Twenty years of sickle cell orphan drug development has ∼12% success rate, with highest rates when targeting early pathophysiological steps.
Failure rates for lack of efficacy were highest in late stages of drug development when vaso-occlusive crisis was the key clinical end point.
Visual Abstract
Sickle cell disease (SCD) is a hereditary red cell disorder with a large disease burden at a global level. In the United States and Europe, medicines may qualify for orphan designation (OD), a regulatory status that provides incentives to boost development. We evaluated the development of new therapies for SCD using data for OD granted in the United States and Europe over the last 2 decades (2000-2021). We analyzed their characteristics, pathophysiological targets, trends, and OD sponsors. We then investigated the approval outcomes, including the phase success rate and reasons for discontinuation across different variables. We identified 57 ODs for SCD: 43 (75.4%) small molecules, 32 (56.1%) for oral administration, and 36 (63.1%) for chronic use to prevent SCD complications. At the end of the study (2021), development of 34 of 57 ODs was completed. Four ODs were approved with a success rate of 11.8%. Products targeting upstream causative events of SCD pathophysiology had a 1.8 higher success rate compared with products targeting disease consequences. Large companies showed a fourfold higher success rate compared with small-medium enterprises. Failures in clinical development were mainly seen in phase 3 for a lack of efficacy on vaso-occlusive crisis as the primary study end point, likely related to variable definitions and heterogeneity of pain scoring and treatment. Both advances in SCD knowledge and regulatory incentives paved the way for new therapies for SCD. Our finding of high failure rates in late-stage clinical development signals the need for better early-stage predictive models, also in the context of meaningful clinical end points.
Introduction
Sickle cell disease (SCD) describes a group of inherited blood disorders in which a single base substitution in the ß-globin gene causes pathological hemoglobin S (HbS) production. When deoxygenated, HbS tends to polymerize, triggering erythrocyte sickling.1 Reflecting Plasmodium falciparum distribution, SCD is most prevalent in sub-Saharan Africa, the Middle East, and India; however, due to migrations from endemic malaria regions, SCD has increasingly become a public health issue in the Americas and Europe, though it is still considered rare.2
Vaso-occlusive crisis (VOC) is among the most important clinical manifestations of SCD based on severity and frequency and can lead to hospitalization with life-threatening complications. VOC is characterized by acute pain of different origins including vascular and inflammatory pain, neuropathic pain, and idiopathic pain.1 Ischemic/reperfusion tissue damage related to the occlusion of small vessels and/or capillaries by sickled erythrocytes is the triggering mechanism for the generation of amplified inflammatory response.1 Not only does the high biocomplexity of acute VOC involve sickling but also adhesion by sticky reticulocytes and neutrophils. Abnormally activated vascular endothelial cells are also involved in VOC pathophysiology, with local and systemic production of cytokines such as endothelin-1, selectin or VCAM-1, and other soluble plasma factors such as ADAMST13 and free heme. These molecules are important in recruitment and adhesion of both neutrophils and sickle erythrocytes to the abnormally activated vascular endothelial surface.3
For decades, the clinical management of SCD has been symptom-based, focused on treating exacerbations of acute painful VOC, infections, acute organ injury or dysfunction, and hemolytic anemia.4 Seminal advances came from the discovery that fetal hemoglobin levels were predictive of SCD morbidity and mortality; increased fetal hemoglobin delays HbS polymerization, thereby blunting an early and critical step in SCD pathophysiology.5 Due to its pleiotropic effect also on vascular and inflammatory components, hydroxyurea showed a marked decrease in the frequency of VOC in patients with HbSS/HbSB0 genotypes. Hydroxyurea was approved in 1998 in the United States and in the following years in the European Union (EU) as the first disease-modifying agent for SCD.6,7
Hydroxyurea has positively impacted patient survival and sickle-related complications in dozens of prospective clinical trials involving children and adults with SCD from across the world and is now globally recognized as the standard of care.8,9 However, the lack of effective therapeutic options for the treatment of VOC, along with the persistence of morbidity and early mortality of many patients with SCD, have prompted the look for new pharmacological targets and novel therapeutic options.
