Intracellular reactive oxygen species (ROS) is acquired in RBCs incubated with P falciparum–conditioned medium.
Glutamine, cysteine, and glycine supplementation led to increased glutathione biosynthesis and reduced ROS levels within stressed RBCs.
Visual Abstract
Malaria is a highly oxidative parasitic disease in which anemia is the most common clinical symptom. A major contributor to the malarial anemia pathogenesis is the destruction of bystander, uninfected red blood cells (RBCs). Metabolic fluctuations are known to occur in the plasma of individuals with acute malaria, emphasizing the role of metabolic changes in disease progression and severity. Here, we report conditioned medium from Plasmodium falciparum culture induces oxidative stress in uninfected, catalase-depleted RBCs. As cell-permeable precursors to glutathione, we demonstrate the benefit of pre-exposure to exogenous glutamine, cysteine, and glycine amino acids for RBCs. Importantly, this pretreatment intrinsically prepares RBCs to mitigate oxidative stress.
Introduction
Anemia is the most common clinical consequence of human malaria, a parasitic disease with nearly 250 million cases annually.1 The pathogenesis of malarial anemia is multifaceted consisting of the loss of both infected and uninfected red blood cells (RBCs), as well as dysregulation of new RBC production.2 In malaria infections caused by Plasmodium falciparum (P falciparum), uninfected RBCs are lost at a much greater rate than infected RBCs. This so-called “bystander effect” occurs before adaptive immune system activation,3 contributing greatly to the development of malarial anemia. The mechanisms of the malarial bystander effect on uninfected RBCs are unclear, although a loss in membrane deformability is known to contribute to the removal of bystander RBCs from circulation.4,5 Bystander RBCs have also been shown to undergo cell surface changes, promoting erythrophagocytosis through complement-mediated activation6 and phosphatidylserine antibody-mediated removal.7 In vivo models for malaria demonstrate that >75% of the RBCs that are lost are uninfected during naïve infection, whereas only ∼5% of all RBCs destroyed during infection are due to direct parasite infection, with inhibition of erythropoiesis making up the remaining portion.8 The culture medium from P falciparum–infected RBCs was shown to impact biological function of nucleated erythroid cells.9 In addition, parasite-derived mitochondrial DNA (mtDNA) within the medium of P falciparum parasite cultures was found to elicit toll-like receptor 9 binding, thereby altering the membranes of healthy RBCs.10 Interrupted glycolysis also increases RBC susceptibility to senescence and oxidative damage, and further highlights the importance of the exogenous metabolic environment for RBCs.11 These previous findings indicate multiple mechanisms contributing to the bystander effect in malaria.
Although mature RBCs have limited metabolic activity owing to a lack of membrane-bound organelles, these cells have multiple active antioxidant components that counter oxidative stress in their environment. These intracellular defenses include reduced glutathione (GSH), catalase, peroxiredoxins, and glutathione peroxidase.12,13 Oxidative stress plays a major role in many anemia-inducing conditions, such as malaria and sickle cell disease (SCD).14,15 In malaria, RBCs from patients have reduced levels of intracellular catalase,16 indicating that these cells are deficient in their ability of fully combating oxidative stress and rendering avenues for antioxidant therapy as a viable treatment to lessen disease severity. Exogenous GSH, a potent antioxidant, is structurally unable to freely permeate cellular membranes. Therefore, individual amino acid building blocks of the tripeptide GSH (glutamine, cysteine, and glycine) are taken up by RBCs, which perform de novo GSH biosynthesis inside the cell.17 Additionally, significant host metabolic alterations occur during malaria infection. This includes markedly reduced levels of plasma-free amino acids such as glutamine and arginine,18-25 suggesting amino acid supplementation could perhaps provide therapeutic benefits in malaria.
Oral treatment with L-glutamine (Gln) is a recently approved therapy for SCD.26 Although the exact cellular mechanism remains unclear, a potential role for Gln is to improve nicotinamide adenine dinucleotide phosphate stores in sickled RBCs,27 thereby reducing the oxidative stress. Gln also serves as a precursor for arginine, an amino acid that has been inversely associated with mortality in cerebral malaria22 and is proposed to be beneficial in both malaria28 and SCD.29 Gln is also implicated in malarial anemia, in which low plasma Gln levels have been found to be associated with pediatric severe malarial anemia (SMA).30 Here, we explored the oxidative impact of the P falciparum culture environment on uninfected catalase-inhibited RBCs as a proxy for the in vivo severe malaria bystander effect. Furthermore, we investigated the role of exogenous amino acid supplementation on bystander RBCs and showed that RBCs pretreated with precursor antioxidant amino acids glutamine, cysteine, and glycine concomitantly have increased intracellular glutathione synthesis and thus these amino acids together confer protection from oxidative stress.
