Key Points
Lysosomal degradation regulates cellular CRTDel52 and MPL levels.
mTOR inhibitors reduce CRTDel52 and MPL levels and CRTDel52–induced cell proliferation.
Visual Abstract
Lysosomal degradation pathways regulate mutant CRT and cell surface MPL levels in mutant CRT-driven MPNs
Lysosomal degradation pathways regulate mutant CRT and cell surface MPL levels in mutant CRT-driven MPNs
Somatic mutants of calreticulin (CRT) drive myeloproliferative neoplasms (MPNs) via binding to the thrombopoietin receptor (MPL) and aberrant activation of the JAK/STAT pathway. Compared with healthy donors, platelets from mutant CRT-expressing patients with MPN display low cell surface MPL. Additionally, coexpression of MPL with an MPN-linked CRT mutant (CRTDel52) reduces cell surface MPL, suggesting that CRTDel52 may induce MPL degradation. We show that lysosomal degradation is relevant to the turnover of CRTDel52 and MPL. Furthermore, CRTDel52 increases the lysosomal localization and degradation of MPL. Mammalian target of rapamycin (mTOR) inhibitors reduce cellular CRTDel52 and MPL, secreted CRTDel52 levels, and impair CRTDel52–mediated cell proliferation. mTOR inhibition also reduces colony formation and differentiation of CD34+ cells from patients with MPN but not from healthy donors. Together, these findings indicate that low-surface MPL is a biomarker of mutant CRT-mediated MPN and that induced degradation of CRTDel52 and MPL is an avenue for therapeutic intervention.
Introduction
Essential thrombocythemia (ET), polycythemia vera, and myelofibrosis (MF) are myeloproliferative neoplasms (MPNs) that induce hyperproliferation of myeloid lineage blood cells.1,2 Somatic mutations of the calreticulin (CRT) gene are found in 20% to 35 % of patients with ET and MF.3,4 Most of these mutations are +1 base pair (bp) frameshift mutations in exon 9, which encodes the carboxy domain of CRT. The type 1 mutation, which involves a 52 bp deletion (Del52), and the type 2 mutation, which involves a 5 bp insertion (Ins5), account for 45% to 53% and 32% to 41% of CRT mutations in MPNs, respectively.3,4 MPN-linked mutant CRT proteins are characterized by the enrichment of basic amino acids in the carboxy domain, in contrast to the acidic amino acids present in the wild-type CRT (CRTWT).3,4 The MPN CRT mutants also lack the Lys-Asp-Glu-Leu (KDEL) motif at the carboxy-terminal end, which is responsible for the endoplasmic reticulum (ER) localization of the WT protein. Loss of the KDEL sequence affects ER retention3-5 and induces secretion of MPN-linked CRT mutants.6-9
The pathogenic effects of mutant CRT proteins in MPNs are attributed in part to their ability to induce ligand-independent constitutive activation of JAK/STAT signaling via the cell surface receptor, MPL (also called the thrombopoietin receptor [TPOR]), which is a known glycoprotein substrate of CRT.10-14 While the interaction between MPL and CRTWT is transient, mutant CRT proteins form stable complexes with MPL via both the glycan-binding site5,14-16 and the novel C-terminal domain.15,17,18 The mutant CRT and MPL complexes cotraffic to the cell surface.5 MPL is expressed on the surface of hematopoietic cells and regulates the differentiation of megakaryocytes and the production of platelets as well as the self-renewal of hematopoietic stem cells.19-21 The expression of MPN-linked mutant CRT proteins and the resultant activation of MPL signaling promote the proliferation of megakaryocytes and excessive production of platelets.10,11,13,22-24
The physiological activation of MPL signaling by thrombopoietin (TPO) is tightly regulated via coordination between the synthesis and release of TPO and its removal from the blood circulation.25 Binding of TPO to MPL and the resultant activation of receptor signaling is followed by internalization and degradation of the receptor-ligand complex, which removes excess TPO from the circulation.26-29 However, little is known about the downstream effects of MPL signaling activated by the binding of MPN-linked mutant CRT proteins. In this study, we measured the relative levels of MPL protein in platelets purified from patients with MPN and healthy donors and found a significant reduction in MPL levels in platelets of patients with MPN. We used model cell lines coexpressing human MPL and mutant CRT proteins to explore the pathways involved in the regulation of surface MPL and mutant CRT levels. Our findings also indicate that the induced degradation of mutant CRT and MPL can be used therapeutically to compromise the cell-transforming effects of mutant CRT.
Methods
Blood/bone marrow samples
Blood/bone marrow samples of patients with MPN were collected from the Myeloproliferative disease at the University of Michigan Medical School repository (study ID: HUM0006778). Healthy donor blood samples were obtained from the University of Michigan Platelet Physiology and Pharmacology Core repository (study ID: HUM00107120) and participants of another research study (study ID: HUM00071750). Cryopreserved mobilized leukopaks from deidentified healthy donors that were discarded by blood banks were collected.
DNA constructs
The pMSCV and pcDNA3.1/Zeo(−) constructs used for the expression of untagged CRTWT and CRTDel52 in mammalian cells have been described earlier.17 A human MPL construct (accession number BC153092) was purchased from DNASU plasmid repository and subcloned into the pMSCV-neomycin vector at the EcoRI and XhoI sites, and the pcDNA-COX2-54 vector by ligation-independent cloning (LIC) as described earlier.17 All primers used are specified in supplemental Table 1.
Proliferation and CFU assays
Proliferation assays of Ba/F3-MPL cells were performed with or without treatment with everolimus or rapamycin for 5 days. For colony-forming unit (CFU) assays, CD34+ cells isolated from bone marrow samples of patients with MPN (supplemental Table 3) or leukopaks from healthy donors were used. Detailed protocols are given in supplemental materials and methods.
Blood/bone marrow samples were collected after obtaining written informed consent from donors and patients with MPN following protocols approved by the University of Michigan Institutional Review Board.
Results
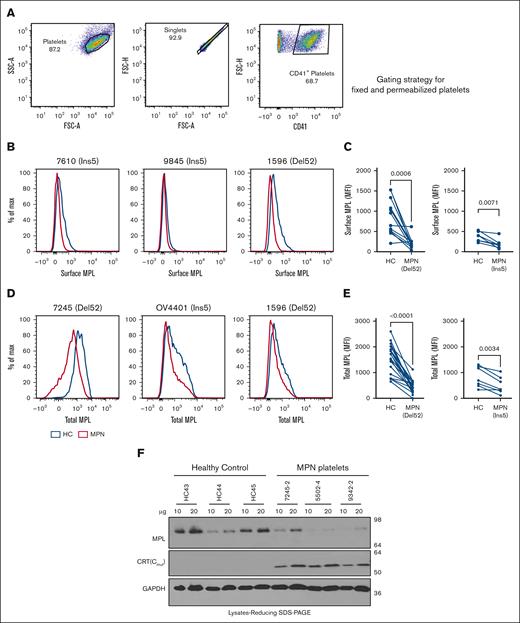
Platelets from patients with MPN are characterized by surface expression of mutant calreticulin
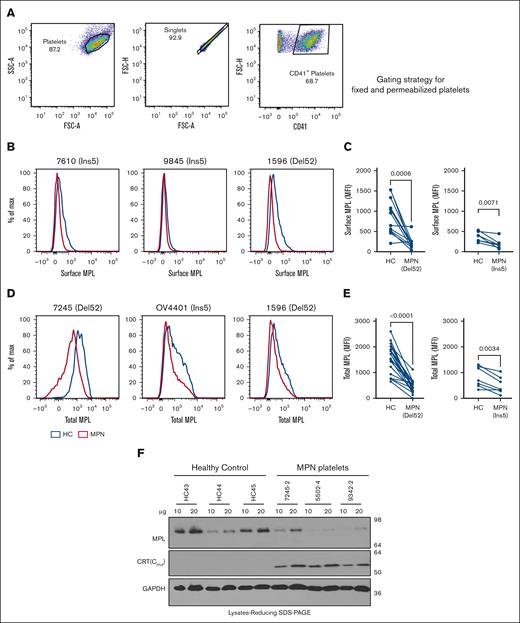
We examined the localization of CRT proteins on the surface of platelets isolated from the blood samples of patients with MPN (supplemental Table 2) or healthy donors that were concurrently processed. Mutant CRT proteins were detected using an antibody (anti-CRT[Cmut]) directed against 22 amino acids unique to the carboxy domain sequence of MPN-linked mutant CRT proteins.17 We also used a commercial antibody that binds to the nonmutated amino-terminal domain (anti-CRT[N]) of the CRT protein present in both WT and mutant CRT proteins. Using flow cytometry, platelets were gated on singlets, followed by gating for CD41 (GPIIb) present on the surface of platelets in a heterodimeric complex with CD61 (GPIIIa).30 CD62p (P-selectin), was used to identify activated platelets 31,32 (Figure 1A).
