Key Points
Patients with multiple myeloma previously treated with T-cell redirection therapy have a significantly lower risk of developing CRS with Tec.
Patients with high-risk cytogenetic features were at a low risk of developing CRS with Tec.
Visual Abstract
Teclistamab (Tec) is a first-in-class BCMA × CD3 bispecific T-cell engager antibody approved for treating multiple myeloma progressing after at least 4 lines of therapy. The objective of this study was to evaluate the rate of cytokine release syndrome (CRS) in patients who were treated with commercial Tec and had prior exposure to other T-cell redirection therapies. A retrospective chart review was performed to identify patients who completed the Tec step-up dosing phase between November 2022 and November 2023. Patients were divided into 2 cohorts based on prior exposure to T-cell redirection therapy (cohort 1: T-cell redirection therapy experienced; cohort 2: T-cell redirection therapy naïve). The primary objective was to compare the differences in the rates of CRS between the 2 cohorts. Univariate and multivariate logistic regression analyses were performed to assess the association between CRS rates with Tec and prior treatment with T-cell redirection therapy. A total of 72 patients were included in the analysis (27 in cohort 1 and 45 in cohort 2). The CRS rates were significantly lower in cohort 1 (37%, n = 10) compared with cohort 2 (80%, n = 36; P = .0004). Based on multivariate logistic regression analysis, patients without prior exposure to T-cell redirection therapy (cohort 2) had about a fourfold increase in the incidence of CRS (95% confidence interval, 1.40-14.90; P = .0002) with Tec. In our study, prior exposure to T-cell redirection therapy reduced the risk of CRS with Tec during the step-up dosing phase. This observation will allow for the optimization of CRS prophylactic strategies for Tec.
Introduction
The therapeutic landscape of multiple myeloma (MM) has witnessed dramatic changes over the past few years, which translated into a significant improvement in survival outcomes.1 Nonetheless, MM remains a chronically managed and incurable disease for the most patients with a relapsing/remitting clinical course.2 With each relapse or progression, the remission period tends to become shorter as drug-resistant clones continue to emerge. A multicenter retrospective analysis that included 275 patients with MM (of whom 70 had penta-refractory disease, ie, refractory to 2 proteasome inhibitors, 2 immunomodulatory agents, and 1 anti-CD38 monoclonal antibody) revealed dismal clinical outcomes particularly among those with penta-refractory disease, in which the median overall survival was ∼6 months.3 Such real-world data suggest that there is an urgent demand to develop novel agents for this heavily pretreated patient population. T-cell redirection therapy (such as chimeric antigen receptor [CAR] T cells and bispecific T-cell engager antibodies) is a type of immunotherapy that has garnered significant attention and interest recently.4 This treatment modality capitalizes on leveraging the inherent power of the immune system to fight the disease, and thereby produce durable remissions. Teclistamab (Tec) is a first-in-class BCMA × CD3 bispecific T-cell engager antibody directed against BCMA, expressed on the myeloma cells.5 Tec received regulatory approval for treating relapsed/refractory (RR) MM (after at least 4 lines of therapy, which include an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody) based on results of the pivotal phase 1/2 MajesTEC-1 clinical study.6 Notably, unprecedented response rates of 63% were reported in a heavily pretreated patient population who received a median of 5 prior lines of therapy. Cytokine release syndrome (CRS) was one of the most prominent side effects of Tec which occurred in ∼72 % of the patients, similar to what has been observed with other T-cell redirection therapies.7-10 The CRS events were mostly mild to moderate in severity (grade 1, 50% and grade 2, 21%) and generally limited to the dose ramp-up or step-up dosing schedule (step-up dose 1, 43%; step-up dose 2, 35%; and first full treatment dose, 24%).6 Because of patient safety concerns, the manufacturer recommends that Tec be initiated in hospital settings where patients could be closely monitored for any signs and symptoms of CRS for at least 48 hours after each dose of the step-up dosing schedule. Because patients who received T-cell redirection therapies before Tec were excluded from the MajesTEC-1 study, the reported rates of CRS cannot be extrapolated to real-world settings. Given this knowledge gap, the primary objective of the study described herein was to interrogate the impact of prior exposure to T-cell redirection therapies on the incidence rates of CRS with Tec (during the step-up dosing phase).
