TO THE EDITOR:
On 28 November 2023, the Food and Drug Administration (FDA) released a statement that the agency is investigating reports of T-cell malignancies, including chimeric antigen receptor (CAR)–positive lymphoma, in patients who received treatment with B-cell maturation antigen (BCMA)– or CD19–directed autologous CAR T-cell immunotherapies. Although CAR-positive T-cell malignancies have been reported in patients treated with next-generation products using the piggyBac transposon system,1 there have been no peer-reviewed publications reporting a CAR-positive T-cell malignancy in patients treated with CAR T-cell therapy produced via retroviral or lentiviral transduction. Although the FDA release clearly states that the benefits of CAR T-cell therapy continue to outweigh the risks, it has reignited concerns over the theoretical risk of insertional mutagenesis, which may generate public trepidation surrounding this potentially life-saving treatment.
A total of 22 cases of postinfusion T-cell malignancies were disclosed by the FDA, all occurring within 2 years after treatment.2-4 Importantly, malignant cells in 3 of the malignancies were noted to express the CAR transgene. There was a paucity of additional details, but a recent abstract reported a CAR-positive T-cell lymphoma developing in an adult with multiple myeloma 5 months after receiving the BCMA-directed CAR, ciltacabtagene autoleucel.5 Lymphoma tissue was remarkable for a clonal population of cells expressing BCMA CAR by quantitative polymerase chain reaction and immunohistochemistry. Integration analysis showed insertion at the 3 prime untranslated region of the PBX2 gene. Notably, a somatic inactivating TET2 mutation and germ line JAK3 activating mutation were concurrently identified. In this context, insertion of the CAR genetic material may have contributed to oncogenic potentiation or, alternatively, been an incidental passenger event in a patient with preexisting clonal hematopoiesis predisposed to a secondary malignancy. The role of CAR T-cell activation in driving T-cell leukemogenesis in the setting of a JAK3 variant was proposed in another recent case.6 Finally, an instance of a CAR-positive malignancy was previously reported in a pediatric patient with B-cell acute lymphoblastic leukemia (B-ALL), whereby the vector integrated in a leukemic blast that had contaminated the leukapheresis product before manufacturing.7 Although further interrogation alleviated any concerns of insertional mutagenesis in this patient, it led to heightened awareness in the field.
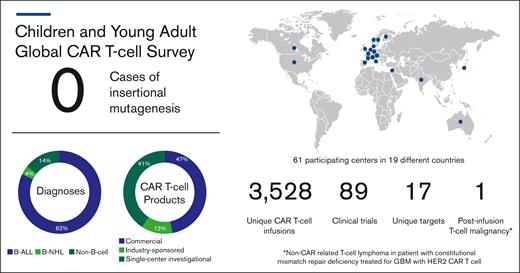
In their announcement, the FDA stated the risk of T-cell malignancies applies to all currently approved BCMA- and CD19-targeting CAR products, which includes tisagenlecleucel (tisa-cel; Kymriah), the sole approved CAR T-cell product for pediatric patients. Members from the pediatric oncology community were compelled to respond to this FDA statement with a clear assessment of the risk of such events for children, adolescents, and young adults (CAYAs). A global task force of pediatric cellular therapy experts was generated to clarify the incidence for CAR-induced T-cell malignancies (Table 1). Retrospective, deidentified data from 61 centers around the world were collected. This captured the number of CAYA patients (aged ≤30 years) with B-cell and non–B-cell malignancies treated with commercial or investigational CAR T-cell products between April 2012 and December 2023 as well as the number of cases of post-CAR subsequent T-cell malignancies. Of the 3038 infusions for B-cell malignancies, no cases of T-cell malignancies were reported after CAR T-cell treatment (Figure 1). There were an additional 490 infusions for non–B-cell malignancies, including 1 patient treated for glioblastoma multiforme who developed a T-cell lymphoma 2 years after infusion. As previously reported, integration analysis revealed absence of retroviral transgene, and because this patient carried a constitutional mismatch repair deficiency, a second primary cancer was considered the most likely diagnosis.8
Postinfusion T-cell malignancy results from global survey of centers treating CAYAs with CAR T cells. B-NHL, B-cell non-Hodgkin lymphoma; GBM, glioblastoma multiforme.
Postinfusion T-cell malignancy results from global survey of centers treating CAYAs with CAR T cells. B-NHL, B-cell non-Hodgkin lymphoma; GBM, glioblastoma multiforme.
