Visual Abstract
TO THE EDITOR:
Clinical practice guidelines (CPGs) inform the practice of medicine, dictate delivery of care, and influence resource distribution. CPGs are developed by subject-matter experts using the most recent evidence-based data. An individual’s relationship with other entities, whether financial, intellectual, or personal in nature, has the potential to influence the content and integrity of CPGs. To ensure the CPGs are as unbiased and evidence based as possible, these relationships, whether encompassing a true conflict-of-interest (COI) or one that appears to be, are therefore important to manage.1
Substantial industry-related financial relationships among CPG authors exist across various medical disciplines,2-10 and multiple specialties have evaluated the nature of financial relationships among their CPG authors.4,11-13 The American Society of Hematology (ASH) produces guidelines that may be used by hematologists and other health care practitioners worldwide. However, the prevalence of financial relationships among ASH CPG authors is limited to 1 CPG.5 We assessed industry-related payments reported in the US Centers for Medicare and Medicaid Services Open Payments Database (CMS-OPD) to physician author positions on ASH CPGs.
ASH requires disclosure of all relevant direct and indirect financial disclosures up to 2 years before application for CPG involvement, and 2016 to 2022 CMS-OPD payment data were available at the time of writing this report. Thus, CPGs published between 2018 and 2023 were analyzed (supplemental Table 1). Two authors collected data from the CMS-OPD for US physician author positions between 2 November 2023 and 13 November 2023. Analysis focused on author positions (vs individual authors), because each position is an opportunity for a unique individual to serve on a CPG. Subgroup analysis of payments to CPG author positions who authored >1 CPG during this period was also performed.
Additional variables included medical school location (US vs international); years elapsed between medical school graduation and 2023; Hirsch index up to 9 November 2023 via Scopus; presence of a doctoral research degree (PhD/DPhil); US census region14 determined by the byline affiliation at the time that each respective CPG was published; and medical specialty. If an individual completed training in multiple specialties, we considered their subspecialty to be their primary specialty (eg, those trained in internal medicine and hematology/oncology were considered to specialize in hematology/oncology). Two authors independently coded gender (100% concordance) via online pronouns (eg, her/she, him/he, and them/they) or gender references (eg, woman, man, nonbinary, etc); if unavailable, photographs were used.
We assessed payments (total, research, and general) to author positions in 4 groups: (1) all CPG author positions; (2) all CPG author positions receiving >$0; (3) all CPG author positions receiving >$600; and (4) all CPG author positions receiving >$5000. The amounts of $600 and $5000 were chosen because they represent the threshold used by the US Internal Revenue Service for independent contractors,15 and the preferred maximum threshold of direct financial payments for ASH CPG authors per the 2019-2023 ASH CPG COI policy, respectively.
For each CPG, the number of US physician author positions that received payment was compared with the total number of US physician author positions on each respective CPG to quantify the total number of CPGs with ≥50% of US physician authors receiving payments (supplemental Table 2).
Statistical analysis was performed using Prism (version 10.0.3, GraphPad Software Inc, Boston, MA). Normality was assessed with the Shapiro-Wilk test. Medians and interquartile ranges were reported. χ2 tests were used to compare observed and expected frequencies. The Mann-Whitney U test was used to compare medians. Multivariable linear regressions (EasyMedStat, version 3.30.2, Paris, France) were performed to assess author characteristics and payment amounts. Data were checked for multicollinearity with the Belsley-Kuh-Welsch technique. Heteroskedasticity and normality of residuals were assessed by the Breusch-Pagan test and the Shapiro-Wilk test, respectively. For all statistical analyses, a P value <.05 was considered statistically significant.