To encourage investments in rare disease areas, both the United States and the EU have created the orphan designation (OD), a special status that can be granted by regulators at any time during drug development to new or repurposed medicines aimed at the treatment of rare diseases. The OD qualifies pharmaceutical developers for financial benefits (specifically additional tax breaks and regulatory fee waivers), protocol assistance, and a pre-defined period of market exclusivity upon drug approval (7 years in the United States and 10 years in the EU).10,11 Such legislations have provided an unprecedented impact in steering pharmaceutical research and development (R&D) toward rare diseases: currently, around 40% of all medicines approved yearly in the United States and in the EU are orphan drugs. However, orphan drugs have also been criticized for the high prices they enter the market despite the governmental support received, thus hampering the access to patients.12
With the aim of providing insights into the crossroads of drug development and regulations in the field of SCD, we describe the characteristics and trends of OD granted by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for SCD and explore those factors contributing to successful or unsuccessful drug development within the United States and the EU regulatory ecosystem.
Methods
Data collection
We retrieved data on the active pharmaceutical ingredient (API), the date of designation, and the sponsor of medicinal products that received OD designation for SCD between 1 January 2000, that is, when both the United States and the EU orphan legislation came into force, to the last follow-up on 31 December 2021 from publicly available databases of the FDA and EU community.13,14 For the identified products, we searched for information on the status and phases of relevant clinical trials on https://clinicaltrials.gov and https://www.clinicaltrialsregister.eu, through the following search strategy: <SCD/Anemia> AND <’OD description’ or ‘acronym’ or ‘API)’>; and on PubMed: [Advanced search: all fields <SCD /Anemia> AND <OD description or acronyms or API> OR <clinical trial number (NTC number or EudraCT Number)>. Filter: Article type <clinical trial>].
OD therapies were categorized as chemical, biological, or gene therapy by matching with the freely accessible database https://go.drugbank.com or the sponsor’s website. Data on sponsor profiles and sizes were collected from companies’ websites and Dun & Bradstreet (http://www.dnb.com/).
Each item was reviewed by 2 authors (E.C. and A.I.), and a pilot-tested Microsoft Excel database was developed and double-checked for identifying potential inconsistencies. Discrepancies were resolved with the assistance of a third author (L.D.F.).
Data analysis
We grouped OD therapies by the main SCD pathophysiological target: (1) HbS genotype; (2) HbS polymerization and red blood cell sickling, together referring to upstream pathophysiological events; as well as (3) inflammation and vasculopathy; and (4) complications/organ damage, together referring to downstream ones.
We considered OD as (1) “approved” when the OD was granted marketing authorization by either the FDA or EMA or both agencies; (2) “in development” when preclinical or clinical testing resulted ongoing or the OD was reported as active in the sponsor’s pipeline; or (3) “discontinued” when drug was shown not to work or the OD was abandoned by the sponsor. For this latter category, the OD was either formally withdrawn by the sponsor, no update was reported in the last 3 years, the development was either declared terminated, or the sponsor resulted inactive or in bankruptcy.15 Reasons for discontinuation were investigated and categorized as R&D-related when it involved lack of efficacy, safety concerns, and enrollment issues; and as strategic when referred to the sponsor’s portfolio rationalization or financial issues of the sponsor.
We used the rate of approval as the main metric of drug development success, defined as the number of approved ODs as a fraction of the approved plus discontinued ones.16 Such rates were stratified by potential drivers for marketing authorization, clustered in the regulatory background, disease targets, and drug- and sponsor-related factors. In addition, we used the “phase success rate,” expressed as the number of ODs that moved from 1 phase of the drug development to the next, divided by the sum of the number of OD that progressed and the number of drugs that were discontinued.17 Finally, we considered an OD as a “new entity” when its active ingredient was not previously licensed in the United States and the EU as medicinal products at the time of SCD designation. Because small-medium enterprises (SME) can benefit from further regulatory and financial incentives, and given their role in the development of drugs for rare diseases, we also investigated their contribution by categorizing the sponsors as large enterprises or SME based on company size (250 employees threshold) or annual financial activity (50 million US dollars or Euros threshold).18
Descriptive statistics were used for displaying all the recorded variables. The trends of ODs over time were analyzed in terms of absolute values and frequencies.