Platelets from patients with MPN are characterized by the cell surface expression of mutant calreticulin. Platelets were isolated from the blood samples of patients with mutant CRT-expressing MPN and healthy controls. (A) Gating strategy used for the analysis of unfixed CD41+ and CD62p+ platelets by flow cytometry. (B-C) Bar graphs indicate flow cytometry-based detection of staining for CD41 (B) and CRT (detected using an N-domain–specific antibody, anti-CRT[N]) (C) in healthy donor platelets either without fixation (surface) or after fixation and permeabilization (total) (n = 7). Relative MFI values after surface and total staining are plotted, assuming MFI values for total CD41 (B) or total CRT (C) as 100%. Paired t tests were used for determining the statistical significance. (D-G) Flow cytometry–based detection of surface CRT on unfixed CD41+ (D-E) and unfixed CD62p+ (F-G) platelets. Representative histograms and bar graphs show the MFI of surface CRT detected on platelets using either mutant CRT-specific antibody (anti-CRT [Cmut]) or anti-CRT (N) antibody. Platelets from patients with MPN and healthy controls (HC) that were processed simultaneously were analyzed as pairs for these measurements. Data are separately analyzed for Del52 (n = 12 for CD41+ platelets and n = 9 for CD62p+ platelets) and Ins5 (n = 8 for CD41+ platelets and n = 8 for CD62p+ platelets) patient samples. Error bars show the standard error of the mean. Statistical significance indicated by P values was determined using GraphPad Prism and paired t test analyses. The heterogeneity of CD41 expression in the representative panel shown in A most likely relates to the limiting amount of antibody, as similar results were obtained based on CD41hi-gating vs gating on all single cells. FSC-H, Forward Scatter-Height; SSC-A, Side Scatter-Area.
Platelets from patients with MPN are characterized by the cell surface expression of mutant calreticulin. Platelets were isolated from the blood samples of patients with mutant CRT-expressing MPN and healthy controls. (A) Gating strategy used for the analysis of unfixed CD41+ and CD62p+ platelets by flow cytometry. (B-C) Bar graphs indicate flow cytometry-based detection of staining for CD41 (B) and CRT (detected using an N-domain–specific antibody, anti-CRT[N]) (C) in healthy donor platelets either without fixation (surface) or after fixation and permeabilization (total) (n = 7). Relative MFI values after surface and total staining are plotted, assuming MFI values for total CD41 (B) or total CRT (C) as 100%. Paired t tests were used for determining the statistical significance. (D-G) Flow cytometry–based detection of surface CRT on unfixed CD41+ (D-E) and unfixed CD62p+ (F-G) platelets. Representative histograms and bar graphs show the MFI of surface CRT detected on platelets using either mutant CRT-specific antibody (anti-CRT [Cmut]) or anti-CRT (N) antibody. Platelets from patients with MPN and healthy controls (HC) that were processed simultaneously were analyzed as pairs for these measurements. Data are separately analyzed for Del52 (n = 12 for CD41+ platelets and n = 9 for CD62p+ platelets) and Ins5 (n = 8 for CD41+ platelets and n = 8 for CD62p+ platelets) patient samples. Error bars show the standard error of the mean. Statistical significance indicated by P values was determined using GraphPad Prism and paired t test analyses. The heterogeneity of CD41 expression in the representative panel shown in A most likely relates to the limiting amount of antibody, as similar results were obtained based on CD41hi-gating vs gating on all single cells. FSC-H, Forward Scatter-Height; SSC-A, Side Scatter-Area.
We first examined CD41 and CRT staining patterns in healthy donor platelets by flow cytometry following the staining of platelets without fixation/permeabilization (surface) or after fixation and membrane permeabilization (total). Setting the detectable protein levels in permeabilized platelets at 100%, a considerable fraction of the total CD41 was detected on the surface of the platelets (Figure 1B). Calreticulin, on the other hand, is predominantly ER localized but translocates to the cell surface under certain conditions.33 Our results showed low background level staining of CRT with the anti-CRT(N) antibody in nonpermeabilized platelets (surface) compared with permeabilized platelets (total), confirming the predominant intracellular localization of CRTWT in platelets (Figure 1C). Compared with healthy donor platelets, we observed significantly higher mean fluorescence intensity (MFI) values of surface staining with both anti-CRT(Cmut) (Figure 1D) and anti-CRT(N) (Figure 1E) antibodies on CD41+ platelets from patients with MPN. A similar trend was noted for CD62p+ platelets (activated platelets; Figure 1F-G). These results demonstrate that MPN-linked mutant CRT proteins (both Ins5 and Del52) were detectable on the surface of platelets isolated from patients with MPN.
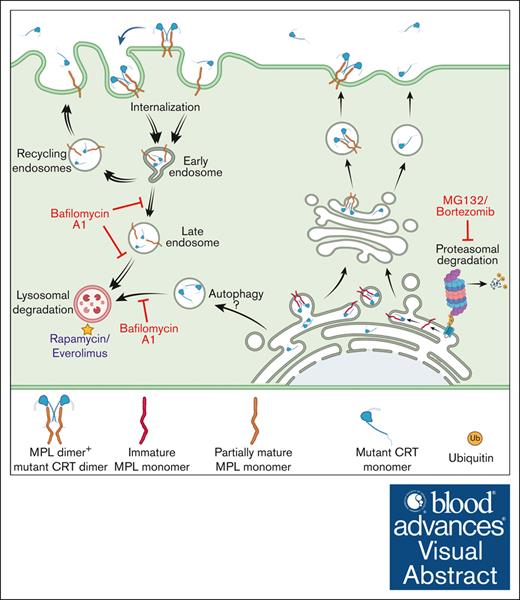
Low-surface MPL levels in platelets of patients with MPN
We also measured the levels of MPL protein in platelets isolated from patients with MPN who expressed mutant CRT proteins and from healthy donors. MPL levels were measured using a commercial anti-MPL antibody (anti-CD110 clone 1.6.1).34 We stained unfixed platelets for surface MPL and fixed/permeabilized platelets for total (surface and intracellular) MPL. The respective gating strategies are shown in Figures 1A and 2A. CD41+ platelets isolated from most patients with MPN showed lower surface MPL levels than healthy donor platelets, suggesting specific downmodulation of surface MPL in the platelets of patients with MPN that overrides normal MPL expression variations among individuals (Figure 2B-C). CD41+ platelets also generally exhibited lower total MPL levels than healthy donor platelets processed in parallel (Figure 2D-E). Platelet lysates from patients with MPN compared with healthy donors exhibit a trend toward lower MPL protein levels, as detected by immunoblotting (Figure 2F). Thus, platelets from patients with MPN-expressing CRT mutants exhibited low surface and total MPL protein levels, which is a potential biomarker for mutant CRT+ MPN.
Downmodulation of MPL in platelets of patients with MPN. (A) Gating strategy used for analysis of fixed and permeabilized platelets by flow cytometry. (B,D) Representative histograms showing surface (B) or total (D) MPL staining with an anti-MPL antibody in platelets from patients with MPN (red histograms) or same-day healthy control (HC) platelets (blue histograms) in unfixed (B) or fixed and permeabilized (D) platelets. (C,E) MFI values of the surface (C; n = 12 for Del52 and n = 7 for Ins5 samples) and total (E, n = 19 for Del52 and n = 7 for Ins5 samples) MPL staining on platelets. Each dot in (C) and (E) represents the MFI value for an individual MPN or HC platelet sample, whereas the lines connect pairs of samples of HC and patients with MPN that were processed in parallel. Statistical significance indicated by P values was determined using GraphPad Prism and paired t test analyses. (F) Representative blots showing MPL and mutant CRT protein levels in the lysates of platelets from HC or patients with MPN subjected to SDS-PAGE under reducing conditions, followed by immunoblotting. Anti-MPL and anti-CRT(Cmut) antibodies were used for the detection of MPL and mutant CRT proteins, respectively. GAPDH blot shows the relative loading of the lysates. The heterogeneity of CD41 expression in the representative panel shown in A most likely relates to the limiting amount of antibody, as similar results were obtained based on CD41hi-gating vs gating on all single cells. FSC-A, Forward Scatter-Area; FSC-H, Forward Scatter-Height; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SSC-A, Side Scatter-Area; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Downmodulation of MPL in platelets of patients with MPN. (A) Gating strategy used for analysis of fixed and permeabilized platelets by flow cytometry. (B,D) Representative histograms showing surface (B) or total (D) MPL staining with an anti-MPL antibody in platelets from patients with MPN (red histograms) or same-day healthy control (HC) platelets (blue histograms) in unfixed (B) or fixed and permeabilized (D) platelets. (C,E) MFI values of the surface (C; n = 12 for Del52 and n = 7 for Ins5 samples) and total (E, n = 19 for Del52 and n = 7 for Ins5 samples) MPL staining on platelets. Each dot in (C) and (E) represents the MFI value for an individual MPN or HC platelet sample, whereas the lines connect pairs of samples of HC and patients with MPN that were processed in parallel. Statistical significance indicated by P values was determined using GraphPad Prism and paired t test analyses. (F) Representative blots showing MPL and mutant CRT protein levels in the lysates of platelets from HC or patients with MPN subjected to SDS-PAGE under reducing conditions, followed by immunoblotting. Anti-MPL and anti-CRT(Cmut) antibodies were used for the detection of MPL and mutant CRT proteins, respectively. GAPDH blot shows the relative loading of the lysates. The heterogeneity of CD41 expression in the representative panel shown in A most likely relates to the limiting amount of antibody, as similar results were obtained based on CD41hi-gating vs gating on all single cells. FSC-A, Forward Scatter-Area; FSC-H, Forward Scatter-Height; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SSC-A, Side Scatter-Area; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
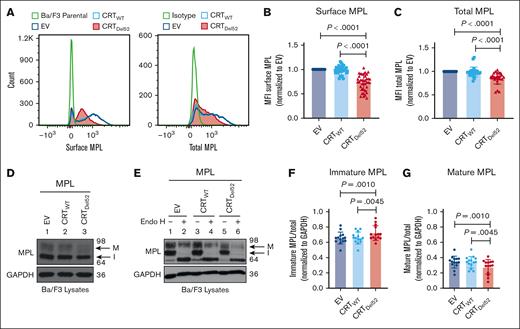
Reduction of total and surface human MPL levels when coexpressed with CRTDel52 in Ba/F3 cells
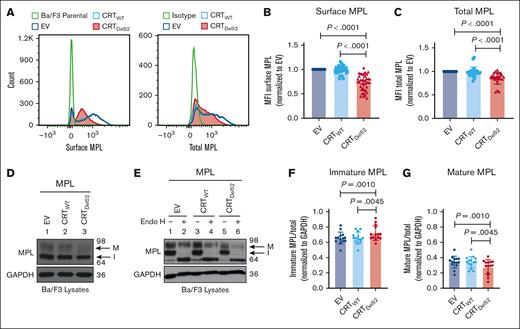
To further investigate whether surface MPL downmodulation is induced by the coexpression of MPN-linked CRT mutants, we expressed human MPL protein in the murine pro–B-cell line, Ba/F3, together with either CRTWT, Del52 (CRTDel52), or the empty vector control (EV). We observed a significant reduction in surface and total MPL protein levels in Ba/F3-MPL CRTDel52 cells compared with those in CRTWT-expressing or EV cells (Figure 3A-C).