Methods
Blood washing and perturbations
Heparinized venous blood, type O+, was collected from healthy adult participants with informed consent and used with approval from the Wake Forest University Institutional Review Board (Study ID: IRB00024199) or commercially purchased from BioIVT Inc and received within 24 hours of collection. Briefly, RBCs were washed 3 times in excess 1× phosphate buffered-saline (PBS), pH 7.4 (Gibco) and centrifuged at 900g to remove plasma and buffy coat layers with each wash. Washed RBCs were stored in 1× PBS supplemented with 5 mM D-glucose (PBS + Gluc) and used on the same day. All RBC incubation experiments were performed at 1% hematocrit (hct) while rocking at 37°C. Overnight 24-hour incubations were performed for amino acid supplementation and P falciparum–conditioned medium (PfCM) stress. For amino acid supplementation experiments, the indicated concentrations of amino acids were thoroughly dissolved in PBS + Gluc and incubated overnight. The amino acid concentrations were selected to be 1000 μM to approximate the average glutamine concentration in the plasma of patients with SCD treated with Gln,27 which is also in the realm of levels of glutamine in Kenyan children with malaria but without SMA (mean of 1361 μM for those without SMA compared with 484 μM for those with SMA).30 The cells were then washed twice with PBS to remove amino acids before stress incubation. All hydrogen peroxide perturbations were performed in the presence of 1 mM sodium azide (NaN3) to block intracellular catalase activity and incubated for 15 minutes while rocking at 37°C, as described previously.31-33PfCM perturbations were also performed using 1 mM NaN3 to block intracellular catalase activity. Control medium conditions for PfCM perturbations included RPMI 1640 (Gibco) supplemented with 1 mM NaN3.
P falciparum culture and conditioned medium
P falciparum from the 3D7 laboratory strain (MRA-102; BEI Resources) was used in the generation of PfCM. RBCs were combined from 2 O+ donors at 2% hct and maintained in standard culture medium consisting of RPMI 1640 (Gibco) supplemented with sodium bicarbonate, HEPES (4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid) buffer, hypoxanthine, gentamicin, and 0.5% (w/vol) Albumax II34 in a gas environment of 1% O2, 5% CO2, and 94% N2. Parasites were synchronized using a 5% sorbitol solution, seeded at 0.4% parasitemia, and allowed to grow with no medium change, but 1 gas exchange for 72 hours. The culture was then centrifuged at 600g for 15 minutes, then 1600g, then 3600g, and then filtered with a 0.45 μm filter, before storing at 4°C, as described previously.35 For glucose supplementation experiments, 5.5 mM D-glucose was dissolved in PfCM and RPMI 1640 base medium. The value of 5.5 mM was selected as being on the high end of the human fasting glucose range (3.9-5.6 mM) and ∼50% of the concentration of D-glucose in fresh RPMI (11.1 mM).
Intracellular ROS detection
Intracellular reactive oxygen species (ROS) was measured using a Cellular ROS Assay Kit (ab113851; Abcam). Washed RBCs were incubated with a 2 μM working concentration of 2’,7’ –dichlorofluorescin diacetate (DCFDA) for 30 minutes. RBCs were then washed twice with 1× PBS and used for either flow cytometry analysis or live-cell imaging.
Flow cytometry
RBCs were analyzed using either a BD LSRFortessa X-20 Flow Cytometer (Becton Dickinson) or CytoFLEX V0-B3-R1 Flow Cytometer (Beckman Coulter). RBCs were passed at a flow rate of ∼5000 cells per second. Samples ran on the BD LSRFortessa X-20 Flow Cytometer (Becton Dickinson) used a 60-milliwatt 488 nm laser at 550 volts for excitation and a green (505 long pass and 530/30 band pass) fluorescein isothiocyanate emission filter for detection. Samples ran on the CytoFLEX V0-B3-R1 Flow Cytometer (Beckman Coulter) used a 50-milliwatt 488 nm laser for excitation and a 525/40 band-pass emission filter for detection. For data analysis, the mean fluorescence intensity was measured for 100 000 events, gated on doublet discrimination (FSC-H vs FSC-A), and analyzed using the FCS Express 7 Research software (De Novo).
Live-cell imaging
Experiments were carried out using the Leica Thunder Live Cell Imaging System and LAS-X acquisition software. Images were acquired as 16-bit data with a Leica K8 sCMOS camera (2048 × 2048 pixels) using a 63× Plan Apo oil immersion lens (1.4NA). RBCs were plated onto 35 mm optical cover glass dishes (ibidi) immediately before imaging. For the time-lapse experiments, images were acquired every 15 seconds. All excitation/acquisition parameters were held constant across the imaging experiments, including the light-emitting diode excitation level and camera exposure time. All analyses were performed using Leica instant computational clearing (ICC) intensity values. For analysis, stationary cells were chosen using Differential Interference Contrast images and isolated using region of interest selection using Fiji image analysis software.36 Fluorescence in the regions of interest was quantified from the ICC-adjusted images at each time frame image. Data were normalized to the average fluorescence of the first 3 time points, before treatment. For visualization purposes, the images were adjusted to maximize the brightness contrast using Fiji.36
Scanning electron microscopy
Perturbed RBCs were washed and fixed in an osmotic-controlled glutaraldehyde solution as previously reported37. Briefly, glutaraldehyde-fixed RBCs were washed and resuspended in distilled H2O at 0.5% hct before air-drying overnight on 12 mm round coverslips at 60°C. Images were collected with Everhart-Thornley secondary electron detection using either an AMRAY 1810 or a Phenom XL scanning electron microscope at 10 Kv accelerating voltage. Typical magnifications used were 2000× to allow for high resolution of RBCs while maintaining a reasonable field size. Samples were prepared for imaging by first dehydrating on carbon tab aluminum stubs, followed by gold sputter coating under argon gas conditions to a thickness of ∼7 to 10 nm. Echinocyte morphology stages were defined as previously described38-40 and the total number of cells in 2 fields of view was imaged and quantified for morphology scoring. Each field of view had varying total cells on a slide (range, 131-384 cells); therefore, the morphology scores and stages were reported per 100 cells to account for this variation per independently imaged field of view.