Results
Trends and traits of orphan drug designations for SCD
Between 2000 and 2021, a total of 57 ODs, corresponding to 52 distinct active substances, were granted for the treatment of SCD in the United States and the EU. Among these 57 ODs, 52 (91.2%) were granted by the FDA and 27 (47.4%) by the EMA, whereas 22 (38.6%) were granted by both agencies.
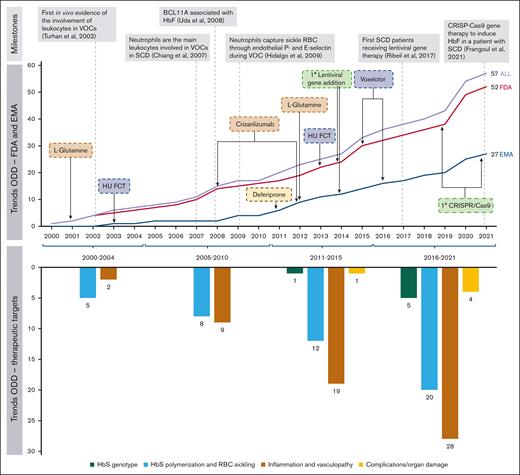
Overall, 5 (8.8%) ODs were aimed at correcting the HbS genotype, 20 (35.1%) interfere with HbS polymerization and red blood cell sickling, 28 (49.1%) target inflammation and vasculopathy, and 4 (7.0%) address clinical complications and organ damage. The number of ODs increased over the study period, from 17 (29.8%) in the first half (2000-2010) to 40 (70.2%) in the second half (2011-2021), also expanding the range of therapeutic targets addressed (Figure 1).
Trends of OD for SCD granted by the FDA and the EMA in the period 2000-2001. The top panel reports some of the milestones that paved the way for the drug development of SCD in the past 2 decades, also showing the main achievements gained in terms of ODs granted by the FDA and the EMA that already have impacted clinical practice and the potential game-changers in development (eg, gene therapies). Below are the main pathopsychological chapters of the disease addressed over time. HbF, fetal hemoglobin; HU, hydroxyurea; HU-FCT, hydroxyurea-film–coated tablets; RBC, red blood cell.
Trends of OD for SCD granted by the FDA and the EMA in the period 2000-2001. The top panel reports some of the milestones that paved the way for the drug development of SCD in the past 2 decades, also showing the main achievements gained in terms of ODs granted by the FDA and the EMA that already have impacted clinical practice and the potential game-changers in development (eg, gene therapies). Below are the main pathopsychological chapters of the disease addressed over time. HbF, fetal hemoglobin; HU, hydroxyurea; HU-FCT, hydroxyurea-film–coated tablets; RBC, red blood cell.
Despite the growing number of gene therapy–granted ODs in recent years, we found that most of the ODs were small-molecule drugs (43, 75.4%), designed for oral administration (32, 56.1%) and developed for chronic use to prevent SCD complications (36, 63.1%). At the time of the designation, half of the ODs (29, 50.9%) were already designated for another rare therapeutic indication (9 specifically for β-thalassemia), and almost one-third (17, 29.8%) were recognized as active substances previously approved for another indication either in the United States or the EU. Small- and medium-sized enterprises accounted for the majority of the OD sponsors (42; 73.7%) (supplemental Table 1).
Factors of successful orphan drug development for SCD
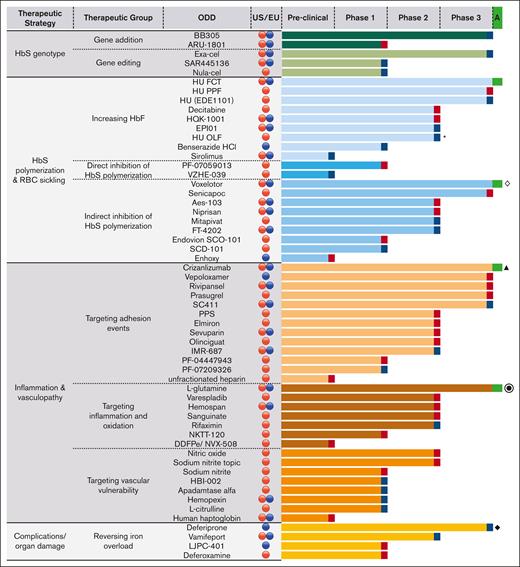
By 2022, 4 of 57 ODs (7.0%) had obtained marketing authorization (4 in the United States and 3 in the EU), whereas 30 (52.6%) were discontinued, and 23 (40.3%) were still in development (Figure 2).