Reduction in total and surface human MPL levels when coexpressed with CRTDel52 in Ba/F3 cells. IL-3-dependent murine Ba/F3 cells were transduced for coexpression of human MPL and either CRTWT or CRTDel52 protein. Ba/F3 cells expressing only human MPL protein (EV) were included as controls. (A) Representative histograms showing surface and total MPL staining detected by flow cytometry in Ba/F3-MPL cells expressing either MPL alone or MPL along with CRTWT or CRTDel52, as indicated. (B) Bar graphs show quantification of MFI values of surface MPL (B) and total MPL (C) measured by flow cytometry in CRTWT or CRTDel52 expressing cells normalized to the MFI values of EV cells within the same experiments. The data points represent measurements taken in 38 (B) and 25 (C) independent experiments using cells from ∼8 different retroviral infections. P values show statistical significance determined by paired t test analyses using GraphPad Prism. (D-E) Representative immunoblots (D, n = 14; E, n = 3) showing the levels of total MPL protein in Ba/F3 cells coexpressing either CRTDel52 or CRTWT compared with EV control cells (D) and without or with Endo H digestion (E). GAPDH is shown as a loading control. The top and bottom bands in the MPL blots represent the mature (M) and immature (I) forms of MPL proteins, respectively. (F-G) Bar graphs show the fraction of immature (F) and mature (G) human MPL protein in EV, CRTWT, and CRTDel52-expressing cells, quantified from the intensities of the mature and immature MPL bands from the immunoblots, normalized to the intensities of GAPDH bands. Image J was used to quantify band intensities. Paired t tests were used in GraphPad Prism to determine statistical significance, as indicated by the P value.
Reduction in total and surface human MPL levels when coexpressed with CRTDel52 in Ba/F3 cells. IL-3-dependent murine Ba/F3 cells were transduced for coexpression of human MPL and either CRTWT or CRTDel52 protein. Ba/F3 cells expressing only human MPL protein (EV) were included as controls. (A) Representative histograms showing surface and total MPL staining detected by flow cytometry in Ba/F3-MPL cells expressing either MPL alone or MPL along with CRTWT or CRTDel52, as indicated. (B) Bar graphs show quantification of MFI values of surface MPL (B) and total MPL (C) measured by flow cytometry in CRTWT or CRTDel52 expressing cells normalized to the MFI values of EV cells within the same experiments. The data points represent measurements taken in 38 (B) and 25 (C) independent experiments using cells from ∼8 different retroviral infections. P values show statistical significance determined by paired t test analyses using GraphPad Prism. (D-E) Representative immunoblots (D, n = 14; E, n = 3) showing the levels of total MPL protein in Ba/F3 cells coexpressing either CRTDel52 or CRTWT compared with EV control cells (D) and without or with Endo H digestion (E). GAPDH is shown as a loading control. The top and bottom bands in the MPL blots represent the mature (M) and immature (I) forms of MPL proteins, respectively. (F-G) Bar graphs show the fraction of immature (F) and mature (G) human MPL protein in EV, CRTWT, and CRTDel52-expressing cells, quantified from the intensities of the mature and immature MPL bands from the immunoblots, normalized to the intensities of GAPDH bands. Image J was used to quantify band intensities. Paired t tests were used in GraphPad Prism to determine statistical significance, as indicated by the P value.
Two distinct forms of MPL protein were detected (with anti-MPL antibody- 06944 from EMD Millipore) in the lysates using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.35 Endoglycosidase H (Endo H) cleaves immature N-glycans acquired by glycoproteins in the ER (Endo H-sensitive) but not complex glycans (Endo H-resistant) that have been processed by enzymes within the Golgi complex during glycoprotein trafficking to the cell surface. The slower migrating MPL band (just below the 98 kDa marker in Figure 3D-E) represents the mature MPL protein (M) based on its resistance to Endo H digestion. The faster migrating band (halfway between the 98 kDa and 64 kDa markers in Figure 3D-E) represents the immature MPL (I) band, as Endo H digestion causes a shift of this band to a lower molecular weight (Figure 3E). We observed an increase in the fraction of immature MPL in CRTDel52-expressing cells (Figure 3F), with a concomitant decrease in the mature MPL fraction (Figure 3G) when compared with EV or CRTWT-expressing cells.
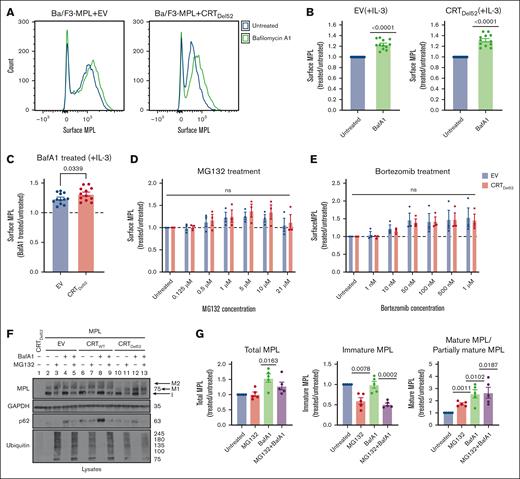
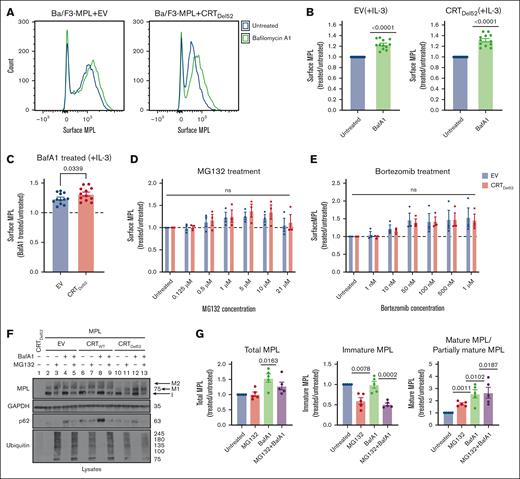
Lysosomal degradation of surface MPL is enhanced in the presence of CRTDel52
We next undertook experiments to better understand the influences of protein degradation on surface MPL levels in CRTDel52-expressing cells, as well as the relative prevalence of distinct MPL glycoforms in the presence of CRTDel52. Ba/F3-MPL cells were treated with bafilomycin A1 (BafA1), an inhibitor of lysosomal acidification, endosomal maturation, and lysosomal degradation.36,37 BafA1 treatment increased surface MPL levels measured by flow cytometry in the EV control cells, as well as in CRTDel52-expressing Ba/F3-MPL cells (Figures 4A-B). However, the rescue of surface MPL upon BafA1 treatment was significantly higher in Ba/F3-MPL cells expressing CRTDel52 than that in EV cells (Figure 4C), indicating increased CRTDel52–mediated MPL degradation. Similar trends were noted when comparing CRTDel52-expressing and CRTWT-overexpressing cells (supplemental Figure 1A). Thus, lysosomal degradation of MPL is not specific to CRTDel52-expressing cells but the presence of CRTDel52 appears to enhance MPL degradation via the lysosomal pathway. We further examined the possible additional role of proteasomal degradation in the downregulation of surface MPL levels in cells expressing CRTDel52. Ba/F3-MPL cells were treated with proteasomal degradation inhibitors, MG132 and bortezomib. Compared with the untreated condition, both EV and CRTDel52-expressing cells exhibited a trend toward an increase in the levels of surface MPL, particularly at the lower concentrations of MG132 (0.5-10 μM; Figure 4D) and upon bortezomib treatment (Figure 4E). However, no significant differences were observed in drug-induced changes in surface MPL levels between EV and CRTDel52 cells (Figure 4D-E). MPL is shown to undergo proteasomal degradation in the presence of TPO.29 When Ba/F3-MPL EV cells cultured in the absence of interleukin-3 (IL-3) but in the presence of human TPO were treated with MG132 and bortezomib, there was a consistent but nonsignificant increase in the cell surface MPL, as observed for Ba/F3-MPL EV cells cultured in the presence of IL-3 but in the absence of TPO (supplemental Figure 1B-C). Overall, it appears that both lysosomal and proteasomal pathways can contribute to MPL degradation, but lysosomal degradation of surface MPL is dominant, particularly in the presence of CRTDel52.