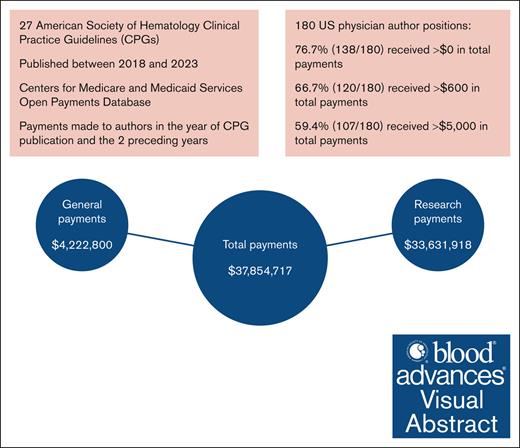
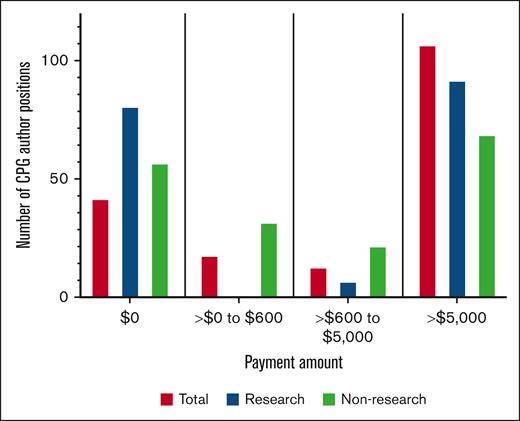
Among the 27 ASH CPGs, 24 CPGs had at least 1 US physician author, and 180 of 582 (30.9%) author positions were held by US physicians (supplemental Table 3). Of US physician author positions, 138 of 180 (76.7%) received at least 1 payment in any category (Figure 1; Table 1), totaling $37 854 717 ($68 148 [$11 451-$307 775]; range, $12-$5 737 631). Among the 24 CPGs with at least 1 US physician author, the median payment per CPG was $676 833 ([$317 799-$1 440 340]; range, $14 134-$14 826 190). For payment thresholds, 120 of 180 (66.7%) received >$600 ($113 880 [$28 970-$321 748]; range, $1189-$5 737 631) totaling $37 852 452 (99.99% of all payments) and 107 of 180 (59.4%) received >$5000 ($145 187 [$49 926-$382 950]; range, $7766-$5 737 631) totaling $37 829 667 (99.93% of all payments; Table 1).
Number of US physician CPG author positions by payment type and amount. Payments are those received during the year of CPG publication and the preceding 2 years.
Number of US physician CPG author positions by payment type and amount. Payments are those received during the year of CPG publication and the preceding 2 years.
Payments to US physician author positions on ASH CPGs during the year of, and 2 years preceding, each respective CPG publication
| Payment type . | Number of positions (% of total) . | Total $ (% of total) . | Median . | IQR . | Range . |
|---|---|---|---|---|---|
| Total payments | |||||
| All positions | 180 | $37 854 717 | $28 970 | $36-$238 724 | $0-$5 737 631 |
| >$0 | 138 (76.7) | $37 854 717 | $68 148 | $11 451-$307 775 | $12-$5 737 631 |
| >$600 | 120 (66.7) | $37 852 452 (99.99) | $113 880 | $28 970-$321 748 | $1 189- $5 737 631 |
| >$5000 | 107 (59.4) | $37 829 668 (99.93) | $145 187 | $49 926-$382 950 | $7 766-$5 737 631 |
| Research payments | |||||
| All positions | 180 | $33 631 918 | $9 525 | $0-$206 785 | $0-$5 732 254 |
| >$0 | 99 (55.5) | $33 631 918 | $181 158 | $44 808-$379 723 | $627-$5 732 254 |
| >$600 | 99 (55.5) | $33 631 918 (100) | $181 158 | $44 808-$379 723 | $627-$5 732 254 |
| >$5000 | 92 (51.1) | $33 622 230 (99.97) | $198 128 | $54 104-$4 418 952 | $7 650-$5 732 254 |
| Non-research payments | |||||
| All positions | 180 | $4 222 800 | $1 069 | $0-$15 039 | $0-$816 901 |
| >$0 | 123 (68.3) | $4 222 800 | $6 629 | $451-$23 731 | $12-$816 901 |
| >$600 | 91 (50.6) | $4 218 694 (99.90) | $14 841 | $5 488-$35 550 | $1 069-$816 901 |
| >$5000 | 69 (38.3) | $4 182 120 (99.04) | $22 987 | $13 160-$49 595 | $5 376-$816 901 |
| Payment type . | Number of positions (% of total) . | Total $ (% of total) . | Median . | IQR . | Range . |
|---|---|---|---|---|---|
| Total payments | |||||
| All positions | 180 | $37 854 717 | $28 970 | $36-$238 724 | $0-$5 737 631 |
| >$0 | 138 (76.7) | $37 854 717 | $68 148 | $11 451-$307 775 | $12-$5 737 631 |