Drug development stage of FDA and EMA OD for SCD categorized by pathophysiological targets and phase of the drug development reached. Blue symbols at the right end of the histograms represent ongoing ODs, red symbols represent discontinued ODs, and green symbols represent ODs approved either in the United States or in the EU.  ODs granted by the FDA;
ODs granted by the FDA;  ODs granted by the EMA; ∗approved in the EU but outside the orphan system; ◊ approved in the United States also for the pediatric population; ⦿ approved in the United States but negative opinion in the EU; ▲ first approved in both the United States and the EU; after a referral, the EMA revoked the marketing authorization in the EU; ◆ approved in the United States but outside the orphan system. DDFPe, dodecafluoropentane emulsion; FCT, film-coated tablets; HU, hydroxyurea; OLF, oral liquid formulation; PPF, powder for pharmaceutical formulation; PPS, pentosan polysulfate sodium.
ODs granted by the EMA; ∗approved in the EU but outside the orphan system; ◊ approved in the United States also for the pediatric population; ⦿ approved in the United States but negative opinion in the EU; ▲ first approved in both the United States and the EU; after a referral, the EMA revoked the marketing authorization in the EU; ◆ approved in the United States but outside the orphan system. DDFPe, dodecafluoropentane emulsion; FCT, film-coated tablets; HU, hydroxyurea; OLF, oral liquid formulation; PPF, powder for pharmaceutical formulation; PPS, pentosan polysulfate sodium.
Drug development stage of FDA and EMA OD for SCD categorized by pathophysiological targets and phase of the drug development reached. Blue symbols at the right end of the histograms represent ongoing ODs, red symbols represent discontinued ODs, and green symbols represent ODs approved either in the United States or in the EU.  ODs granted by the FDA;
ODs granted by the FDA;  ODs granted by the EMA; ∗approved in the EU but outside the orphan system; ◊ approved in the United States also for the pediatric population; ⦿ approved in the United States but negative opinion in the EU; ▲ first approved in both the United States and the EU; after a referral, the EMA revoked the marketing authorization in the EU; ◆ approved in the United States but outside the orphan system. DDFPe, dodecafluoropentane emulsion; FCT, film-coated tablets; HU, hydroxyurea; OLF, oral liquid formulation; PPF, powder for pharmaceutical formulation; PPS, pentosan polysulfate sodium.
ODs granted by the EMA; ∗approved in the EU but outside the orphan system; ◊ approved in the United States also for the pediatric population; ⦿ approved in the United States but negative opinion in the EU; ▲ first approved in both the United States and the EU; after a referral, the EMA revoked the marketing authorization in the EU; ◆ approved in the United States but outside the orphan system. DDFPe, dodecafluoropentane emulsion; FCT, film-coated tablets; HU, hydroxyurea; OLF, oral liquid formulation; PPF, powder for pharmaceutical formulation; PPS, pentosan polysulfate sodium.
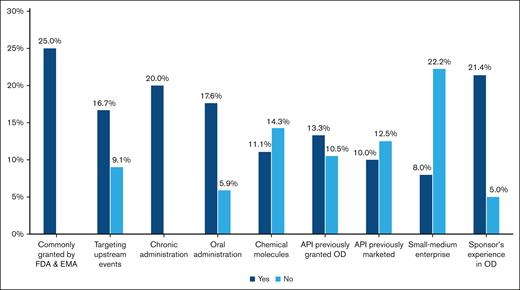
Not counting the 23 ODs still in development, the overall rate of approval of ODs for SCD was 4 out of 34 (11.8%). Across the different examined variables, we observed trends toward positive approval for OD-targeting upstream events of SCD pathophysiology compared with downstream ones (16.7% vs 9.1%); chronic administration rather than acute or single-time treatment (20.0% vs 0.0%); oral administration rather than parenteral or topical use (17.6% vs 5.9%); marketing by larger companies compared to smaller ones (22.2% vs 8.0%); and sponsors with rare diseases experience in the United States or EU regulatory space (21.4% vs 5.0%) (Figure 3).