Osmotic gradient ektacytometry
A Technicon osmotic gradient ektacytometer (Technicon Instrument Corp) facilitated the deformability measurements of RBCs. Approximately 31 g/L of polyvinylpyrrolidone polymer (437190; Sigma) mixed with 0.9 g/L of sodium phosphate dibasic anhydrous Na2HPO4 (7558-79-4; Fisher Biotec), 0.24 g/L of sodium phosphate monobasic NaH2PO4 (10049-21-5; Fisher Biotec), and 0.544 g/L of sodium chloride NaCl (S7653; Sigma) was prepared in Milli-Q ultrapure water with the pH of 7.4. The “low” solution (40 mOsm) was used to make the “high” (750 mOsm) and “sample” solutions by dissolving 11.25 g of NaCl in 500 mL of the low solution and 1.9782 g of NaCl in 250 mL of the low solution (290 mOsm), respectively. After calibration using known osmolality mixtures and determining the required parameters, as well as laser alignment of the ektacytometer, 150 μL of the blood samples (40% hct) was diluted into 4 mL of sample solution. The population of RBCs suspended in the highly viscous medium was directed into the gap between the 2 coaxial cylinders. The outer cylinder stayed motionless whereas the inner cylinder rotated with a distinct angular velocity to apply a defined shear stress of 159 dynes/cm2 (∼16 pascals) at a controlled temperature. During the operation, the focused laser beam passed through the suspension and generated elliptical diffraction patterns of the flowing RBCs projected onto the detector. The LabVIEW software recorded the diffraction patterns, corresponding osmoscans, and quantitative statistics. The data were fitted using the Origin 2016 software and the averages of 3 separate scans from each blood sample were analyzed. GraphPad Prism was used for the graphing of the recorded result.
DNA extraction and amplification
After PfCM exposure, RBCs were washed in 1× PBS (Gibco) and DNA was extracted from the samples using a commercially available kit (K0781; Thermo Fisher Scientific). Quantitative polymerase chain reaction amplification of a 500 bp fragment of the P falciparum mitochondrial cytochrome c oxidase subunit III (coxIII) gene using primers previously developed10 was performed using PowerTrack SYBR green Master Mix (A46012; Thermo Fisher Scientific) and a Roche LightCycler 480. Cycle threshold and baseline threshold values were calculated using Roche LightCycler 480 software.
Metabolite extraction
After perturbation experiments, 1 × 107 RBCs were pelleted and the supernatant was carefully removed. The pelleted cells were resuspended in 1× PBS with 100 mM N-ethylmaleimide (NEM) (catalog no. 128-53-0; Millipore Sigma) for 15 minutes at room temperature.31 After NEM incubation, cells were washed twice with 1× PBS. After the thorough removal of NEM from the supernatant, PBS was added at a final cell concentration of 20% hct, and cell concentration was recorded using a hemocytometer. Methanol was added (4:1), followed by vortexing and storing the cells on ice for 30 minutes. Sample tubes were then centrifuged at 18 000g and the supernatant was carefully removed and stored at −80°C for mass spectrometry analysis.
Targeted mass spectrometry
Targeted liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was performed at the Proteomics and Metabolomics Shared Resource (Wake Forest University School of Medicine, Winston-Salem, NC). Briefly, extracted samples were dehydrated and reconstituted in H2O, followed by mass spectrometry (SCIEX 7500 MS) analysis41 for relative quantification of reduced glutathione (GSH alkylated by NEM) and oxidized glutathione (GSSG) metabolites, without any further derivatization.42 Separation was performed on a Thermo Scientific Hypersil GOLD aQ reverse phase column (2.1 × 150 mm, 3 m) with a gradient mobile phase system consisting of an aqueous phase of 0.1% formic acid (A) and an organic phase of acetonitrile (B) at a flow rate of 0.5 mL/min (0-0.5 minute, 0.5%-5% B; 0.5-6.5 minute, 5%-98% B; 6.5-9 minute, 98% B). The mass spectrometer used the following source parameters: ion source gas 1, 35 psi; ion source gas 2, 70 psi; curtain gas, 40 psi; CAD gas, 9 psi; source temperature, 250°C; and spray voltage, 5500 V. Transition masses for targeted analysis were 433.00 > 304.00 m/z (NEM-labeled GSH), and 613.20 > 355.25, 613.20 > 484.20, and 613.20 > 231.05 m/z for GSSG. Relative peak intensity values were normalized to 106 cells per sample. Total glutathione levels were determined by adding the intensities of GSH-NEM and GSSG, with correction for ionization efficiency.