Inhibition of lysosomal acidification rescues cell surface levels of MPL protein more significantly in the presence of CRTDel52. (A-C) Murine Ba/F3-MPL control (EV) cells or those expressing CRTDel52 were treated with 100 nM BafA1 for 4 hours in media with IL-3. Untreated cells are included for comparison. The surface MPL levels were detected using flow cytometry. (A) Representative histograms of the surface MPL levels with and without BafA1 treatment in both cell lines. (B) Average MFI for surface MPL levels after BafA1 treatment plotted as a ratio to the levels in untreated Ba/F3 cells. One-sample t tests were used for determining the statistical significance. (C) Comparison of BafA1-mediated rescue of surface MPL in control (EV) and CRTDel52 cells. The P value was calculated using a paired t test analysis. Panels B and C include data from 12 experiments from 4 independent transductions of BaF3-MPL cells. (D- E) Ba/F3-MPL control (EV) and Ba/F3-MPL CRTDel52 cells were treated with inhibitors of proteasomal degradation, MG132 (D) or bortezomib (E) at the indicated concentrations for 4 hours at 37°C in media with IL-3. Surface MPL levels measured by flow cytometry are plotted as a ratio of MFI values in treated relative to untreated cells (n = 3). Multiple paired t-tests were used for determining the statistical significance. (F) Representative blots (n = 5) for MPL in lysates of Ba/F3-MPL (EV), Ba/F3-MPL-CRTWT, or Ba/F3-MPL-CRTDel52 cells as indicated, treated with 21 μM MG132, 100 nM BafA1, or both. The immature form of MPL protein (I) and 2 distinct mature forms, M1 (partially mature MPL expressed in CRTDel52 cells) and M2 (detected in CRTWT-expressing cells), are indicated within the MPL blot. Ubiquitin and p62 were probed as markers to show successful inhibition of proteasomal and lysosomal degradation, respectively. (G) Bar graphs show quantification of total, immature, and mature/partially mature MPL proteins in Ba/F3-MPL CRTDel52 cells treated with MG132 and/or BafA1 normalized to the values in untreated cells based on immunoblots. ImageJ was used for the quantification of blots. One-sample t test was used to determine the statistical significance. Graphs were plotted using GraphPad Prism. ns, nonsignificant.
Inhibition of lysosomal acidification rescues cell surface levels of MPL protein more significantly in the presence of CRTDel52. (A-C) Murine Ba/F3-MPL control (EV) cells or those expressing CRTDel52 were treated with 100 nM BafA1 for 4 hours in media with IL-3. Untreated cells are included for comparison. The surface MPL levels were detected using flow cytometry. (A) Representative histograms of the surface MPL levels with and without BafA1 treatment in both cell lines. (B) Average MFI for surface MPL levels after BafA1 treatment plotted as a ratio to the levels in untreated Ba/F3 cells. One-sample t tests were used for determining the statistical significance. (C) Comparison of BafA1-mediated rescue of surface MPL in control (EV) and CRTDel52 cells. The P value was calculated using a paired t test analysis. Panels B and C include data from 12 experiments from 4 independent transductions of BaF3-MPL cells. (D- E) Ba/F3-MPL control (EV) and Ba/F3-MPL CRTDel52 cells were treated with inhibitors of proteasomal degradation, MG132 (D) or bortezomib (E) at the indicated concentrations for 4 hours at 37°C in media with IL-3. Surface MPL levels measured by flow cytometry are plotted as a ratio of MFI values in treated relative to untreated cells (n = 3). Multiple paired t-tests were used for determining the statistical significance. (F) Representative blots (n = 5) for MPL in lysates of Ba/F3-MPL (EV), Ba/F3-MPL-CRTWT, or Ba/F3-MPL-CRTDel52 cells as indicated, treated with 21 μM MG132, 100 nM BafA1, or both. The immature form of MPL protein (I) and 2 distinct mature forms, M1 (partially mature MPL expressed in CRTDel52 cells) and M2 (detected in CRTWT-expressing cells), are indicated within the MPL blot. Ubiquitin and p62 were probed as markers to show successful inhibition of proteasomal and lysosomal degradation, respectively. (G) Bar graphs show quantification of total, immature, and mature/partially mature MPL proteins in Ba/F3-MPL CRTDel52 cells treated with MG132 and/or BafA1 normalized to the values in untreated cells based on immunoblots. ImageJ was used for the quantification of blots. One-sample t test was used to determine the statistical significance. Graphs were plotted using GraphPad Prism. ns, nonsignificant.
Rescue of surface MPL upon BafA1 treatment was also reflected by an increase in the mature and partially mature MPL fractions in lysates of Ba/F3-MPL CRTDel52, as well as in Ba/F3-MPL CRTWT and EV cells, based on immunoblots (Figure 4F, lane 2 compared with 4, 6 compared with 8, and 10 compared with 12; Figure 4G; supplemental Figure1D). On the other hand, 21 μM MG132 treatment induced an increase in mature MPL along with a parallel reduction in the immature MPL levels (band indicated as ‘I’ in Figure 4F; lane 2 compared with 3, 6 compared with 7, and 10 compared with 11; Figure 4G; supplemental Figure 1D) in all cells. Similar trends were noted in cells treated with 100 nM bortezomib (supplemental Figure 1D). These effects were observed at higher concentrations of MG132 or bortezomib (supplemental Figure 1E) and may correspond to forced MPL exit from the ER and glycan maturation under conditions in which ER-associated degradation is blocked via proteasome inhibition.
The immunoblots also showed that cells coexpressing CRTDel52 and MPL had a lower molecular weight glycoform of mature MPL (Figure 4F, lanes 10-13; supplemental Figure 1D, lanes 7-12, band labeled M1) compared with EV or CRTWT-expressing cells (Figure 4F, lanes 2-9; supplemental Figure 1D, band labeled M2). This observation is consistent with previous findings that the interaction of CRTDel52 with MPL prevents the maturation of N117-linked glycans in MPL.16 Overall, the findings in Figure 4 show that in cells expressing CRTDel52, BafA1 treatment significantly increased the surface (Figure 4A-C), partially mature, and total MPL, but not immature MPL (Figure 4G). Thus, lysosomal degradation of the cell surface and partially mature MPL is likely to underlie the lower cell surface levels of MPL, as well as the higher fraction of immature MPL (Figure 3E-G) observed in cells expressing CRTDel52. Conversely, proteasomal inhibition increased the partially mature MPL fraction, but not the total MPL (Figure 4G), and there was a nonsignificant increase in surface MPL (Figure 4D-E).
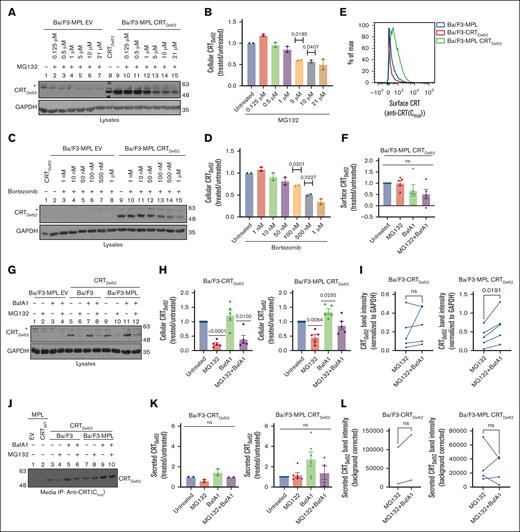
Inhibiting lysosomal degradation rescues CRTDel52 levels
We next assessed pathways relevant to CRTDel52 degradation. When Ba/F3-MPL CRTDel52 cells were treated with different concentrations of MG132 and bortezomib, we observed a small, nonsignificant increase in the amount of CRTDel52 in Ba/F3-MPL cells at low MG132 and bortezomib concentrations. However, CRTDel52 levels notably decreased upon treatment with higher concentrations of MG132 (1-21 μM; Figure 5A-B) and bortezomib (50-1 μM; Figure 5C-D), and the levels of CRTDel52 were the lowest upon treatment with the highest concentrations of MG132 (21 μM) and bortezomib (1 μM). Thus, higher concentrations of proteasomal inhibitors promote CRTDel52 degradation in Ba/F3-MPL cells.