Drivers of marketing authorization for OD for SCD in the United States and the EU in 2000 to 2021. Histograms show the rate of approval of ODs for SCD over the different variables analyzed.
Drivers of marketing authorization for OD for SCD in the United States and the EU in 2000 to 2021. Histograms show the rate of approval of ODs for SCD over the different variables analyzed.
With a consistent trend across the OD-targeting upstream or downstream pathophysiology, we found the lowest success rate in the last step of drug development, whereby 37.5% of all the ODs reaching phase 3 progressed to phase 4 (supplemental Figure 1).
Reasons for discontinuation of orphan drug designations for SCD
In analyzing the reasons for 30 ODs that were discontinued, 16 were attributable to strategic and 14 to R&D-related issues; the former was relatively prevalent in the early phases, whereas the latter increased in the latest phases of drug development (supplemental Figure 2).
Among ODs discontinued for strategic issues, clinical trials were prematurely stopped for financial reasons (eg, sponsor bankruptcy, cease of sponsor operations, or portfolio rationalization), and despite non-negative results (eg, ARU-1801). Out of the 16 ODs discontinued for strategic reasons, 12 (75.0%) were associated with SME.
In contrast, out of the 14 ODs discontinued for R&D-related issues, 2 cases were due to low recruitment in the phase; 2 cases for safety concerns; 1 case was terminated early by the sponsor due to unblinding; 1 case for perceived futility in a phase 2 study, whereby the baseline pain score in first patients enrolled was too low to be able to demonstrate an improvement; 3 cases for lack of pharmacological effect in phase 1 and phase 2; and 5 cases (sevuparin, prasugrel, senicapoc, rivipansel, vepoloxamer) for lack of clinical efficacy (supplemental Table 2).
Clinical end points in trials involving patients with SCD
Focusing on 51 ODs that entered clinical development, we identified 106 clinical trials. Most of these trials aimed at assessing the maximum tolerated dose, adverse events, and pharmacokinetics parameters, whereas 38 of 106 (35.8%) were designed to assess the efficacy of the new treatment. Twenty (52.7%) of these 38 trials included VOC as a primary or co-primary end point, either for prevention (13 trials) or treatment (7 trials).
Although in the advanced phases of clinical development senicapoc and prasugrel failed in reducing the VOC rate, OD like velopoxamer, rivipansel, and sevuparin, were discontinued for a lack of efficacy in reducing the VOC duration. By contrast, voxelotor, an antisickling and left-shifting oxygen affinity drug, successfully increased hemoglobin, as primary end point, in comparison with placebo, although the VOC rate was unchanged in the initial trial and in subsequent long-term follow-up (Table 1).
On the other hand, in a 48-week phase 3 study, L-glutamine reduced the median number of VOC from 4 in the placebo group to 3 in the L-glutamine group. Based on the results, the FDA granted approval, whereas the EMA expressed a negative opinion on its benefit/risk ratio. Crizanlizumab, which significantly reduced the VOC rate in a phase 2 study, was approved by both the FDA and conditionally by the EMA. However, as results from a subsequent phase 3 trial (STAND, NCT03814746) did not confirm the efficacy of crizanlizumab in reducing the VOC rate, the EMA revoked the conditional marketing authorization (Table 1).
Discussion
Four drugs, hydroxyurea, L-glutamine, crizanlizumab, and voxelotor, are currently approved by the FDA specifically for SCD (and 3 of them by EMA), but trials of multiple other promising drugs have been unsuccessful. Although this is, in large part, due to a lack of clinical efficacy of the tested drugs, there is some concern that the negative results in some clinical trials may have been due to the choice of the primary clinical end point.27
In this study, we describe a rising trend of pharmaceutical products developed for the treatment of SCD within the framework of the United States and EU orphan drug legislation over the past 2 decades. Echoing advances in SCD, we observed an ever-widening array of therapeutic targets addressed, whereby most of the ODs tackled the consequences of SCD pathophysiology, such as vasculopathy and inflammation, but those interfering with HbS polymerization and erythrocyte sickling showed a higher rate of approval.