Methods
Study design
This is a retrospective, single-center study seeking to discern the relationship between prior exposure to T-cell redirection therapies, and the incidence of CRS with Tec.
Patient identification and selection
The Memorial Sloan Kettering Cancer Center plasma cell disorders research database was queried to identify patients who were hospitalized to receive commercial Tec between November 2022 and November 2023 for RR MM (Figure 1). Only patients who completed the Tec step-up dosing schedule (received the first step-up, second step-up, and the first full doses and were monitored for at least 48 hours after each of these 3 doses) were included. Based on prior exposure to T-cell redirection therapies (CAR T cells and/or bispecific antibody therapies), patients were classified into 2 cohorts: cohort 1 comprised of patients who had prior exposure to T-cell redirection therapies (T-cell redirection therapy experienced) whereas cohort 2 had no prior exposure to T-cell redirection therapies (T-cell redirection therapy naïve).
Intervention/treatment and data collection
Patients received premedications at least 45 minutes before each of the 3 step-up doses of Tec per the Memorial Sloan Kettering Cancer Center clinical practice standards. For CRS monitoring, vital sign checks were performed at baseline and every 6 hours during the 48-hour time interval between the step-up doses. The immune effector cell–associated encephalopathy assessment was performed daily (during the step-up dosing schedule) to screen patients for immune effector cell–associated neurotoxicity syndrome (ICANS). CRS and ICANS events were managed per institutional guidelines and the discretion of the treating physician after ruling out an underlying infectious disease.
The electronic medical records were reviewed to extract the following data: patient demographics, disease characteristics (including the presence of high-risk cytogenetic features: t(4;14), t(14;16), t(14;20), TP53 mutations, 17p deletion, and 1q gain/amplification), prior lines of therapy, administration dates of the Tec step-up doses, CRS/ICANS grades, dates of CRS/ICANS events, and supportive therapy for the management of CRS and/or ICANS. For patients in cohort 1, the type and dates of prior T-cell redirection therapy (CAR T cells and/or bispecific antibody) were also collected during the medical record review.
Statistical analysis
Baseline characteristics were described as either median (interquartile range [IQR]) or percentage (frequency). The χ2/Fisher exact time (with 1 degree of freedom) was used to compare the differences in the rates and the grades of CRS between the 2 study cohorts. Univariate logistic regression analysis was performed initially to identify and select significant variables to include in the multivariate logistic regression analysis (only variables with a P value <.05 were selected and incorporated into the model). Multivariate logistic regression analysis was performed to determine whether prior exposure to T-cell redirection therapy could predict the incidence of CRS with Tec after adjusting for significant covariates identified in univariate logistic regression analysis. For cohort 1, the Mann-Whitney test was used to compare the difference in the median time elapsed between the dates of last dose of the prior T-cell redirection therapy and Tec initiation among patients who developed CRS with any of the Tec step-up doses and those who did not. All statistical analyses were performed using IBM SPSS software (version 29).
An institutional review board approved protocol allowed retrospective data collection for research purposes on all patients with RR MM receiving treatment with T-cell redirection therapies such as Tec.
Results
A total of 75 patients were identified; 72 (cohort 1: 27 and cohort 2: 45) were included in the final analysis as shown in Figure 1. The remaining 3 patients were excluded because they did not complete the step-up dosing schedule of Tec. Baseline characteristics were comparable between the 2 cohorts except for the presence of high-risk cytogenetic features and number of prior lines of therapy (Table 1). In cohort 1 (n = 27), 21 (78%) received CAR T cells before Tec, directed against BCMA in 19 (90%; ciltacabtagene autoleucel, and idecabtagene vicleucel), GPRC5D in 1 (5%), and CD38 in 1 patient (5%); 10 patients received bispecific antibodies targeting BCMA in 3 (30%; elranatamab), GPRC5D in 3 (30%; talquetamab) and Fc receptor homolog 5 in 4 (40%; cevostamab). Of note, the percentages of patients with prior CAR T cells and bispecific antibodies did not add up to 100% because 4 patients received both types of T-cell redirection therapies (CAR T cells and bispecific antibodies) before initiation of Tec therapy.