Inhibition of lysosomal degradation rescues CRTDel52 levels, whereas proteasomal pathway inhibition promotes CRTDel52 degradation. (A-D) Representative immunoblots (n = 2) showing CRTDel52 levels (A,C) in the lysates of control (EV) or CRTDel52 expressing Ba/F3-MPL cells treated for 4 hours with different concentrations of MG132 (A) or bortezomib (C). Bar graphs (B,D) show quantification of CRTDel52 band intensities from blots of Ba/F3-MPL CRTDel52 cells (n = 2) treated with different concentrations of MG132 (B) or bortezomib (D), normalized to the CRTDel52 band intensities in untreated cells. (E) Representative histograms of surface CRT detected by flow cytometry using anti-CRT(Cmut) antibody in Ba/F3-MPL, Ba/F3-CRTDel52, and Ba/F3-MPL CRTDel52 cells. (F) The bar graph shows the MFI of surface CRT, detected by anti-CRT(Cmut) antibody, on Ba/F3-MPL CRTDel52 cells treated with 21 μM MG132 and/or 100 nM BafA1, normalized to the MFI values for untreated cells (n = 5). (G-I) Ba/F3-MPL, Ba/F3-CRTDel52, or Ba/F3-MPL CRTDel52 cells as indicated were treated with 21 μM MG132, 100 nM BafA1, or both drugs for 4 hours at 37°C in media with IL-3. Representative blots (n = 5) showing the levels of cellular CRTDel52 (G). Graphs show band intensities of CRTDel52 quantified from immunoblots of lysates from drug–treated Ba/F3-CRTDel52 or Ba/F3-MPL CRTDel52 cells normalized to the band intensities of untreated cells (H) or paired comparisons of band intensities from MG132-treated cells relative to those of cells treated with MG132 + BafA1 (I). (J) Representative blot of secreted CRTDel52 IP with anti-CRT(Cmut) antibody from the cell culture media of Ba/F3-CRTDel52 cells (n = 2) and Ba/F3-MPL CRTDel52 cells (n = 6) that were either untreated or treated with 21 μM MG132 and/or 100 nM BafA1 for 4 hours at 37°C in media with IL-3. (K-L) Quantification of secreted CRTDel52 band intensities from IP/immunoblots of drug-treated cells, normalized to the values for untreated cells (K). Line graphs show secreted CRTDel52 band intensities from MG132-treated cells compared to those of cells treated with MG132 + BafA1 (L). Each line in graphs I and L represents an individual experiment and lines connect data points for the indicated treatments within the experiments. CRTDel52 (A,C,G,J) was probed using an anti-CRT(Cmut) antibody. GAPDH blots in panels A,C,G show equal loading of lysates in the different lanes. ImageJ was used for the quantification of blots. Graphs were plotted using GraphPad Prism. Statistical significance was determined using one-sample t tests in panels B,D,F,H,K and paired t tests in panels I,L. P value <0.05 are shown. The bands marked by asterisk in panels A, C and G are non-specific bands. ns, nonsignificant.
Inhibition of lysosomal degradation rescues CRTDel52 levels, whereas proteasomal pathway inhibition promotes CRTDel52 degradation. (A-D) Representative immunoblots (n = 2) showing CRTDel52 levels (A,C) in the lysates of control (EV) or CRTDel52 expressing Ba/F3-MPL cells treated for 4 hours with different concentrations of MG132 (A) or bortezomib (C). Bar graphs (B,D) show quantification of CRTDel52 band intensities from blots of Ba/F3-MPL CRTDel52 cells (n = 2) treated with different concentrations of MG132 (B) or bortezomib (D), normalized to the CRTDel52 band intensities in untreated cells. (E) Representative histograms of surface CRT detected by flow cytometry using anti-CRT(Cmut) antibody in Ba/F3-MPL, Ba/F3-CRTDel52, and Ba/F3-MPL CRTDel52 cells. (F) The bar graph shows the MFI of surface CRT, detected by anti-CRT(Cmut) antibody, on Ba/F3-MPL CRTDel52 cells treated with 21 μM MG132 and/or 100 nM BafA1, normalized to the MFI values for untreated cells (n = 5). (G-I) Ba/F3-MPL, Ba/F3-CRTDel52, or Ba/F3-MPL CRTDel52 cells as indicated were treated with 21 μM MG132, 100 nM BafA1, or both drugs for 4 hours at 37°C in media with IL-3. Representative blots (n = 5) showing the levels of cellular CRTDel52 (G). Graphs show band intensities of CRTDel52 quantified from immunoblots of lysates from drug–treated Ba/F3-CRTDel52 or Ba/F3-MPL CRTDel52 cells normalized to the band intensities of untreated cells (H) or paired comparisons of band intensities from MG132-treated cells relative to those of cells treated with MG132 + BafA1 (I). (J) Representative blot of secreted CRTDel52 IP with anti-CRT(Cmut) antibody from the cell culture media of Ba/F3-CRTDel52 cells (n = 2) and Ba/F3-MPL CRTDel52 cells (n = 6) that were either untreated or treated with 21 μM MG132 and/or 100 nM BafA1 for 4 hours at 37°C in media with IL-3. (K-L) Quantification of secreted CRTDel52 band intensities from IP/immunoblots of drug-treated cells, normalized to the values for untreated cells (K). Line graphs show secreted CRTDel52 band intensities from MG132-treated cells compared to those of cells treated with MG132 + BafA1 (L). Each line in graphs I and L represents an individual experiment and lines connect data points for the indicated treatments within the experiments. CRTDel52 (A,C,G,J) was probed using an anti-CRT(Cmut) antibody. GAPDH blots in panels A,C,G show equal loading of lysates in the different lanes. ImageJ was used for the quantification of blots. Graphs were plotted using GraphPad Prism. Statistical significance was determined using one-sample t tests in panels B,D,F,H,K and paired t tests in panels I,L. P value <0.05 are shown. The bands marked by asterisk in panels A, C and G are non-specific bands. ns, nonsignificant.
We further examined the changes in cell surface CRTDel52 levels in Ba/F3 treated with 21 μM MG132, 100 nM BafA1, or both. Under all conditions, flow cytometric analyses using the anti-CRT(Cmut) antibody 17 indicated a low to undetectable surface expression of CRTDel52 in the absence of MPL (Figure 5E). On the other hand, cell surface CRTDel52 was better detectable in cells expressing MPL but neither MG132 nor BafA1 significantly affected surface CRTDel52 expression (Figure 5E-F). Because MPL and CRTDel52 are known to interact at the cell surface,5 it is somewhat surprising that BafA1 treatment rescued surface MPL (Figure 4B-C) but did not affect surface CRTDel52 levels (Figure 5F). It is possible that, although the partially mature forms of MPL and CRTDel52 are internalized from the cell surface as a complex, the proteins are not necessarily recycled to plasma membrane as a complex. Although MPL recycling rescues surface MPL levels, CRTDel52 is mostly secreted, as indicated by our findings below. Notably however, as observed for MPL (Figure 4), BafA1 treatment increased cellular (lysate) CRTDel52 levels in Ba/F3-MPL cells compared with untreated cells (CRTDel52 blot in Figure 5G, lanes 9 vs 11). BafA1-mediated increase in CRTDel52 levels was also observed in Ba/F3 cells in the absence of MPL coexpression; however, the rescue of cellular CRTDel52 levels was only significant in cells coexpressing MPL (Figure 5H). On the other hand, treatment with MG132 (21 μM) promoted CRTDel52 degradation independently of MPL coexpression (CRTDel52 blot in Figure 5G, lanes 5 vs 6 and lanes 9 vs 10; Figure 5H).
Paired comparisons of the CRTDel52 band intensities in the MG132 alone and MG132 + BafA1 conditions showed increased recovery of CRTDel52 in the MG132 + BafA1 condition, which was statistically significant in Ba/F3-CRTDel52 cells coexpressing MPL but showed similar trends in the absence of MPL (Figure 5I). Similar trends were also noted in a representative experiment comparing 100 nM bortezomib alone with a combination of 100 nM each of bortezomib and BafA1 (supplemental Figure 1D). Thus, high concentrations of proteasome inhibitors may increase lysosomal degradation of cellular CRTDel52. There is precedence regarding such effects of proteasome inhibitors in the literature.38
To compare the effects of degradation inhibitors on CRTDel52 secretion, we immunoprecipitated (IP) CRTDel52 using the anti-CRT(Cmut) antibody17 from the culture supernatant of Ba/F3 cells following treatment with MG132 and/or BafA1. As shown by the representative blot, the absence of bands after IP from EV- or CRTWT–expressing control cells (Figure 5J, lanes 1 and 2) demonstrated the specificity of IP of CRTDel52 from Ba/F3 cells expressing CRTDel52. BafA1 treatment resulted in a trend toward rescue of secreted CRTDel52 in the culture supernatant of Ba/F3 cells, irrespective of MPL coexpression (Figure 5J, lanes 5 and 9; Figure 5K). MG132 treatment resulted in loss of secreted CRTDel52 in the absence of MPL (Figure 5J-K). The combination of BafA1 treatment with MG132 showed nonsignificant changes in secreted CRTDel52 levels in the culture supernatants of Ba/F3 cells (with or without MPL coexpression) when compared with untreated cells (Figure 5K) and with cells treated with MG132 alone (Figure 5L).
CRTDel52 exhibits increased lysosomal localization compared with CRTWT and increases the fraction of MPL in lysosomes
We used a microscopy-based approach to probe the lysosomal localization of CRTDel52 in BafA1-treated cells. Human embryonic kidney 293T (HEK293T) cells were transfected to overexpress human MPL, together with either CRTWT or CRTDel52 and processed for confocal microscopy. Anti-CRT(Cmut) was used to stain CRTDel52, whereas the CRTWT protein was stained with anti-CRT(N). Mutant CRT is previously shown to colocalize with markers of Golgi apparatus5 whereas CRTWT colocalizes with ER markers.3,5 In addition to the perinuclear Golgi-like localization described earlier,5 we observed a vesicular or punctate pattern of CRTDel52 localization in HEK cells (Figure 6A), which has also been reported earlier.9 This was observed under both the untreated and BafA1-treated conditions. CRTWT, on the other hand, exhibited a more homogenous ER-like subcellular localization with fewer puncta, if any (Figure 6A). MPL also showed vesicular/punctate localization in addition to the Golgi-like perinuclear and membrane localization under both untreated and BafA1-treated conditions (Figure 6A). Lysosomes were stained with lysosomal-associated membrane protein 1 (LAMP1). Object-based quantification was undertaken using CellProfiler on BafA1-treated cells (Figure 6B-F). A significantly higher number of puncta were observed for CRTDel52 than for CRTWT (Figure 6B). CRTDel52-expressing cells also showed a significantly higher number of MPL puncta (Figure 6C) and a significantly higher colocalization of MPL and CRTDel52 puncta (Figure 6D). CRTDel52 and MPL puncta displayed colocalization within or in close proximity to LAMP1 structures (Figure 6A, arrowheads in the zoomed inset). Compared with CRTWT, a significantly higher fraction of CRTDel52 puncta colocalized with LAMP1-positive lysosomal structures (Figure 6E). In agreement with this, a significantly higher fraction of lysosomes (LAMP1 structures) displayed colocalization with CRTDel52 and MPL puncta in CRTDel52-expressing cells (Figure 6F) compared with CRTWT-overexpressing cells. The increased lysosomal localization of CRTDel52 supports our finding that the lysosomal pathway is relevant to the regulation of cellular CRTDel52 levels (Figure 5). Furthermore, enhanced lysosomal localization of MPL in CRTDel52-expressing cells supports our finding of a CRTDel52-mediated increase in lysosomal degradation of surface MPL (Figure 4C,F-G).