Results
Exogenous Gln alone is not sufficient but exogenous amino acid cocktail reduces oxidative stress acquired from H2O2
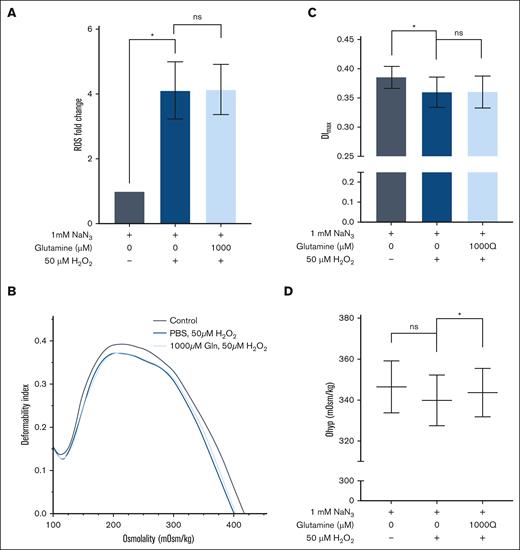
The relevance of Gln in both malaria and sickle cell anemia, along with it being a cell-permeable precursor to GSH, lead us to first explore the impact of exogenous Gln supplementation on oxidative stress in RBCs. For these studies, we used the intracellular fluorescent ROS indicator DCFDA. To verify the measured changes were indeed due to ROS and not the result of nonspecific effects from the DCFDA probe, we (1) tested and verified that mean fluorescent intensity increases linearly with hydrogen peroxide concentrations (supplemental Figure 1) and (2) tested and verified that cells pretreated with amino acids in the absence of H2O2 stress have no observable change in mean fluorescence intensity compared with the cells incubated with PBS + Gluc (supplemental Figure 2). Despite a clear significant difference in intracellular ROS levels upon H2O2 treatment (fold change of 1.0 for 0 μM H2O2 vs 4.1 for 50 μM H2O2; P = .036), we found no difference between H2O2-stressed RBCs pre-exposed to control null medium or medium supplemented with Gln overnight (Figure 1A).
Glutamine pretreatment benefited oxidatively stressed RBCs through osmotic protection. (A) Fold change in intracellular ROS detected by DCFDA staining and flow cytometry in RBCs. (B) Representative ektacytometry curve as cells pass through an osmotic gradient at a constant shear stress of 16 Pa. (C) Maximum deformability index (DImax) values, and (D) RBC hydration graphed as Ohyper (n = 3). The mean ± standard error of the mean is denoted by the error bars. A 1-way paired t test was used to analyze the data. ns, not significant; ∗P < .05.
Glutamine pretreatment benefited oxidatively stressed RBCs through osmotic protection. (A) Fold change in intracellular ROS detected by DCFDA staining and flow cytometry in RBCs. (B) Representative ektacytometry curve as cells pass through an osmotic gradient at a constant shear stress of 16 Pa. (C) Maximum deformability index (DImax) values, and (D) RBC hydration graphed as Ohyper (n = 3). The mean ± standard error of the mean is denoted by the error bars. A 1-way paired t test was used to analyze the data. ns, not significant; ∗P < .05.
As H2O2 is known to impair RBC function through reduced RBC membrane deformability and cellular dehydration,32,33,43 we assessed the osmotic effect44 of Gln and saw an expected decrease in membrane deformability, as measured by DImax, in response to H2O2 (0.385 DImax for 0 μM H2O2 vs 0.360 DImax for 50 μM H2O2; P = .035). However, RBCs supplemented with Gln had a similar loss in deformability when stressed with H2O2, suggesting no impact of Gln on parameters (Figure 1B-C).
In terms of cell hydration, we observed slight dehydration in RBCs stressed by H2O2 bordered on significance (347 mOsm/kg for 0 μM H2O2 vs 340 mOsm/kg for 50 μM H2O2; P = .061), but more interestingly, we found that Gln supplementation significantly improved hydration status compared with RBCs not supplemented with Gln (340 mOsm/kg for 0 μM Gln vs 344 mOsm/kg for 1000 μM Gln; P = .031) (Figure 1D). These data demonstrate that Gln supplementation before oxidative stress is osmotically advantageous for RBCs hydration but does not improve RBC membrane deformability or reduce intracellular ROS generation in RBCs.
Although Gln supplementation alone did not seem to protect RBCs from oxidative stress, Gln is a precursor for glutamate, which is required, in addition to cysteine and glycine, for de novo GSH synthesis inside RBC to combat high levels of oxidative stress.45,46 We incorporated these additional amino acids into our approach and found that RBCs exogenously pretreated with Gln (Q), cysteine (C), and glycine (G) together, hereby referred to as QCG, incurred significantly less intracellular ROS when stressed with H2O2 (mean fold change of 5.0 without QCG vs 4.0 with 1000 μM QCG pretreatment; P = .009; Figure 2A). This oxidative protection was most notably conferred from 1000 μM QCG supplementation. We also found that RBCs pre-exposed to QCG had a significant reduction in the loss of membrane deformability once stressed with H2O2 (Figure 2B-C), although the 1000 μM QCG concentration of pretreatment did not reach significance (P = .068). Similar to Gln supplementation, QCG pretreatment resulted in a significantly higher hydration status in RBCs (340 mOsm/kg without QCG vs 350 mOsm/kg with QCG; P = .021), as measured by Ohyp (Figure 2B,D). The reduction in oxidative stress in RBCs pre-exposed to QCG was synergistic compared with the effects of individual amino acid pretreatment alone (supplemental Figure 2). For comparison with QCG, we also tested the impact of arginine amino acid supplementation, as it has been reported to be beneficial in both sickle cell anemia and malaria studies; however, exogenous arginine supplementation did not provide a significant intracellular oxidative advantage to RBCs (supplemental Figure 2).