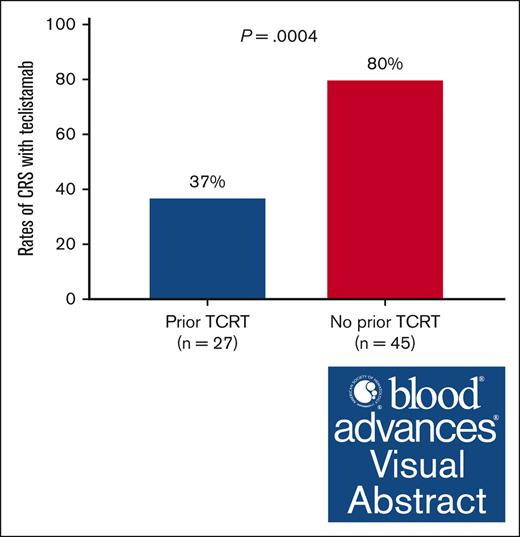
The overall incidence rate of CRS in the entire cohort was 63% (n = 46), and none had an underlying infection at the time when the CRS event occurred; the CRS rates differed significantly between cohorts 1 and 2 (37% vs 80%; P = .0004; Figure 2A). Although grade 2 CRS rates were higher in cohort 2 compared to cohort 1, the difference did not reach statistical significance (Figure 2B). The breakdown of CRS occurrence based on the Tec step-up dosing schedule was illustrated in Figure 2C; the first step-up dose was associated with the highest rates of CRS in either cohort. Tocilizumab was administered to 4 patients (40%) in cohort 1 and 13 patients (36%) in cohort 2. Among the patients who developed CRS, 4 (11%) had a recurrent CRS event in cohort 2 and 2 (20%) patients in cohort 1.
Rates of CRS with Tec. (A) Differences in the rates of CRS between cohort 1 (TCRT experienced) and cohort 2 (T-cell redirection therapy naïve). (B) Differences in the grades of CRS between cohort 1 (TCRT experienced) and cohort 2 (TCRT naïve). (C) Differences in the rates of CRS between cohort 1 (TCRT experienced) and cohort 2 (TCRT naïve) based on Tec step-up dose. TCRT, T-cell redirection therapy.
Rates of CRS with Tec. (A) Differences in the rates of CRS between cohort 1 (TCRT experienced) and cohort 2 (T-cell redirection therapy naïve). (B) Differences in the grades of CRS between cohort 1 (TCRT experienced) and cohort 2 (TCRT naïve). (C) Differences in the rates of CRS between cohort 1 (TCRT experienced) and cohort 2 (TCRT naïve) based on Tec step-up dose. TCRT, T-cell redirection therapy.
Based on univariate logistic regression analysis, cohort 1 had about a sixfold increase in the incidence of CRS with Tec (95% confidence interval, 2.41-20.80; P = .002) compared with cohort 2 (Table 2). High-risk cytogenetic features and number of prior lines of therapy were also significantly associated with the risk of CRS with Tec in univariate logistic regression analysis (Table 2). In multivariate logistic regression analysis, the effect of prior exposure to T-cell redirection therapy remained significant with an odds ratio of 4.44 (P = .0002; Table 2) after adjusting for high-risk cytogenetic risk features and prior lines of therapy. In cohort 1 (n = 27), 59% (n = 16) of the patients experienced CRS with previous T-cell redirection therapies (CAR T cells or bispecific antibodies) before they were treated with Tec. Among these patients, only 9 (56%) developed CRS with Tec. Alternatively, there was only 1 patient who experienced CRS with Tec but not with prior T-cell redirection therapy (Figure 3).
Change in number of patients in cohort 1 who developed CRS with prior TCRT and with Tec initiation.
Change in number of patients in cohort 1 who developed CRS with prior TCRT and with Tec initiation.
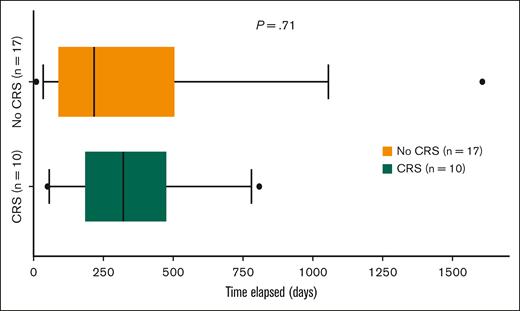
There was no statistically significant difference in the median time elapsed from previous T-cell redirection therapy exposure to Tec initiation between patients who developed CRS (median, 322 days[ IQR, 249]) compared with those who did not (median, 217 days [IQR, 374]; Figure 4) in cohort 1.