Inhibition of lysosomal degradation promotes the localization of MPL and CRTDel52 in LAMP1 compartments. HEK293T cells transfected to express human MPL along with either CRTWT or CRTDel52 were either left untreated or treated with 100 nM BafA1 for 4 hours at 37°C, fixed, and immunostained for LAMP1 (green), MPL (red) and either CRTWT (anti-CRT[N]) or CRTDel52 (anti-CRT[Cmut]) (blue) proteins. The nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). (A) Representative Airyscan microscopy images (n = 2) showing a single z-slice of untreated and treated HEK293T cells expressing specific proteins and stained with specific antibodies, as indicated. Zoomed view of the inset is shown and arrowheads point to the CRTDel52 and MPL puncta colocalizing within the LAMP1 compartments. Scale bar is 5 μm in all the images except the zoomed inset images in which the scale bar is 2 μm. (B-F) Graphs show quantification of CRT puncta (B), MPL puncta (C), fraction of MPL puncta colocalizing with CRT (D), fraction of CRT puncta colocalizing with LAMP1 structures (E), and fraction of LAMP1 structures colocalizing with CRT or MPL (F) in BafA1-treated HEK293T cells expressing either CRTDel52 (82 cells from 2 experiments) or CRTWT (91 cells from 2 experiments). Data were quantified using the segmentation and analysis platform CellProfiler. Graphs were plotted using GraphPad Prism and statistical significance was determined using unpaired t tests.
Inhibition of lysosomal degradation promotes the localization of MPL and CRTDel52 in LAMP1 compartments. HEK293T cells transfected to express human MPL along with either CRTWT or CRTDel52 were either left untreated or treated with 100 nM BafA1 for 4 hours at 37°C, fixed, and immunostained for LAMP1 (green), MPL (red) and either CRTWT (anti-CRT[N]) or CRTDel52 (anti-CRT[Cmut]) (blue) proteins. The nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). (A) Representative Airyscan microscopy images (n = 2) showing a single z-slice of untreated and treated HEK293T cells expressing specific proteins and stained with specific antibodies, as indicated. Zoomed view of the inset is shown and arrowheads point to the CRTDel52 and MPL puncta colocalizing within the LAMP1 compartments. Scale bar is 5 μm in all the images except the zoomed inset images in which the scale bar is 2 μm. (B-F) Graphs show quantification of CRT puncta (B), MPL puncta (C), fraction of MPL puncta colocalizing with CRT (D), fraction of CRT puncta colocalizing with LAMP1 structures (E), and fraction of LAMP1 structures colocalizing with CRT or MPL (F) in BafA1-treated HEK293T cells expressing either CRTDel52 (82 cells from 2 experiments) or CRTWT (91 cells from 2 experiments). Data were quantified using the segmentation and analysis platform CellProfiler. Graphs were plotted using GraphPad Prism and statistical significance was determined using unpaired t tests.
mTOR inhibition reduces cytokine-independent proliferation mediated by CRTDel52
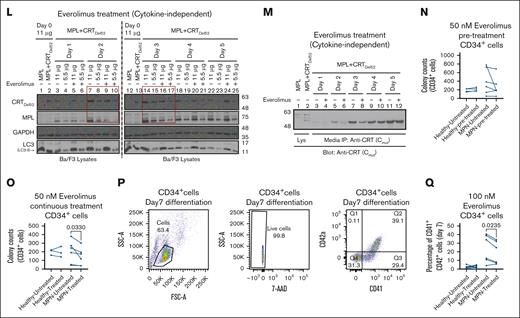
Figures 4 and 5 show that CRTDel52 and MPL were degraded via the lysosomal pathway more significantly in the presence of one another, and inhibition of lysosomal degradation rescued both proteins. Based on these results, we examined whether direct activation of lysosomal degradation using Food and Drug Administration (FDA)-approved clinical drugs could promote CRTDel52 and MPL degradation and interfere with the cell proliferative effects of CRTDel52. For this, we used rapamycin and everolimus, both of which are inhibitors of the mammalian target of rapamycin (mTOR), a protein kinase that inhibits autophagy and promotes cell growth and anabolism in response to several growth factors, nutrients, and other factors.39 We expected that one of the effects of these drugs would be the induction of lysosomal degradation. Ba/F3-MPL EV cells cultured in the presence of IL-3 generally showed normal growth patterns upon treatment with either 50 nM or 100 nM rapamycin (Figure 7A-B) or 100 nM everolimus (Figure 7C-D). A low inhibition of proliferation of Ba/F3-MPL EV cells was measured at early time points upon treatment with rapamycin or everolimus (Figure 7B,D), which could result from the inhibition of the IL-3–dependent signaling pathway by mTOR inhibitors.40 However, EV cells recovered from the effects of mTOR inhibitors at later time points (Figure 7B,D). In contrast, Ba/F3-MPL CRTDel52 cells showed significantly reduced cytokine-independent proliferation upon treatment with rapamycin (Figure 7E-F) and everolimus (Figure 7G-H). A significant reduction of cytokine-independent proliferation was observed after treatment of Ba/F3-MPL CRTDel52 cells with rapamycin starting on day 2 (Figure 7F), as well as with 100 nM everolimus starting on day 1 (Figure 7H). In contrast, mTOR inhibitors had smaller and largely nonsignificant effects on the proliferation of Ba/F3-MPL EV cells cultured in the presence of TPO (Figure 7I-J). Everolimus-mediated inhibition of Ba/F3-MPL CRTDel52 cell proliferation was accompanied by an increase in the fraction of cells in the early stage of apoptosis and a concomitant decrease in the fraction of cells in the S phase of the cell cycle (supplemental Figure 2A-E).
To examine whether the reduced proliferation of Ba/F3-MPL cells treated with rapamycin and everolimus was mediated by the enhanced degradation of MPL and/or CRTDel52, we measured cellular CRTDel52 and MPL levels in the lysates of untreated or treated cells collected at different time points. Immunoblots confirmed that at least at earlier time points (lanes highlighted by boxes), both CRTDel52 and MPL levels were lower in cells treated with rapamycin (Figure 7K, lanes 3-6 and lanes 7-10 corresponding to lysates from days 1 and 2, respectively, after the start of drug treatment) and everolimus (Figure 7L, lanes 7-10 and lanes 14-17 corresponding to lysates from days 2 and 3, respectively, after the start of drug treatment) compared with untreated cells. An everolimus-mediated reduction in CRTDel52 levels was also observed for secreted CRTDel52 in the media based on immunoprecipitation analyses (Figure 7M). Thus, the reduction in cellular CRTDel52 and MPL levels triggered by treatment with mTOR-inhibiting (lysosomal degradation-activating) drugs could partly explain the observed reduction in the cytokine-independent proliferation of Ba/F3-MPL CRTDel52 cells in the presence of rapamycin and everolimus.
Primary CD34+ cells from patients with MPN demonstrate lower colony-forming capacity and reduced differentiation after treatment with everolimus
To check the susceptibility of primary CD34+ cells from patients with CRT-mutated MPN to mTOR inhibition, CFU assays were performed with CD34+ cells isolated from bone marrow samples of patients with MPN (supplemental Table 3) or from healthy donor leukopaks. In the first set of experiments, CD34+ cells were either left untreated or pretreated with everolimus for 24 hours before plating (Figure 7N, pre-treatment). In subsequent experiments, everolimus was incorporated into the plating medium (Figure 7O; continuous treatment). Compared with colony counts under untreated conditions, CD34+ cells from patients with MPN exhibited a decrease in the number of CFUs after pre-treatment with 50 nM everolimus, but the reduction was nonsignificant (Figure 7N; n = 6). Under more stringent conditions involving continuous exposure to 50 nM everolimus (Figure 7O; n = 6), a significant decrease was observed in the number of CFUs derived from CD34+ cells of patients with MPN when compared with untreated cells. There was significant heterogeneity in the measured response, which included a heterogeneous group of available patient samples from different disease states (supplemental Table 3). However, healthy donor CD34+ cells exhibited little to no effect of pre-treatment (n = 4) or continuous treatment (n = 3) with 50 nM everolimus when compared with untreated healthy CD34+ cells (Figure 7N-O). Furthermore, when differentiated into megakaryocytes, CD34+ cells from patients with MPN showed reduced fractions of differentiated CD41+CD42a+ cells in the presence of everolimus, but this was not observed in CD34+ cells from healthy donors (Figure 7Q). Thus, despite the expected variability in primary human samples, the results from Figure 7 clearly indicate that mutant CRT–expressing Ba/F3-MPL cells and primary CD34+ cells from patients with MPN are more susceptible to mTOR inhibition than Ba/F3-MPL (EV) cells and healthy donor CD34+ cells, respectively. The parallel loss in CRTDel52 and MPL is consistent with the model that mTOR inhibitor-mediated activation of the autophagy/lysosomal pathway is detrimental to CRTDel52-mediated cell proliferation.