QCG preincubation reduces oxidative impact of H2O2 induced stress. (A) Fold change in intracellular ROS detected by DCFDA staining and flow cytometry in RBCs (n = 6). (B) Representative ektacytometry curve as cells pass through osmotic gradient at a constant shear stress of 16 Pa. (C) Maximum deformability and (D) hydration level of RBCs from ektacytometry curves (n = 3). Mean ± standard error of the mean denoted by error bars. A 1-way paired t test was used to analyze the data. ns, not significant; ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005.
QCG preincubation reduces oxidative impact of H2O2 induced stress. (A) Fold change in intracellular ROS detected by DCFDA staining and flow cytometry in RBCs (n = 6). (B) Representative ektacytometry curve as cells pass through osmotic gradient at a constant shear stress of 16 Pa. (C) Maximum deformability and (D) hydration level of RBCs from ektacytometry curves (n = 3). Mean ± standard error of the mean denoted by error bars. A 1-way paired t test was used to analyze the data. ns, not significant; ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005.
PfCM induces stress in human RBCs
To determine the oxidative impact of PfCM on uninfected catalase-depleted RBCs, we measured RBC echinocytosis, a morphological change associated with oxidative stress.47 We observed that RBCs exposed to PfCM had significantly higher morphology scores (mean score of 116 for control vs 138 for PfCM; P = .0002) (Figure 3A) and a higher percentage of echinocytes (mean of 10.5% for control vs 24.9% for PfCM; P = .002; Figure 3B), than RBCs in control RPMI medium. Although the overall RBC morphology stages showed slight variations between participants, more severe echinocyte stages were present in RBCs from all participants exposed to PfCM (supplemental Figure 3). Next, we aimed to determine whether the redox state was affected in mature RBCs alongside the change in morphology. RBCs incubated with PfCM had a significant increase in intracellular ROS as detected by DCFDA, compared with RBCs from the same donor exposed to control medium (fold change of 1.0 for control vs 1.8 for PfCM; P = .011; Figure 3C-D). To confirm that PfCM–induced oxidative stress was not due to known depleted levels of glucose during P falciparum in vitro cultivation,48 we supplemented PfCM and control medium with excess glucose and found that although oxidative stress was slightly improved when PfCM was supplemented with excess glucose compared with PfCM without excess glucose, there was still a significant increase in intracellular ROS (supplemental Figure 4A). As recent work has demonstrated that free mtDNA can stimulate toll-like receptor 9 responses in RBCs and induce morphological changes (eg, echinocytosis and increased rigidity),10 we wanted to know whether our PfCM also had elevated mtDNA and whether this coincided with the increase in intracellular ROS. Indeed, when we performed quantitative polymerase chain reaction for CpG-containing mtDNA in PfCM-exposed RBCs, we corroborated recently published findings by Lam et al and observed an association between conditions with elevated mtDNA and elevated intracellular ROS (supplemental Figure 4B).10
PfCM increases RBC echinocytosis and intracellular ROS. RBCs incubated in control medium or PfCM overnight were imaged using scanning electron microscopy (AMRAY 1810×, 2000× total original magnification) and assessed based on (A) morphology scores and (B) percentage echinocytes (n = 7). RBCs incubated in control medium or PfCM overnight were washed after incubations and stained with DCFDA to quantify intracellular ROS using flow cytometry. Representative flow cytometry histogram shown (C) and bar graph of ROS fold change compared with the control (D); n = 4. Mean ± standard error of the mean denoted by error bars. A 1-way paired t test was used to analyze the data. ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005.
PfCM increases RBC echinocytosis and intracellular ROS. RBCs incubated in control medium or PfCM overnight were imaged using scanning electron microscopy (AMRAY 1810×, 2000× total original magnification) and assessed based on (A) morphology scores and (B) percentage echinocytes (n = 7). RBCs incubated in control medium or PfCM overnight were washed after incubations and stained with DCFDA to quantify intracellular ROS using flow cytometry. Representative flow cytometry histogram shown (C) and bar graph of ROS fold change compared with the control (D); n = 4. Mean ± standard error of the mean denoted by error bars. A 1-way paired t test was used to analyze the data. ∗P < .05; ∗∗P < .005; ∗∗∗P < .0005.
QCG supplementation lessens PfCM–induced oxidative stress in RBCs
We next aimed to determine whether QCG supplementation also conferred protection to RBCs exposed to PfCM. Indeed, RBCs pretreated with QCG amino acids were found to have a significant reduction in intracellular ROS after PfCM incubation (mean fold change of 1.72 for 0 μM QCG vs 1.15 for 1000 μM QCG; P = .027; Figure 4A). Morphologically, we observed RBCs pretreated with QCG had overall improved morphology scores after PfCM stress (mean score of 130 for 0 μM QCG vs 122 for 1000 μM QCG; P = .040; Figure 4B). Therefore, we found that QCG supplementation confers protection against PfCM-induced oxidative stress in RBCs and that decreased intracellular ROS levels coincided with improved RBC morphology. However, we did not find that QCG supplementation had any effect on lessening the amount of mtDNA, suggesting that QCG supplementation does not work by depleting the amount of mtDNA stressor in the medium (supplemental Figure 4B).