Differences in the time elapsed from prior exposure to TCRT to Tec initiation between patients who developed CRS and those who did not in cohort 1.
Differences in the time elapsed from prior exposure to TCRT to Tec initiation between patients who developed CRS and those who did not in cohort 1.
As for ICANS, 2 patients in cohort 2 developed grade 1 ICANS with Tec, but none in cohort 1. No statistical analysis was performed to compare the difference in rates of ICANS between the 2 cohorts because of its infrequent occurrence.
Discussion
CRS is one of the most common complications of bispecific T-cell redirecting antibody therapy. Its occurrence predominantly during the Tec step-up dosing phase has been considered a barrier to treatment initiation in the outpatient settings.
To our knowledge, this study is the first to provide real-world evidence on the incidence rates of CRS with Tec among patients with RR MM who had prior exposure to T-cell redirection therapy. Dima et al also generated real-world data on the tolerability of Tec in the RR setting.11 In this study, however, the primary end point (CRS rates) was evaluated based on age stratification (ie, comparing CRS rates among patients age of >70 years vs <70 years) and not previous treatment with T-cell redirection therapy. Dima et al11 demonstrated that the CRS rates were similar between the 2 age groups (67% in older patients vs 64% in younger patients, P = .7).
In our study, patients with prior exposure to T-cell redirection therapy had a significantly lower risk of developing CRS during the Tec step-up dosing phase than those who were naïve to prior T-cell redirection therapies. Multivariate logistic regression analysis showed that previous T-cell redirection therapy exposure was a significant independent predictor associated with reduced incidence of CRS with Tec. The study by Touzeau et al sought to assess the efficacy of Tec among patients who had prior anti-BCMA therapies where CRS events occurred at a rate of 63%.12 The discordance with our findings could be attributed to several factors. The study cohort entailed 38 patients, of whom 13 received non-BCMA targeting agents (such as anti-CD38 monoclonal antibodies, immunomodulatory agents, and proteasome inhibitors) as their last line of therapy before initiating the Tec step-up dosing phase. For the group of patients (n = 25) who were treated with BCMA-targeted therapies (before Tec), 16 had BCMA antibody drug conjugates which from a mechanistic standpoint, are not considered T-cell redirection therapies. In another study involving talquetamab, CRS rates among patients who had prior T-cell redirection therapy exposure (51/288) were comparable with those who did not (77% vs 75%-79%).13 Nonetheless, it was not discernible whether this treatment modality (T-cell redirection therapy) was the last line of therapy before talquetamab. It is worth noting that talquetamab does not target BCMA but rather a different antigen on the myeloma cells which is GPRC5D. This may also provide a plausible explanation for the discrepancy in the results.
In the overall patient population, the CRS rate was lower than what was reported in the MajesTEC-1 trial (63% vs 73%). However, for patients who were T-cell redirection therapy naïve (cohort 2), the CRS rates were slightly higher than MajesTEC-1 trial, thereby corroborating that the reduced CRS rates with Tec in our real-world patient population could be a ramification of prior exposure to T-cell redirection therapy. From a biological perspective, this observation suggests either T-cell anergy or BCMA antigen loss in the T-cell redirection therapy exposed cohort since CRS is merely a manifestation but not a cause of T-cell activation.14 Interestingly, the presence of high-risk cytogenetic risk features also resulted in significantly lower rates of CRS; whether this finding is secondary to the reduced efficacy of Tec is an area that warrants further investigation.