| >$600 | 120 (66.7) | $37 852 452 (99.99) | $113 880 | $28 970-$321 748 | $1 189- $5 737 631 |
| >$5000 | 107 (59.4) | $37 829 668 (99.93) | $145 187 | $49 926-$382 950 | $7 766-$5 737 631 |
| Research payments | |||||
| All positions | 180 | $33 631 918 | $9 525 | $0-$206 785 | $0-$5 732 254 |
| >$0 | 99 (55.5) | $33 631 918 | $181 158 | $44 808-$379 723 | $627-$5 732 254 |
| >$600 | 99 (55.5) | $33 631 918 (100) | $181 158 | $44 808-$379 723 | $627-$5 732 254 |
| >$5000 | 92 (51.1) | $33 622 230 (99.97) | $198 128 | $54 104-$4 418 952 | $7 650-$5 732 254 |
| Non-research payments | |||||
| All positions | 180 | $4 222 800 | $1 069 | $0-$15 039 | $0-$816 901 |
| >$0 | 123 (68.3) | $4 222 800 | $6 629 | $451-$23 731 | $12-$816 901 |
| >$600 | 91 (50.6) | $4 218 694 (99.90) | $14 841 | $5 488-$35 550 | $1 069-$816 901 |
| >$5000 | 69 (38.3) | $4 182 120 (99.04) | $22 987 | $13 160-$49 595 | $5 376-$816 901 |
IQR, interquartile range.
Overall, 99 of 180 (55.0%) of positions received at least 1 research payment ($181 158 [$44 808-$379 723]; range, $627-$5 732 254), totaling $33 631 918 (88.84% of all payments; Figure 1; Table 1). All author positions that received a research payment received >$600. 51.1% (92/180) received >$5000 in research ($198 128 [$54 104-$418 952]; range, $7650-$5 732 254), totaling $33 622 230 (99.97% of total research payments).
Of 180 positions, 123 (68.3%) received at least 1 general payment ($6629 [$451-$23 731]; range, $12-$816 901) totaling $4 222 800 (11.16% of all payments; Figure 1; Table 1); 91 of 180 (50.6%) received >$600 ($14 841 [$5488-$35 550]; range, $1069-$816 901) totaling $4 218 694 (99.90% of total general payments) and 69 of 180 (38.3%) received >$5000 ($22 987 [$13 160-$49 595]; range, $5376-$816 901) totaling $4 182 120 (99.04% of total general payments).
Overall, 21 individuals authored >1 CPG (2 [2-4]; range, 2-10), and held 38.9% (70 of 180) of all ASH CPG author positions occupied by US physicians. Of these positions, 54 of 70 (77.1%) received >$0 ($50 506 [$1622-$219 221]; range, $36-$486 605), 46 of 70 (65.7%) received >$600 ($56 796 [$25 041-$232 876]; range, $1189-$486 605), and 39 of 70 (55.7%) received >$5000 ($68 148 [$45 884-$272 838]; range, $14 841-$486 605), accounting for $6 413 113 (16.94%) of total payments. Of these individuals, 14 of 21 (66.7%) received >$0, 12 of 21 (57.1%) received >$600%, and 11 of 21 (52.4%) received >$5000.
In multivariable analysis (supplemental Table 4), specializing in a non–internal medicine specialty was associated with lower total payment, research payment, and general payment values compared with individuals specializing in hematology/oncology. Individuals located in the US Midwest region received higher research payment amounts, whereas individuals in the US South region and those with a doctoral research degree (PhD/DPhil) received higher general payment.