QCG supplementation protects RBCs from PfCM-induced oxidative stress. (A) Fold change of intracellular ROS detected by DCFDA staining and flow cytometry in RBCs (n = 3). (B) The RBC morphology score assessed from scanning electron microscopy images (n = 5). Mean ± standard error of the mean denoted by error bars. One-way paired t test. ns, not significant; ∗P < .05.
QCG supplementation protects RBCs from PfCM-induced oxidative stress. (A) Fold change of intracellular ROS detected by DCFDA staining and flow cytometry in RBCs (n = 3). (B) The RBC morphology score assessed from scanning electron microscopy images (n = 5). Mean ± standard error of the mean denoted by error bars. One-way paired t test. ns, not significant; ∗P < .05.
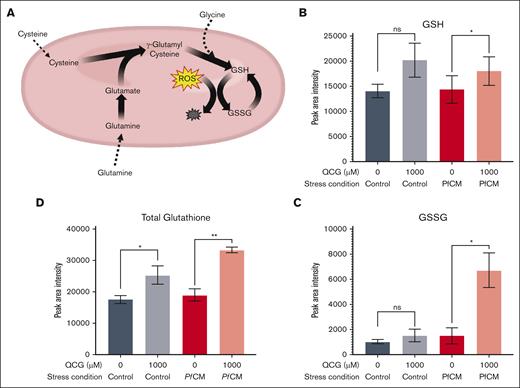
QCG supplementation induces intracellular RBC glutathione synthesis
We hypothesized that QCG protection occurs via intracellular GSH metabolic pathways, given that each amino acid is a known precursor of de novo GSH synthesis (Figure 5A). We found increased levels of total glutathione in QCG-supplemented RBCs (Figure 5B-D; supplemental Figure 5A-C). This activity was evident from QCG supplementation with or without induced oxidative stress. We also observed an increased level of GSSG in QCG-supplemented RBCs after exposure to either PfCM (1525 mean peak area intensity for 0 μM QCG vs 6725 for 1000 μM QCG; P = .009; Figure 5C) or H2O2 (111 650 mean peak area intensity for 0 μM QCG vs 211 245 for 1000 μM QCG, P = .103; supplemental Figure 5B) compared with RBCs without QCG supplementation or exposure to oxidative stress. These results indicate that QCG-supplemented RBCs have increased intracellular glutathione biosynthesis.
Glutathione metabolic changes in RBCs supplemented with QCG followed by PfCM stress. (A) Schematic of metabolic processes known to occur within RBCs from QCG amino acids. Peak area intensity of (B) GSH, (C) GSSG, and (D) total glutathione, as measured by LC-MS/MS (n = 3). A 1-way paired t test was used to analyze the data. ns, not significant; ∗P < .05; ∗∗P < .005.
Glutathione metabolic changes in RBCs supplemented with QCG followed by PfCM stress. (A) Schematic of metabolic processes known to occur within RBCs from QCG amino acids. Peak area intensity of (B) GSH, (C) GSSG, and (D) total glutathione, as measured by LC-MS/MS (n = 3). A 1-way paired t test was used to analyze the data. ns, not significant; ∗P < .05; ∗∗P < .005.
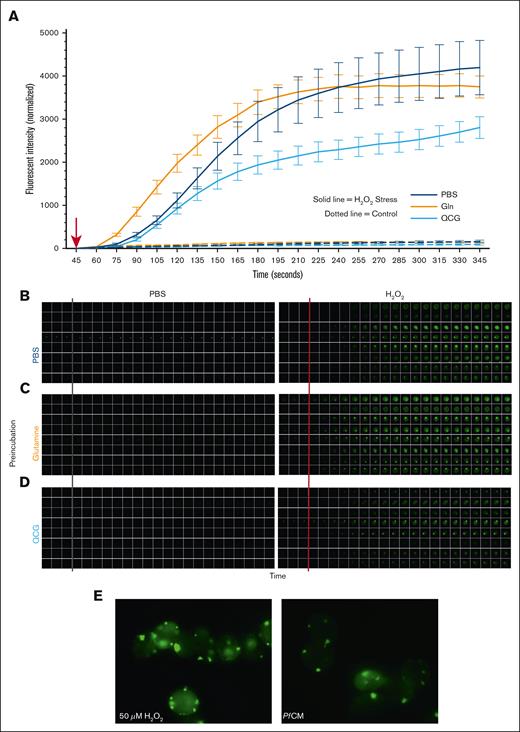
Supplementation with QCG promotes RBC intrinsic antioxidant properties
Given the aforementioned findings, it remained unclear whether QCG-mediated protection was a generalized RBC response or whether QCG protection occurred specifically under oxidative stress exposure. To determine this, we analyzed the kinetic response of QCG-supplemented RBCs to oxidative stress. RBCs pre-exposed to QCG showed lower levels of intracellular ROS within 2.5 minutes of H2O2–induced oxidative stress whereas RBCs pre-exposed to either PBS or Gln had higher levels of ROS that developed quicker within the cells (Figure 6A). Cellular response to oxidative stress among all preincubation conditions appeared to be heterogenous across cells (Figure 6B-D) and interestingly, we observed punctate-like areas of increased fluorescence in each preincubation condition, suggesting internal organization of intracellular ROS with morphology consistent with that of Heinz-Ehrlich bodies. This phenomenon was observed in both H2O2- and PfCM-stressed RBCs (Figure 6E). Together, these data indicate that QCG preincubation intrinsically prepares RBCs to counter oxidative stress before any oxidative stress exposure, rather than mounting an antioxidant response directly after oxidative stress.
