated by the presence of mosaic chromosomal alterations.44,45,73,86 Inflammation is involved in the pathogenesis of many diseases and normal aging. CHIP has been associated with increased levels of proinflammatory cytokines, which cause tissue damage associated with the disease.105 The key role of inflammation in mediating CHIP-related diseases may also contribute to variations in risk estimates across populations with similar study designs, as participant characteristics and particularly comorbidities may contribute to an inflammatory phenotype. Our subgroup meta-analyses confirm that larger clone sizes and non-DNMT3A mutations are important predictors of myeloid cancer risk in people with CHIP, consistent with the inclusion of these variables in risk prediction models.44,45
Our review updates previous reviews on this topic, most of which have been narrative reviews. The only prior systematic review106 was published 2 years ago and included only 32 studies. This review also identified that CHIP was associated with an increased risk of CV disease (VAF ≥10% but not <10%), all-cause mortality and hematologic cancers. They also meta-analyzed 4 studies reporting specifically on therapy-related myeloid neoplasms; however, 2 of those studies were limited to patients with hematological (nonmyeloid) malignancy and therefore were excluded from our review.50,107 CV disease has a very heterogenous outcome, including CHD, stroke, HF, CV mortality, and composite outcomes. With a greater number of studies included in our review, we were able to look at these CV outcomes separately, reducing the heterogeneity from outcome definitions. Furthermore, we were able to assess additional outcomes (including lung cancer, cancer mortality, renal impairment, and severe COVID-19), as well as assess for small study effects for some outcomes, and consider additional causes of heterogeneity, such as participant characteristics.
Limitations of our review include that all studies were observational. We tried to minimize confounding by extracting risk estimates adjusted for age and other relevant covariates but these differed between studies and there may be residual unmeasured confounding. There was also heterogeneity between studies for some outcomes. We used a random-effects meta-analysis to try and account for this to an extent and where possible tried to explore the cause of heterogeneity using exploratory subgroup analyses. Using a random-effects (rather than fixed-effect) meta-analysis also meant that summary measures were less driven by only the largest cohorts.108 We tried to minimize publication bias by including manuscripts from preprint servers; however, a limitation of this approach is that such manuscripts, as well as papers published as letters to the editor rather than in a full research report format, tended to provide fewer details on the methodology, resulting in a greater risk of bias. Another limitation is that the subgroup analyses generally had fewer participants, limiting the power to detect clinical associations. We were not able to perform subgroup analyses for all reported outcomes due to limited data being available and were not able to evaluate the impact of rare mutations such as spliceosome mutations that likely have a greater impact on clinical outcomes, including myeloid cancer15,44-46 and CV disease.15,25 Additionally, we could not evaluate the impact of multiple mutations, including in individuals with DNMT3A mutations. Future research would benefit from standardized methods of assessing CHIP as well as consistency in outcome definitions and measurement. Although individual studies may lack the power to detect subgroup differences, reporting variant- and VAF-specific effects would allow for meaningful meta-analyses.
In conclusion, we found that CHIP confers an approximately fourfold increased risk of myeloid malignancy but also a 16% to 76% increased risk of nonhematologic diseases, including CV diseases (such as CHD, stroke, and HF), all-cause mortality, lung cancer, renal impairment, and severe COVID-19 disease. Clone size is an important determinant of the risk for hematologic malignancy, CHD, and CV mortality. DNMT3A mutations in isolation were not associated with an increased risk of myeloid malignancy, all-cause mortality, or renal disease but were associated with composite CVE. Direct application of these findings is hampered by heterogeneity in definitions and measurements of CHIP and clinical outcomes, and greater consistency in these in future research would allow greater applicability of study results to individuals with CHIP. A better understanding of the prognostic implications of CHIP is essential in guiding further research into the mechanisms of the disease and potential therapeutic strategies.
Acknowledgments
J.S. is supported by the Haematology Society of Australia and New Zealand/Leukaemia Foundation PhD scholarship. Z.K.M. is supported by the Australian National Health and Medical Research Council (NHMRC) Emerging Leader Investigator grant (GNT1194811) and E.M.W. is supported by an NHMRC Leadership Fellow Investigator grant (GNT1177784).
Authorship
Contribution: J.S., Z.K.M., E.M.W., L.T.P.T., and D.J.C. designed the study; J.S., N.L., E.A., and Z.K.M. conducted the review and analyses; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jasmine Singh, Monash University, School of Public Health and Preventive Medicine, 553 St Kilda Rd, Melbourne, VIC 3004, Australia; email: jasmine.singh@monash.edu.
References
Author notes
Data will be available on reasonable request from the corresponding author, Jasmine Singh (jasmine.singh@monash.edu).
The full-text version of this article contains a data supplement.