In line with other analyses carried out in the area of rare diseases and drug development successes industry-wide, we found that only around 1 of 10 OD products developed for SCD gained the status of marketing authorization.18,28-30
Although small companies accounted for most sponsors that invested in OD for SCD, OD developed by large companies showed a higher success rate for marketing authorization.31 This might partially be explained by the tendency of big corporations to buy small companies committed to early-stage R&D, but with limited capacity in marketing and regulation. Shedding light on these trajectories in future research might contribute to rethinking regulatory incentives to support small companies in moving forward in the regulatory space, to avoid monopolies from big companies in a field already marked by low competition.32 Unlike with other analyses on pharmaceutical R&D, whereby the lowest phase success rate is generally observed in phase 2, our findings showed that for SCD the lowest success rate was in phase 3,17,29 thus highlighting the potential limitations of the chosen clinical end points to define the efficacy of new treatments.
The ambiguity of the definition of VOC has affected the success of the clinical study in patients with SCD, either for their treatment or prevention. Indeed, the failure of clinical studies in patients with SCD was related to lack of efficacy in reducing either the rate (eg, senicapoc) or the duration (eg, rivipansel) of VOCs. This is extremely interesting because VOC describes the pathophysiology of a series of cumulative and synergic events, which are difficult to reduce to a single measurement (yes/no, number of VOC/year), or to consider as a surrogate of the severity of SCD. In addition, the symptoms of VOC are acute pain and often concomitant worsening fatigue.33 Thus, we might better consider the intensity of pain with or without signs of organ dysfunction as more objectively measurable end points than the number of VOC per se. Indeed, pain perception is a personal experience, deeply affected by psychological and socio-cultural dimensions.34,35 Other disorders characterized by acute and/or chronic pain such as cancer have generated similar discussions in the context of valid pain assessment in clinic studies.36 Up till now, the assessment of “recalled worst pain” seems to be better than scores on pain intensity in patients with cancer enrolled in clinical trials. This is even more important in patients with SCD, moving into a global scenario characterized by differences in pain management (single agent, early use of opioids, multimodal pain analgesia) as well as in the definition of pain threshold for the intensity of pain management among countries, especially for agents targeting a single pathway, such as P-selectin, are evaluated when compared with a molecule with multimodal action such as hydroxyurea.37
Accordingly, the development of patient-centered complementary strategies to enhance the performance of pain and fatigue evaluation is crucial not only for daily clinical management of patients with SCD, but also for clinical study purposes. A combination of friendly mobile (m)Health applications for pain scores and treatment plus a medical evaluation at comprehensive SCD centers may be ideal. This is routinely used in other home settings such as post-surgery pain management for children38-40 or pain management in cancer patients41
One paradigmatic example is senicapoc, an oral Gardos channel inhibitor with potent effects on hemolysis and anemia in SCD, recognized in phase 2 but not enough to prevent premature trial closure in phase 3 due to failure in preventing pain in patients with SCD.42,19 Ten years later, voxelotor, an oral antisickling agent was approved by the FDA and EMA for the treatment of SCD based on the amelioration of chronic anemia without significant impact on VOC rate.20 Although this was an uncontrolled open-label trial, it opens non-pain end points for SCD trials because hemolysis is a risk factor for early death and severe organ complications.43,44 Due to the fact that SCD is a chronic disease, the impact of non-pain end points for SCD trials on the natural history of the disease needs to be evaluated in long-term studies.43,44 The use of this new end point was supported by FDA-led multistakeholder discussions with physicians and patient communities to identify unmet needs and potential clinical trial end points, as well as by a company-sponsored analysis of external patient-level data to demonstrate a correlation between hemoglobin change and stroke risk.45
It should be noted that multiple laboratory biomarkers have been described in SCD, and many of these have limited clinical value and require further assessment in prospective studies to validate their prognostic importance before they are accepted as surrogate end points.46
This is a crucial aspect in drug regulation, as surrogate end points have increasingly been accepted by regulatory bodies to foster the development of medicines, but also largely criticized as they led health systems to pay high prices for medicines with unclear or unconfirmed efficacy. Such criticism is amplified in the context of accelerated approval pathways, whereby regulators tend to accept more flexible evidence to expedite the review and approval of medicines in areas of unmet medical need.47
Uncertainties in expediting the approval of medicines for SCD can be better understood from crizanlizumab, which was granted conditional marketing authorization by the EMA upon results from the phase 2 trial, but revoked in the EU when no reduction of annualized rates of VOC were observed in comparison with placebo in phase 3. Surrogate measures only reasonably predict clinical benefit, and therefore should require a stringent post-approval evidence-generating system to confirm their linkage to accepted clinical outcomes.