QCG supplementation rapidly protects RBCs from intracellular ROS formation during oxidative stress. RBCs pretreated with either PBS, Gln (Q), or QCG were labeled with DCFDA, placed in a glass bottom petri dish per sample condition, and allowed to settle for 45 seconds before given a bolus (red arrow, A) of PBS (gray line, B-D) or 50 μM H2O2 (red line, B-D). Stationary cells (total 8 cells per condition) were quantified (A) and imaged (B-D) per condition at each 15 second time point for 5 minutes. Images were taken with a Leica Thunder at 63× oil immersion (A-D). Representative images of DCFDA labeled RBCs stressed with 50 μM H2O2 (left) or PfCM (right) (E). Images taken with Echo Revolve fluorescent microscope at 100× oil immersion (E).
QCG supplementation rapidly protects RBCs from intracellular ROS formation during oxidative stress. RBCs pretreated with either PBS, Gln (Q), or QCG were labeled with DCFDA, placed in a glass bottom petri dish per sample condition, and allowed to settle for 45 seconds before given a bolus (red arrow, A) of PBS (gray line, B-D) or 50 μM H2O2 (red line, B-D). Stationary cells (total 8 cells per condition) were quantified (A) and imaged (B-D) per condition at each 15 second time point for 5 minutes. Images were taken with a Leica Thunder at 63× oil immersion (A-D). Representative images of DCFDA labeled RBCs stressed with 50 μM H2O2 (left) or PfCM (right) (E). Images taken with Echo Revolve fluorescent microscope at 100× oil immersion (E).
Discussion
In this study, we investigated the metabolic role of amino acid supplementation in oxidatively stressed RBCs with the overall goal of alleviating the RBC oxidative burden. We first explored Gln supplementation in RBCs stressed by H2O2 and observed that while Gln supplementation alone was not sufficient to reduce oxidative stress levels within RBCs, Gln supplementation promoted RBC hydration (Figure 1). In contrast, we identified a significant decrease in intracellular ROS in RBCs that had been supplemented with QCG amino acids (Figure 2). These data suggest that observed ROS protection is conferred through the RBC GSH biosynthesis pathway, as total glutathione was increased in RBCs supplemented with QCG (Figure 5) and this protection from intracellular ROS development was found to be rapid (2.5 minutes) upon induction of oxidative stress (Figure 6). Interestingly, single amino acid supplementation did not reduce intracellular ROS, an effect we only saw with simultaneous supplementation with QCG (supplemental Figure 2). Postexposure of QCG to RBCs did not have comparable protection to RBCs that were oxidatively stressed first (data not shown), suggesting that function of QCG is to equip RBCs to defend themselves against any oxidative stress they may encounter in the future. Here, we also report for the first time to our knowledge that conditioned medium from P falciparum culture increases intracellular ROS in uninfected, catalase-depleted RBCs (Figure 3) and is a likely contributor to the malaria bystander effect. Additionally, we found QCG supplementation does indeed confer protection in the form of reduced intracellular ROS and improved echinocyte morphology to PfCM-stressed RBCs (Figure 4) despite the inhibition of catalase in these RBCs, highlighting a therapeutic prospect for malarial anemia.
Recently, PfCM was shown to increase oxidative stress in erythroid precursor cells9 and alter the membrane structure and binding in uninfected mature RBCs.10 However, it was unclear whether PfCM also perturbed the oxidative status of uninfected mature RBCs. We confirmed 1 mode of action that PfCM has on mature, catalase-depleted RBCs is through oxidative stress, as measured by the induction of intracellular ROS. In general, elevated ROS reduces RBC survivability in vivo,49 suggesting that this could be an additional contributor to the pathogenesis of the bystander effect in malaria.
Gln is implicated in both sickle cell anemia and malarial anemia, highlighting a possible role of metabolic intervention in anemic conditions. Lower plasma Gln levels are associated with pediatric malarial anemia,30 and oral Gln supplementation is an approved treatment for sickle cell anemia, although the cellular mechanisms of this therapy are still under investigation. Here, we show that Gln supplementation improves the RBC hydration status, a potential mechanistic role that warrants more investigation, specifically in sickle RBCs. It is long appreciated that RBCs respond to exogenous metabolites and that the lack of necessary exogenous metabolites negatively impacts RBC lifespan.50,51 Before this study, it was unknown how pre-exposure to exogenous amino acids impacted RBCs in the context of oxidative stress and malaria bystander effect. Our study design of amino acid supplementation focused on recapitulating the plasma environment if key metabolites were supplemented in advance of infection or oxidative stress. We found that RBCs supplemented with QCG amino acids were equipped to counter oxidative stress from both H2O2 and PfCM. We showed that this benefit was intrinsic to RBCs and that ROS development was mitigated within minutes in the cell. Because intracellular GSH synthesis occurs in the order of hours within RBCs,17,52 this suggests that GSH stores increase in response to QCG preincubation and are not a combative cellular response to oxidative stress. Glutamine is an important precursor to arginine in vivo via citric acid cycle and mitochondrial pathways and supplementation with arginine is reported to be beneficial in both malaria28 and sickle cell anemia.29 Within the context of RBCs, as reported in this study, arginine supplementation did not confer oxidative protection intracellularly, suggesting that the beneficial role of arginine, and therefore glutamine as an arginine precursor, requires pathways that are active in nucleated cells rather than in RBCs. Collectively, our results highlight the beneficial role of exogenous QCG in RBCs before oxidative stress. These findings suggest a prophylactic or therapeutic role for amino acid supplementation in oxidative anemias, including malaria.