We found that 77% of author positions held by US-based physicians on 27 ASH CPGs received at least 1 industry-related payment during the year of CPG publication and the preceding 2 years. These payments totaled more than $37 million, with a median of ∼$677 000 per CPG among those with at least 1 US physician author. Approximately 90% of all payments were related to research. Although the range of payments was wide ($12 to >$5 million), almost 60% of US physician author positions received >$5000 in total payments. Specializing in a non–internal medicine specialty was associated with lower total payments, research payments, and nonresearch payments compared with individuals specializing in hematology/oncology, paralleling findings of high prevalence of financial payments within oncology.16
These results demonstrate substantial payments to individuals holding CPG author positions, mirroring prior studies indicating that influential positions in medicine and science, such as journal editors, receive significant payment amounts.17-20 It is important to note that the findings in this study do not necessarily represent payments that would cause conflict for an author, because they may or may not have been related to the content of the particular guideline(s) authored by the recipient. Moreover, industry-related payments are not inherently detrimental, and we do not suggest that these payments should be entirely prohibited, or that all industry payments represent a financial COI. Similar to other medical societies, ASH maintains strict COI and disclosure policies for CPG authors; however, studies from multiple specialties have found varying rates of adherence to such policies.4 Failure to disclose can evoke skepticism regarding individuals’ motives and credibility and, by extension, their published or promoted material.
Limitations include potential data inaccuracies in CMS-OPD, although reporting entities are subject to audit by the Office of Inspector General to ensure the reported data are accurate. However, accuracy is only known at the level of the reporting entity.21 We attempted to mitigate potential bias by analyzing groups receiving >$600 and >$5000. Despite its limitations, CMS-OPD is the most comprehensive database for industry payments, and has improved since its inception.22 However, the extent to which payment recipients are aware of CMS-OPD or should query CMS-OPD for payment accuracy is unknown. Additionally, a database equivalent to CMS-OPD does not exist for non-US individuals.
US physician author positions on ASH CPGs receive significant financial payments from industry, predominantly related to research. Future research regarding the impact of industry-related payments on CPG content, as well as methods for ensuring the accuracy and completeness of reported payments, is warranted.
In accordance with the Code of Federal Regulations, 45 CFR 46.102, this study was deemed exempt by the Mayo Clinic institutional review board.
Contribution: J.W.J., J.S.W., and L.D.S. performed the research; J.S.W. and J.W.J. drafted the manuscript; G.S.B., A.A.M., L.D.S., B.D.A., R.G., and S.K. revised the manuscript; J.S.W., G.S.B., and J.K.S. provided supervision; and all authors approved the final version.
Conflict-of-interest disclosure: G.S.B. has no disclosures related to this study; unrelated, he reports compensation for serving as a faculty speaker for a nonaccredited continuing education program and consulting for Grifols Diagnostics. L.D.S. has no disclosures related to this study; unrelated, she reports attending an educational event including food and beverage from GPI USA. A.A.M. has no disclosures related to this study; unrelated, she reports compensation for participating on an accredited educational program, and for being a speaker at a local nonaccredited medical professional conference. G.D.L. has no disclosures related to this study; unrelated, he reports serving as an independent contractor for Cancer Commons as the Editor in Chief. J.K.S. has no disclosures related to this study; unrelated, she reports participating in research funded by the Binational Scientific Foundation and National Institutes of Health and community programming from the Cummings Foundation; she is an adviser to Simplifed and a venture partner at Third Culture Capital; and she receives royalties from books that she has authored and edited. J.S.W. has no disclosures related to this study; unrelated, she reports attending an education event including food and beverage from Immucor and GPI USA, and food and beverage from Instrumentation Laboratory; she also reports serving as a consultant for Instrumentation Laboratories, for which she has not received an industry payment although CMS Open Payments has reported a payment. The remaining authors declare no competing financial interests.
Correspondence: Jeremy W. Jacobs, Department of Laboratory Medicine and Pathology, Mayo Clinic, 210 2nd St SW, Rochester, MN 55905; email: jacobs.jeremy@mayo.edu.
References
Author notes
Data are available on request from the corresponding author, Jeremy W. Jacobs (jacobs.jeremy@mayo.edu).
The full-text version of this article contains a data supplement.