There has been great interest in optimizing the premedication regimen administered before the step-up doses of bispecific antibodies to facilitate treatment initiation in outpatient settings and thereby avoid hospitalization. A handful of recent studies set out to discern whether tocilizumab prophylaxis (administered with dexamethasone, acetaminophen, and diphenhydramine) mitigated the incidence of CRS events with bispecific antibodies. In 1 study that included a total of 53 patients, a single dose of tocilizumab administered 4 hours before the second priming dose (based on the median onset of CRS) decreased the CRS rates from 73.3% (11/15 patients who did not receive tocilizumab prophylaxis) to 26.3% (10/38 patients who received tocilizumab prophylaxis).15 Similarly, the studies by van de Donk N et al and Kowalski et al reported CRS rates of 29% and 13%, respectively, among patients who received prophylactic tocilizumab administered within 4 hours before the first Tec step-up dose.16,17 Most of the CRS events were mild in severity with grade 2 CRS reported in 5% to 14% of the patients. Notably, the rates of grade 2 CRS with tocilizumab prophylaxis were comparable with what was detected in our cohort of patients who were treated with prior T-cell redirection therapies (grade 2, 7%). Tocilizumab prophylaxis was also investigated with other bispecific antibodies such as cevostamab, which targets Fc receptor homolog 5. In the study by Trudel et al, which included 72 patients (28 in the tocilizumab pretreatment arm and 44 in the no pretreatment arm), pretreatment with tocilizumab (2 hours before first dose of cevostamab) was associated with a significant reduction in CRS rates (90.9% in the no tocilizumab pretreatment arm vs 35.7% in the tocilizumab pretreatment arm; P < .05) with grade 2 CRS reported at a lower frequency in the prophylaxis arm (14% and 34% in the pretreatment and no pretreatment arms, respectively).18 This data provided some preliminary evidence to support the implementation of a tocilizumab proactive approach, which was not only deemed to be safe, but also did not compromise the efficacy of bispecific antibodies. Of note, this strategy reduced the incidence of CRS events, but it did not abrogate it.
Our findings are important for optimizing best clinical practices since the MajesTEC-1 trial precluded patients who received CAR T cells or bispecific T-cell engager antibodies. The significantly low incidence rate and most importantly severity/grade of the CRS events support the notion that Tec administration can be safely instituted in outpatient rather than hospital settings in patients had prior exposure to T-cell redirection therapies, especially in this day of age when it is feasible to set up virtual telehealth visits for CRS/ICANS assessment after each step-up dose. If preemptive tocilizumab becomes part of the standard premedication regimen for CRS prevention with Tec or other bispecific T-cell engager antibodies in the near future, it is imperative that this approach be streamlined in a manner that promotes the judicious use of tocilizumab. This can be accomplished by reserving prophylactic tocilizumab to patients who are T-cell redirection therapy naïve as evidenced by our data.
One of the main limitations of our study is the small number of patients in cohort 1 (prior exposure to T-cell redirection therapies, which were primarily CAR T cells rather than bispecific T-cell engager antibodies). For this reason, we opted to group these patients into a single cohort for study end point analyses. In addition, bone marrow biopsies for disease burden assessment as well as baseline levels of inflammatory markers (ferritin, C-reactive protein, etc) were not available when Tec was initiated. Because these studies were not routinely performed for all patients at the time of disease progression, we were not able to conclusively ascertain whether they affect CRS rates with Tec.
In conclusion, prior exposure to T-cell redirection therapies was associated with a significantly lower incidence of CRS during the Tec step-up dosing phase, which might be indicative of T-cell exhaustion. This observation will allow for optimization of CRS prophylactic strategies in patients receiving treatment with bispecific antibodies such as Tec for RR MM. Nonetheless, further research is needed to uncover specific predictive biomarkers that can be utilized as clinical tools to help identify patients at risk of developing CRS with Tec and other bispecific antibodies.
Authorship
Contribution: I.S.H., S.Z.U., and C.R.T. contributed to designing the study; I.S.H., T.S., C.R., R.S.F., A.X.W. contributed to patient identification and data collection; I.S.H. provided statistical analyses of the data; I.S.H., S.Z.U., and C.R.T. wrote the manuscript; R.S.F., N.K., M.L.H., A.M.L., S.M., H.H., U.A.S., K.M., S.R., D.P., G.L.S., M.S., O.B.L., D.J.C., H.J.L., S.G., S.Z.U., and C.R.T. provided medical care for the study patients; and all authors substantially contributed to critically reviewing or revising the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlyn R. Tan, Plasma Cell Disorders Division, Department of Hematologic Oncology & Blood Disorders, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: TanC4@mskcc.org; and Saad Z. Usmani, Myeloma Service, Division of Hematologic Oncology, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: usmanis@mskcc.org.
References
Author notes
Original data are available on request from the corresponding author, Carlyn R. Tan (tanc4@mskcc.org).