Our study had limitations. First, these experiments were performed in vitro with only RBCs present. However, in an in vivo context, RBC exogenous amino acid availability and use would be complicated by other cells present, which also can use these substrates. Second, we induced oxidative stress in RBCs in the presence of sodium azide, a known inhibitor of the catalase enzyme, to model stressed uninfected RBCs from P falciparum culture and malaria-infected patients that have been shown to have significantly reduced catalase levels.16,53 It is important to acknowledge that complete ablation using sodium azide is a nonphysiological model and is more severe than what is experienced by RBCs under physiological conditions. Third, we are unable to comment on the exogenous bioavailability and use of QCG by RBCs in an in vivo context, as these studies focused on the cellular, in vitro effects of QCG supplementation. More studies would need to be performed in in vivo systems. In terms of our measurement of ROS, we performed 1 assay, DCFDA, which provides a general measure of intracellular ROS, but further studies using additional probes to better confirm and define the type(s) of ROS in the cell would be valuable. It is also challenging to determine the extent of clinical significance by measuring deformability. Although the effects may be small, any amount of reduced deformability reflects rheological deficiencies that could be associated with increased blood viscosity and propensity for hemolysis. The fact that QCG supplementation showed significant improvement after an acute insult with H2O2 coupled with catalase inhibition may translate to clinical improvements in contexts in which insults are prolonged. For example, in severe malarial anemia, prolonged exposure to oxidative stress could reduce the endogenous antioxidant capacity of RBCs (eg, intracellular GSH stores) and exogenous amino acids can serve as essential building blocks for building back up those internal stores. A strength but also a limitation of our study is that we used different human participants in the various experiments shown. On the one hand, this shows the broad applicability of these findings to different humans, but on the other hand, we do not have 1 set of human participants that we analyzed side by side in all experiments. As such, there is a certain amount of human-to-human variability that we have as part of this study. Finally, although we found an association between intracellular ROS and altered RBC morphology as have others,54 we cannot definitively conclude that this is a causative relationship. Future work must be done to fully decipher the mechanistic processes underlying RBC morphology change in the context of exogenous stressors.
Acknowledgments
The authors thank Betty Pace and Mohandas Narla for their mentorship and feedback. The authors also thank Jordan Buzzett, Kevin Coffey, and Ryan Marie Kelly for their laboratory assistance. Portions of Figure 5 and the visual abstract were created with BioRender.com.
This research was supported in part by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute Career Development Award (K01HL143112) and the Small Research Project Award from the Programs to Increase Diversity Among Individuals Engaged in Health-Related Research – Functional and Translational Genomics of Blood Disorders Program at Augusta University (R25HL106365) (R.J.C.); the NIH/National Institute of General Medical Sciences Training Grant (T32GM127261 [H.C.B] and T32GM149818 [C.E.S]); and the NIH/Office of the Director Shared Instrumentation Grant (S10OD032281 [G.S.M.]). This research was also supported by awards from the Wake Forest University Center for Molecular Signaling and Wake Forest University School of Medicine Center for Redox Biology and Medicine. The authors also acknowledge the Wake Forest Baptist Comprehensive Cancer Center Proteomics and Metabolomics Shared Resource supported by the National Cancer Institute (support grant award number P30CA012197) and the Microscopic Imaging Core supported by the Wake Forest Department of Biology.
Authorship
Contribution: R.J.C. and H.C.B. designed and conceptualized the project; H.C.B. performed and analyzed the results of the experiments, designed the figures, and wrote the initial manuscript; E.A. performed and analyzed ektacytometry experiments; C.E.S. performed flow cytometry experiments; D.S.N. performed parasite culture and conditioned medium generation; J.F.W. contributed technical expertise for flow cytometry experiments; A.O.C. performed mass spectrometry experiments; C.M.F. supervised mass spectrometry and contributed redox biology expertise; G.S.M. assisted with microscopy experiments and contributed technical expertise for fluorescent and scanning electron microscopy imaging; D.B.K-S. supervised ektacytometry, contributed to red blood cell biophysics expertise, and contributed to the overall experimental design; R.J.C. supervised the experiments, data analysis, and revising of the manuscript; and all authors contributed to and endorsed the final version of the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
Correspondence: Regina Joice Cordy, Wake Forest University, Department of Biology, 1834 Wake Forest Rd, Winston Hall, Room 211, Winston-Salem, NC 27109; email: cordyrj@wfu.edu.
References
Author notes
Data are available on request from the corresponding author, Regina Joice Cordy (cordyrj@wfu.edu).
The full-text version of this article contains a data supplement.