T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma (T-ALL/LBL) is a rare hematologic malignancy most commonly affecting adolescent and young adult males. Outcomes are dismal for patients who relapse, thus, improvement in treatment is needed. Nelarabine, a prodrug of the deoxyguanosine analog 9-β-arabinofuranosylguanine, is uniquely toxic to T lymphoblasts, compared with B lymphoblasts and normal lymphocytes, and has been developed for the treatment of T-ALL/LBL. Based on phase 1 and 2 trials in children and adults, single-agent nelarabine is approved for treatment of patients with relapsed or refractory T-ALL/LBL, with the major adverse effect being central and peripheral neurotoxicity. Since its approval in 2005, nelarabine has been studied in combination with other chemotherapy agents for relapsed disease and is also being studied as a component of initial treatment in pediatric and adult patients. Here, we review current data on nelarabine and present our approach to the use of nelarabine in the treatment of patients with T-ALL/LBL.
Introduction
T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/LBL) is a rare hematologic malignancy characterized by the unconstrained proliferation of T lymphoblasts.1,2 T-ALL is classically defined as disease with ≥25% marrow lymphoblasts whereas T-LBL has <25% marrow blasts. T lymphoblasts express marker(s) of immaturity (CD34 and terminal deoxynucleotidyltransferase), CD3 (T-lineage defining), and variably other T-lineage markers. There is significant overlap in the clinical presentations of T-ALL and T-LBL with variable involvement of the marrow, blood, lymph nodes, and central nervous system (CNS).2-4 Bulky mediastinal disease is the classic feature of LBL. Typically, T-ALL and T-LBL are considered divergent presentations of the same disease (hereafter, T-ALL), although there is evidence of distinct biology and chemotherapy responsiveness, including to nelarabine, the topic of this review.5
T-ALL is less common than B-cell ALL (∼15% and ∼25% of pediatric and adult ALL, respectively) and most commonly affects adolescent and young adult males with a higher incidence in Black indiviudals.4,6-8 In pediatric ALL, T lineage is associated with high risk features including age of >10 years, hyperleukocytosis, CNS disease, and slow response.4,9-12 In contrast, adults with T-ALL have equivalent or better outcomes than adults with B-ALL when treated with conventional chemotherapy, although outcomes of adults with ALL are inferior to that of children.8,9,13 Of note, asparaginase-based pediatric-style regimens may be particularly effective for T-ALL.14,15 The prognostic value of cytogenetic abnormalities is not well established in T-ALL but disruption of NOTCH pathway signaling (NOTCH/FBXW7 mutations) is common, and associated with a more favorable prognosis.1,16-18
Early T-cell precursor ALL (ETP-ALL) is a rare (∼10%) T-ALL subset that is biologically and prognostically distinct.1 ETP-ALL arises from a primitive stem cell–like hematopoietic precursor, with genetic and immunophenotypic features that resemble myeloid leukemias, and usually lacks NOTCH pathway mutations.19-21 It can affect individuals of any age, does not present with bulky mediastinal disease or hyperleukocytosis, and is defined immunophenotypically as being CD8−, CD1a−, and CD5dim/CD5−.1,22 ETP-ALL is considered to be adverse risk both in pediatric and adult patients although chemotherapy intensification and allogeneic hematopoietic stem cell transplantation (allo-HSCT) may overcome treatment resistance.19,21,23-25
In summary, T-ALL is a rare leukemia that may be cured with conventional chemotherapy.8,13-15 However, patients with relapsed or refractory (R/R) T-cell disease have few treatment options, and almost always die of their disease.8,26-30 Thus, improvements in initial and salvage approaches are needed. The role of nelarabine, a chemotherapy with unique activity in T-ALL, in the management of patients with T-ALL is reviewed here.
Nelarabine: drug overview
The severe T-cell immune deficiency observed in patients with purine nucleoside phosphorylase (PNP) deficiency was found to be the result of abnormal accumulation of deoxyguanosine triphosphate (dGTP) in lymphocytes.31 This led to investigation of dGTP and its analogs as potential antileukemic drugs.32 However, dGTP is rapidly degraded by PNP in red blood cells.33 Nelarabine (compound 506U78; trade name Arranon) is a prodrug, which is demethylated by adenosine deaminase in the blood to the active deoxyguanosine analog, 9-β-arabinofuranosylguanine (ara-G).34 Ara-G is resistant to degradation by PNP and is more lymphotoxic to T lymphoblasts than to mature T cells.35 After accumulation within T-lymphoblasts, ara-G is converted to 5′-triphosphate (ara-GTP) via deoxycytidine kinase and mitochondrial deoxyguanosine kinase and results in DNA synthesis inhibition and cell death.36,37 Because ara-G is challenging to synthesize and is poorly water soluble, the use of nelarabine as the prodrug, which is 8 times more water soluble then ara-G, has overcome practical obstacles.38,39 After intravenous administration of nelarabine, it is rapidly converted to ara-G, with a half-life of 2 hours and an area under the concentration time curve 10-fold higher compared with that of nelarabine, demonstrating that nelarabine as an efficient prodrug for ara-G.34 The exquisite sensitivity of T cells compared with B-cells is thought to be associated with higher concentrations of ara-G accumulation in T cells, which leads to greater exposure to ara-GTP.37,40,41
Nelarabine monotherapy for R/R ALL
An initial multi-institutional phase 1 study of nelarabine was conducted in pediatric (n = 59) and adult (n = 34) patients with refractory lymphoid and myeloid malignancies who are heavily pretreated to identify the maximum tolerated dose (MTD), toxicity profile, pharmacokinetics, and pharmacodynamics of the drug (Table 1).42 Neurologic toxicity was identified as the dose-limiting toxicity whereas myelosuppression and other organ toxicity were not significant concerns. In total, 72% (n = 67) of patients were affected with mostly reversible neurotoxicity, with more severe neurotoxicity at higher dose levels (DLs). Neurologic toxicities were sensory and motor; central and peripheral; including events of encephalopathy, seizure, ataxia, and peripheral neuropathy. There was 1 grade 5 event of status epilepticus. A specific MTD was not identified but a dose range of 30 to 40 mg/kg was recommended for phase 2 trials as responses were observed at all DLs. In the phase 1 cohort, there was an overall response rate (ORR) of 31%, with a higher response rate noted in patients with T-cell malignancies: 54% of patients with T-ALL achieved a complete response (CR, 23%) or partial response (PR, 31%) after 1 or 2 five-day courses of treatment. The phase 1 experience prompted phase 2 studies in children and adults.