Discussion
Our results indicate a significant increase in the localization of CRT mutants on the surface of both resting (CD41+) and activated (CD62p+) platelets isolated from patients with MPN when compared with healthy donor platelets (Figure 1D-G), consistent with previous findings in other cells.8-10,16 Although early studies have reported the downregulation of surface MPL in platelets of patients with ET, polycythemia vera, and MF,41,42 whether patients with CRT mutations also have low MPL levels is unknown. Our findings indicate that platelets from most patients with MPN that harbor mutations in CRT gene exhibit low MPL/TPOR levels compared with healthy donor platelets (Figure 2). These findings prompted studies on the role of proteasomal and lysosomal degradation pathways in regulating the dynamics of MPL and mutant CRT proteins in MPNs. The low surface and mature MPL levels measured in Ba/F3 cells expressing both MPL and CRTDel52 confirmed that the observed MPL downmodulation is more acute in cells expressing mutant CRT proteins (Figure 3).
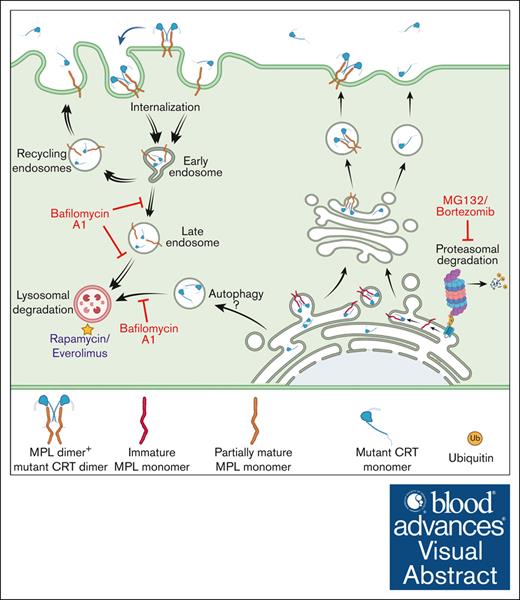
Mature MPL has been suggested to be targeted for degradation by both lysosomal and proteasomal degradation pathways in response to TPO.28,29 On the other hand, MPL downmodulation in Ba/F3-MPL cells expressing the JAK2V617F mutant is attributed to increased ubiquitination and degradation of the receptor by the proteasomal degradation pathway.43 By analogy to these previous findings, we suggest a model wherein low MPL levels on the cell surface in mutant CRT-expressing cells result from MPL signaling activated by mutant CRT proteins, followed by internalization and lysosomal degradation of MPL-mutant CRT complexes. Notably, lysosomal degradation rather than proteasomal degradation of these complexes is prominently induced in the context of the CRT mutants. In support of this model (see the visual abstract), we found that inhibition of lysosomal acidification markedly increased cellular CRTDel52 levels, particularly in the presence of MPL (Figure 5G-H). Additionally, lysosomal degradation of cell surface MPL became more prominent (Figure 4C) and an increased lysosomal localization of MPL was observed when CRTDel52 was coexpressed (Figure 6). Indeed, a synergistic increase in both MPL and CRTDel52 levels was observed upon the inhibition of lysosomal degradation (Figure 4-5).
In contrast to the measured accumulation of cellular CRTDel52 and cell surface MPL after treatment with the inhibitor of lysosomal degradation, BafA1, drug-mediated inhibition of proteasomal degradation reduced cellular CRTDel52 levels while not increasing secreted mutant calreticulin levels (Figure 5A-D,G-K; supplemental Figure 1D). This observation suggests that proteasomal inhibitors enhance the degradation of CRTDel52. These findings are consistent with the observed decrease in mutant CRT protein levels after the inhibition of proteasomal degradation, as reported in an earlier study.7 Reduced CRTDel52 levels after inhibition of proteasomal degradation were likely caused by enhanced CRTDel52 degradation via activation of the autophagy/lysosomal pathway38 (Figure 5I). Recent studies have shown that the proteasomal pathway is upregulated in mutant CRT-expressing cells from patients with MPN.44 Treatment with the proteasomal inhibitor, bortezomib, inhibits the proliferation of mutant CRT–expressing hematopoietic stem cells and megakaryocytes,44,45 which is attributed to the combined targeting of the proteasome and the ER stress response prevalent in cells expressing CRTDel52. Although these studies did not show the effect of proteasomal inhibitors on the expression levels of CRT mutants, it is likely that the proliferation-suppressive effects of proteasomal inhibitors are, at least in part, driven by their ability to induce CRTDel52 degradation.
In contrast to MPL protein levels, the expression of MPN-linked mutant CRT proteins is associated with increased MPL transcription.7,46 In one of these studies, enhanced MPL transcription has been attributed to the enhanced binding of ERp57 and transcription factor Fli1 on the MPL promoter in CRTDel52 expressing cells.46 Although MPL protein levels were not measured in these studies, the data in Figure 2 of this study indicate a dominant loss of MPL expression in platelets despite any gain in mRNA expression, further supporting the model that protein degradation contributes to the low measured MPL protein levels. Downregulation of MPL protein levels, despite increased MPL mRNA expression, was also observed in Ba/F3-MPL cells expressing the JAK2V617F mutant.43
mTOR activity plays a crucial role in lysosomal function by regulating lysosomal biogenesis, lysosomal positioning, and activity of lysosomal proteins.47 mTOR inhibition has been correlated with enhanced lysosomal degradation via different mechanisms, including enhanced lysosomal biogenesis 48 or enhanced lysosomal acidification through the assembly of active V-ATPase at the lysosomal membranes.49 The mTOR inhibitors, rapamycin and everolimus, inhibit the proliferation and survival of cancer cells.50,51 We further showed here that treatment with these drugs suppressed the cytokine-independent proliferation of CRTDel52 expressing Ba/F3-MPL cells (Figure 7A-J). The drugs also induced a decrease in the levels of cellular CRTDel52 and MPL and secreted CRTDel52, which was readily detectable at early time points after drug treatment (Figure 7K-M). We also observed a decrease in the number of CFUs recovered from CD34+ cells isolated from patients with MPN after treatment with everolimus and a reduction in CD34+ cell differentiation into megakaryocytes (Figure 7N-Q). In contrast, everolimus treatment had no measured impact on colony formation or differentiation of CD34+ cells from healthy donors (Figure 7N-Q).
Overall, our findings demonstrate the importance of cellular degradation pathways in the regulation of MPL and mutant CRT protein levels, which are the 2 major factors that control oncogenesis in mutant CRT-linked MPNs. Everolimus (or RAD001) has been approved as an anticancer drug for the treatment of breast cancer, renal cell carcinoma, and certain types of pancreatic and lung cancers. Some studies have indicated that mTOR pathway inhibitors are potential drugs for the treatment of MPNs.52,53 The combination of an mTOR inhibitor, BEZ235 and JAK2 inhibitor, ruxolitinib, showed synergistic effects in the treatment of JAK2V617F mice models of MPNs.54 Our study suggests that mTOR inhibitors are promising targets for the treatment of patients with MPN-expressing CRT mutants and highlight their therapeutic potential.
Acknowledgments
The authors are grateful to all donors and patients who volunteered to donate blood and/or bone marrow samples for this work. The authors thank Amanda Prieur from the University of Michigan Platelet Physiology and Pharmacology Core for collecting healthy donor blood samples. They thank Polk Avery from Talpaz laboratory for coordinating and collecting blood and bone marrow samples of patients with MPNs. They thank Eric Rentchler from the University of Michigan Microscopy Core for providing consultation on quantitative analysis of microscopy images. They also thank Timothy Gondre-Lewis for the support of this work.
This work was funded by the National Institutes of Health (grant R01 AI123957) (M.R.) and the University of Michigan Fast Forward Protein Folding Diseases Initiative.
Authorship
Contribution: A.K. and A.V. designed and performed the experiments, analyzed the data, wrote the original draft, and edited the manuscript; M.K. collected patient blood and bone marrow samples, purified platelets, and edited the manuscript; M.T. is the director of the MPN repository at the University of Michigan; and M.R. designed and supervised the study, obtained funding, analyzed data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: M.T. serves as an advisory board member for Sierra Oncology, Bristol Myers Squibb, Sumitomo, and GlaxoSmithKline/Pfizer; and has received research support from Bristol Myers Squibb, Novartis, Sumitomo, and MorphoSys. The remaining authors declare no competing financial interests.
The current affiliation for A.V. is Department of Ophthalmology & Visual Sciences, Upstate Medical University, Syracuse, NY.
Correspondence: Malini Raghavan, Department of Microbiology and Immunology, University of Michigan Medical School, 5641 Medical Science Building II, Ann Arbor, MI, 48109; email: malinir@umich.edu.
References
Author notes
The original data can be accessed using the ImmPort shared data platform (ImmPort study ID: SDY2663).
The full-text version of this article contains a data supplement.