Although the FDA provided a list of surrogate end points for a wide spectrum of diseases that medicines developers can consider for drug development,48 the acceptability of such end points is determined on a case-by-case basis and might change based on accumulated post-approval evidence.
In the evolving scenario we describe, the FDA and the EMA showed different approaches in evaluating clinical evidence of treatments for SCD, even with the same data set of information, adopting different decisions, such as in the case of L-glutamine and crizanlizumab.
It is out of scope to assess the efficiency of the 2 regulatory processes in terms of decision-making, as they are based on different conceptual and legal approaches. However, given the potential impact that medicines licensed in the United States and the EU might have on SCD patients worldwide, especially in low-resource settings where the disease reaches the highest frequencies, a global discussion on the use and evaluation of clinical end points for regulatory decisions should occur.49 Seeking common regulatory ground would strengthen the whole R&D framework for SCD, not to mention homogeneous access for patients, an aspect that will increasingly be crucial with the operationalizing of the African Medicines Agency.50
As with all the analyses of public data, there are some limitations to consider. Data on the development stage at the time of OD granting and those on rejected or withdrawn applications were not always publicly available and therefore, were not included in the analysis. Being undisclosed, we did not collect data on potential scientific advice or protocol assistance provided by regulators once the OD was granted, a piece of information that could have improved our knowledge of the input provided by the FDA and EMA to the development of such therapies.
Conclusions
Advances in SCD pathophysiology have played a major role in drug development, but our findings highlight how the interplay between drug development requires a holistic approach to bring new and effective treatments to the market. Moving forward in the SCD treatment realm will require providing clinical evidence on the benefit of new treatments—either as a single agent or as add-on therapy on top of those already on the market with a different mechanism of action—and in a timely manner. Although discussion within the scientific community to harmonize the use of new and innovative clinical end points in regulatory decision-making has been implemented over the past few years, our analysis supports a more general discussion on the impact of end points of clinical trials for drug development within the United States and the EU framework. We predict this will improve the transferability of results in regions of the world where the SCD prevalence and burden are higher, and where differences in pain management are even more pronounced.
Acknowledgments
The authors are very grateful to Lucio Luzzatto from Muhimbili University for his thoughtful input and achieving discussions during the conceptualization of the study. The authors also wish to acknowledge Aaron Kesselheim from Harvard University for his comments on a preliminary version of this article.
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated or employed.
This article has partially been supported by internal funding of the University of Verona: Public Research Projects of National Relevance grant (PRIN2020-MIUR:2020Z22PM7).
Authorship
Contribution: E.C., H.G.M.L., and L.D.F. conceived the study; E.C., A.I., and L.D.F. were responsible for data gathering and processing; E.C. and A.I. performed the analyses; M.d.M., R.E.W., and L.D.F. verified the underlying data of the analysis; E.C., A.I., and H.G.M.L. contributed to the regulatory interpretation of data; E.C., A.I., M.d.M., R.E.W., and L.D.F. contributed to the therapeutic interpretation of data; E.C. drafted the first version of the manuscript; and all authors participated in reviewing the manuscript and approved the final version.
Conflict-of-interest disclosure: M.d.M. declares support for attending the American Society of Hematology meeting from Addmedica and for participation in steering committees from Vertex, Novartis, and Addmedica. R.E.W. receives research funding from Bristol Myers Squibb and Addmedica and participated in a data safety monitoring board for Novartis. L.D.F. received research grants from Agios, Baxalta, and Bristol; honoraria for lectures from Novartis; and for participation in advisory boards from Roche and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Enrico Costa, Division of Pharmacoepidemiology and Clinical Pharmacology, World Health Organization Collaborating Centre for Pharmaceutical Policy and Regulation, Utrecht University, N/A 99|3584 Utrecht, The Netherlands; email: e.costa@uu.nl.
References
Author notes
For academic purposes, raw data are available upon reasonable request from the corresponding author, Enrico Costa (e.costa@uu.nl).
The full-text version of this article contains a data supplement.