Since the first reports of T-cell malignancies arising in children receiving retrovirally transduced hematopoietic stem cells for the treatment of inherited immunodeficiencies,9 the risk of insertional mutagenesis has been of particular concern in the field of pediatric gene therapy. A goal for any potentially curative gene therapy is minimization of late effects particularly in a pediatric context in which the time-dependent burden may be greatest. Because most CAR T cells use lentiviral vectors, which have a predilection for inserting in noncoding introns and therefore are less likely to disrupt tumor suppressor genes or activate proto-oncogenes near integration sites, it was anticipated that the risk of insertional mutagenesis would be lower for CAR T cells. Additionally, mature lymphocytes are potentially less prone to transformation compared with hematopoietic stem cells due to proapoptotic and epigenetic mechanisms that potentially prevent clonal outgrowth.10
The low risk of insertional mutagenesis has been borne out in clinical practice to date, with several groups characterizing their early experience with late effects after CAR T-cell therapy. Specifically as it relates to CAYA patients, a multi-institutional collaboration reported on their retrospective experience with 420 patients treated with CD19 CARs and reported a subsequent malignant neoplasm (SMN) incidence of 1.7% after CAR infusion, none of which were T-cell malignancies.11 This experience was similar to the pooled safety analysis of tisa-cel in CAYA patients with B-ALL.12 Both reports were limited by short median follow-ups of 30.1 and 24 months, respectively. In contrast, the incidence of SMN has been higher in adult reports, potentially related to more preexisting risk factors in this population.13,14 Further support includes the complete lack of replication-competent retrovirus or lentivirus across testing of >1000 infusion products, because replication-defective vectors should have a lower risk of insertional mutagenesis.15,16
Separate from the risk of insertional mutagenesis, the FDA’s heightened concern may also be related to the frank incidence of non–CAR-positive T-cell malignancies after infusion. Speculation has existed surrounding the indirect impact of CAR T cells on leukemogenesis, including the robust cytokine production and the iatrogenic immunodeficiency that is created and how these factors may affect normal T-cell development. The observation that our group recorded no T-cell malignancies after any B-cell targeting CAR infusion could be related to a lower overall risk of malignant transformation in CAYA patients or may be due to the lower number of treated patients to date. Furthermore, it is not clear whether the incidence of postinfusion T-cell malignancies is indeed higher than the baseline incidence of secondary T-cell malignancies after a primary B-cell malignancy.17
Since the first pediatric patient with B-ALL was treated with CAR T-cell therapy in 2012, there have been no reported cases of post-CAR T-cell malignancies, including insertional mutagenesis, in the pediatric B-ALL setting. Although the FDA warns of post-CAR T-cell malignancies after all approved CAR products, including tisa-cel, we demonstrate in this global survey that if secondary T-cell malignancy is a risk in the CAYA post-CAR setting, the incidence is likely very low. Every patient and family consented for CAR T-cell treatment is informed of expected and possible risks, with each toxicity being factored into shared decision-making. As a result, we urge that excess risk should not attributed to the development of CAR-induced SMNs in CAYAs receiving CARs for B-cell malignancies, based on data generated in adult cohorts, in which the prevalence of clonal hematopoiesis is higher and accumulation of proto-oncogenic somatic lesions more likely.18 Although secondary CAR-induced malignancies have not emerged as a major challenge for CAYA patients over the first decade of CAR translation, due to the seriousness of this risk, ongoing monitoring and longitudinal surveillance remain a clinical standard in the post-CAR setting. Based on our extensive collective experience to date, we remain committed to the ongoing utilization, safe implementation, and advancement of CAR T-cell therapies in CAYA patients with life-threatening diseases.
Acknowledgments: This work was supported in part by the Intramural Research Program, Center of Cancer Research, National Cancer Institute and National Institutes of Health (NIH) Clinical Center, NIH (ZIA BC 011823 [N.N.S]). S.L.M. is a scholar in Clinical Research of the Leukemia & Lymphoma Society.
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Contribution: A.J.L., L.M.S., N.N.S., and S.L.M. wrote the first draft of the manuscript; and all authors contributed to data collection and provided relevant details, substantially contributed to the final version of the manuscript, and approved the submission.
Conflict-of-interest disclosure: N.N.S. receives research funding from Lentigen, Vor Bio, and CARGO Therapeutics, and has attended advisory board meetings (no honoraria) for Vor Bio, ImmunoACT, and Sobi. S.G. reports honoraria from Novartis; patents with University College London Business Ltd; and trial steering committee participation for Autolus. L.M.S. reports advisory board fees from Novartis Pharmaceuticals and CARGO Therapeutics. R.A.G. receives royalty payments related to patents from Juno Therapeutics, unrelated to this work. S.L.M. has received clinical trial support from and has served on advisory and study steering committees for Novartis and Wugen, and has a patent pending and licensed to Novartis Pharmaceuticals without royalty for PCT/US2017/044425: “Combination Therapies of Chimeric Antigen Receptors and PD-1 Inhibitors.” E.M.H. consults for Novartis. R.H.R. has served as a consultant for Pfizer; received honoraria from Novartis; and reports research support from Tessa Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Adam J. Lamble, Division of Hematology/Oncology, University of Washington, Seattle Children's Hospital, 4800 Sand Point Way NE, Seattle, WA 98105; email: adam.lamble@seattlechildrens.org.
References
Author notes
A.J.L. and L.M.S. are joint first authors.
N.N.S. and S.L.M. are joint senior authors.
Data are available on request from the corresponding author, Adam J. Lamble (adam.lamble@seattlechildrens.org).