![Platelets from patients with MPN are characterized by the cell surface expression of mutant calreticulin. Platelets were isolated from the blood samples of patients with mutant CRT-expressing MPN and healthy controls. (A) Gating strategy used for the analysis of unfixed CD41+ and CD62p+ platelets by flow cytometry. (B-C) Bar graphs indicate flow cytometry-based detection of staining for CD41 (B) and CRT (detected using an N-domain–specific antibody, anti-CRT[N]) (C) in healthy donor platelets either without fixation (surface) or after fixation and permeabilization (total) (n = 7). Relative MFI values after surface and total staining are plotted, assuming MFI values for total CD41 (B) or total CRT (C) as 100%. Paired t tests were used for determining the statistical significance. (D-G) Flow cytometry–based detection of surface CRT on unfixed CD41+ (D-E) and unfixed CD62p+ (F-G) platelets. Representative histograms and bar graphs show the MFI of surface CRT detected on platelets using either mutant CRT-specific antibody (anti-CRT [Cmut]) or anti-CRT (N) antibody. Platelets from patients with MPN and healthy controls (HC) that were processed simultaneously were analyzed as pairs for these measurements. Data are separately analyzed for Del52 (n = 12 for CD41+ platelets and n = 9 for CD62p+ platelets) and Ins5 (n = 8 for CD41+ platelets and n = 8 for CD62p+ platelets) patient samples. Error bars show the standard error of the mean. Statistical significance indicated by P values was determined using GraphPad Prism and paired t test analyses. The heterogeneity of CD41 expression in the representative panel shown in A most likely relates to the limiting amount of antibody, as similar results were obtained based on CD41hi-gating vs gating on all single cells. FSC-H, Forward Scatter-Height; SSC-A, Side Scatter-Area.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/13/10.1182_bloodadvances.2023011432/3/m_blooda_adv-2023-011432-gr1.jpeg?Expires=1769393954&Signature=oUypxhJDO-wKtBju69UTf-ojExYrsfbdyHtX0gmxZCQCpdqm1Zs7O12B5PpcXwzufaA8usRNB5zQagWlRGdMhUD10rzE24SXcpOfz9g3uftTdEFZJxYeFR-0LDcwhIxdw73DhUkmatD25kNxHzPZkq8oRuHco6n5StPXMKRbbKBSGPLivC~W~5lCX-8TrDnI9Z3vznwSyVqiCIth8j~9e0eJ17U~PoemHVz~YmPTmd1cjBPhGhb0~Chnm~zQnuAtRstSKdiUkbblr-4ITjhuS3-hE9~bOYEGM7~ST6fCOsy8ewv2FCKzidHjCNQ2hQZqtoo6Qm~Th3gPJ-OOwqQH~w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Inhibition of lysosomal degradation promotes the localization of MPL and CRTDel52 in LAMP1 compartments. HEK293T cells transfected to express human MPL along with either CRTWT or CRTDel52 were either left untreated or treated with 100 nM BafA1 for 4 hours at 37°C, fixed, and immunostained for LAMP1 (green), MPL (red) and either CRTWT (anti-CRT[N]) or CRTDel52 (anti-CRT[Cmut]) (blue) proteins. The nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). (A) Representative Airyscan microscopy images (n = 2) showing a single z-slice of untreated and treated HEK293T cells expressing specific proteins and stained with specific antibodies, as indicated. Zoomed view of the inset is shown and arrowheads point to the CRTDel52 and MPL puncta colocalizing within the LAMP1 compartments. Scale bar is 5 μm in all the images except the zoomed inset images in which the scale bar is 2 μm. (B-F) Graphs show quantification of CRT puncta (B), MPL puncta (C), fraction of MPL puncta colocalizing with CRT (D), fraction of CRT puncta colocalizing with LAMP1 structures (E), and fraction of LAMP1 structures colocalizing with CRT or MPL (F) in BafA1-treated HEK293T cells expressing either CRTDel52 (82 cells from 2 experiments) or CRTWT (91 cells from 2 experiments). Data were quantified using the segmentation and analysis platform CellProfiler. Graphs were plotted using GraphPad Prism and statistical significance was determined using unpaired t tests.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/13/10.1182_bloodadvances.2023011432/3/m_blooda_adv-2023-011432-gr6.jpeg?Expires=1769393954&Signature=YV4PgsOA4vJ7P3Bci9XjHciuHL581xTBNNGnBrO7H4GOjEwF4V5fYlLhzAAOkQQrKa6cgnCFgqAFSbiEjj6jJVq6cZyEQyJzjfCrp0fhKKwaM9x63sew2rdL2V0Tk9OkarE2ngiDjsncZ16hVJkS9rY-dFqv3eSDzFTjBK~hB6wlJ8n~3U7yhfRr7jJfIUov7vYyEGRI~09Rm94vciW09ysileZjIFcAtDxCdwzznf9ReEPJK~CMJ8oTSQ5B5Zq9ispvgi70oDYCCeibvjp-5vKhahzXclY5PnBuBm6~P6hp8JQz09d4sVcmRhtnAZsBs1BcKohFzFAJEpGVaV9uFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Platelets from patients with MPN are characterized by the cell surface expression of mutant calreticulin. Platelets were isolated from the blood samples of patients with mutant CRT-expressing MPN and healthy controls. (A) Gating strategy used for the analysis of unfixed CD41+ and CD62p+ platelets by flow cytometry. (B-C) Bar graphs indicate flow cytometry-based detection of staining for CD41 (B) and CRT (detected using an N-domain–specific antibody, anti-CRT[N]) (C) in healthy donor platelets either without fixation (surface) or after fixation and permeabilization (total) (n = 7). Relative MFI values after surface and total staining are plotted, assuming MFI values for total CD41 (B) or total CRT (C) as 100%. Paired t tests were used for determining the statistical significance. (D-G) Flow cytometry–based detection of surface CRT on unfixed CD41+ (D-E) and unfixed CD62p+ (F-G) platelets. Representative histograms and bar graphs show the MFI of surface CRT detected on platelets using either mutant CRT-specific antibody (anti-CRT [Cmut]) or anti-CRT (N) antibody. Platelets from patients with MPN and healthy controls (HC) that were processed simultaneously were analyzed as pairs for these measurements. Data are separately analyzed for Del52 (n = 12 for CD41+ platelets and n = 9 for CD62p+ platelets) and Ins5 (n = 8 for CD41+ platelets and n = 8 for CD62p+ platelets) patient samples. Error bars show the standard error of the mean. Statistical significance indicated by P values was determined using GraphPad Prism and paired t test analyses. The heterogeneity of CD41 expression in the representative panel shown in A most likely relates to the limiting amount of antibody, as similar results were obtained based on CD41hi-gating vs gating on all single cells. FSC-H, Forward Scatter-Height; SSC-A, Side Scatter-Area.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/13/10.1182_bloodadvances.2023011432/3/m_blooda_adv-2023-011432-gr1.jpeg?Expires=1769393955&Signature=JBXU4H9Jj3BoA0JTzntQwgFLdtmNr7lx~ocxMBknQNmjG~11rSpYA~h3ULQWwV1Rc0o-hKEUOnkSdv9pf61uA5xr~H33oIM32zIPD9-opANY5JbVcaboADE90O-uKFC6rZwb5QMToz6cW8ocwLUL9C3vW44~5WZkNJahN3gU1XN2ooKEgo2gbsfBZKpmpMcLej8V8Z~88UFMa6woN1~M4KMxCvupNB979ayNVAQcAHHFijioF7Vi4LnLSNhH~evxKu8sdAhVqGO8K7HFlFLD5MDUH0goJzmoNRS-KX8kvYrZ8kfzjS~niSbWL0Ml5MXHdX~8w~Mq6Hv8qVMA8WpNCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Inhibition of lysosomal degradation promotes the localization of MPL and CRTDel52 in LAMP1 compartments. HEK293T cells transfected to express human MPL along with either CRTWT or CRTDel52 were either left untreated or treated with 100 nM BafA1 for 4 hours at 37°C, fixed, and immunostained for LAMP1 (green), MPL (red) and either CRTWT (anti-CRT[N]) or CRTDel52 (anti-CRT[Cmut]) (blue) proteins. The nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). (A) Representative Airyscan microscopy images (n = 2) showing a single z-slice of untreated and treated HEK293T cells expressing specific proteins and stained with specific antibodies, as indicated. Zoomed view of the inset is shown and arrowheads point to the CRTDel52 and MPL puncta colocalizing within the LAMP1 compartments. Scale bar is 5 μm in all the images except the zoomed inset images in which the scale bar is 2 μm. (B-F) Graphs show quantification of CRT puncta (B), MPL puncta (C), fraction of MPL puncta colocalizing with CRT (D), fraction of CRT puncta colocalizing with LAMP1 structures (E), and fraction of LAMP1 structures colocalizing with CRT or MPL (F) in BafA1-treated HEK293T cells expressing either CRTDel52 (82 cells from 2 experiments) or CRTWT (91 cells from 2 experiments). Data were quantified using the segmentation and analysis platform CellProfiler. Graphs were plotted using GraphPad Prism and statistical significance was determined using unpaired t tests.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/13/10.1182_bloodadvances.2023011432/3/m_blooda_adv-2023-011432-gr6.jpeg?Expires=1769393955&Signature=2c7nqExyqnZm8Hyvh2ib7lrRu4T68rnvje9Yoz5v-~qQtvOmui0yXX-uG45YG7X-82uGmCNy0~Lf4Eu7dndVH1zaApjIv89BeiEwWJCAL7TulZ-2jYFZ-5WnkavAGzYsCKOkU9N0izsjXNSjdNgALyDfp~V04iQBRJDK4ZjneOPWY-ZF5bo~5EqPz1~rjtPlZWLDjnoAqd4WZFaFemntDehoc5J5Bj-6b-fWnZwRv0DlGPMq~V9EmYNqrLnPc8Kbo74BfvE0bufqJdZicRXD5e3MJKMHjeBTBEspD0lgilizaziLr9mNHw8w~NFBFGhJ6I0Wy8riMa~Db~yKyTQuPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)