A multi-institutional Children’s Oncology Group (COG)-led phase 2 study (COG P9693) of single-agent nelarabine in children (aged ≤21 years, n = 153) with T-ALL in first or later relapse demonstrated a CR in ∼50% of patients in first relapse (“stratum 1”) treated with 650 mg/m2 per day, 5 days per cycle (48% CR [16/33]; 55% CR + PR).43 Notably, patients in later relapse, with CNS relapse, and with lymphomatous relapse responded less frequently (eg, 27% CR + PR in patients with second or subsequent relapse). Consistent with the phase 1 data, the primary adverse event was neurotoxicity (18% experienced ≥3 grade neurologic adverse events), which prompted de-escalation from 1.2 g/m2 per day, ×5 days; to 650 mg/m2 per day, × 5 days. Although there was no association between neurologic adverse events and nelarabine dose, prior or current CNS disease, or concurrent medications (including intrathecal [IT] chemotherapy), the study was not powered to evaluate these associations. There was no significant myelosuppression.
In parallel to the COG study, a multi-institutional Cancer and Leukemia Group B (CALGB) phase 2 study (19801) was conducted in adults (n = 39) with R/R T-ALL, with nelarabine administered on an alternate-day schedule in an attempt to minimize neurotoxicity (the initial dose was 2.2 g/m2 per day, reduced to 1.5 g/m2 per day on days 1, 3, and 5 after 3 patients).44 The cohort (82% male, median age 34 years [range, 16-66 years], 67% T-ALL/33% T-LBL) was heavily pretreated with 72% (28/39) having ≥2 prior regimens. The ORR was 41% (16/39) with 31% (12/39) achieving a CR. Similar to the COG study, patients in first relapse were more likely to respond (ORR 55% [6/11]) compared with patients with >1 prior regimen (ORR 36% [10/28]). There was no difference in response rates between T-ALL and T-LBL. Seven patients, including 5 responders, ultimately received allo-HSCT. For all patients who received treated, the 1-year overall survival (OS) was 28% (median, 20 weeks). Neurotoxicity was again frequently reported, although here most events were grade 1/2 sensory (37%) or motor (21%) peripheral neuropathy. Neurologic adverse events grade ≥3 were reported in only 4 patients (6 episodes) including 1 case each of grade 3 motor neuropathy, grade 3 seizure (in setting of imipenem and renal dysfunction), grade 3 aphasia, and reversible grade 4 depressed level of consciousness. There were no treatment-related deaths.
The German GMALL group conducted a phase 2 study of nelarabine in adults with R/R T-ALL (n = 126; median age, 33 years; range, 18-81 years) and enrolled a heavily pretreated population. This study confirmed the CALGB findings in a larger cohort, reporting that 36% (45/126) achieved a CR after 1 or 2 cycles.45 Most patients achieved a CR after the first cycle, but 39% (7/18) of patients with a PR after cycle 1 achieved a CR after cycle 2. No late responses were reported. In this study, an initial diagnosis of T-ALL (vs LBL), lack of extramedullary involvement, and thymic phenotype (vs early or mature immunophenotype) increased the likelihood of achieving a CR (disease status was not significant in this analysis). Most patients who achieved CR (80% [36/45]) were able to bridge to allo-HSCT, without excess toxicity, with reasons for nontransplantation being age (n = 3), previous allo-HSCT (n = 4), and lack of donor (n = 1). However, the authors noted that posttransplant relapse remained frequent with 3-year OS and relapse-free survival of 31% and 37%, respectively. Neurologic toxicity again was reported but manageable with grade ≥3 neurologic toxicities reported in just 4% of cycles and 7% of patients. There were no grade 5 neurologic or nonneurologic adverse events related to nelarabine.
A recently published “real-world” analysis of 118 patients treated at 27 Italian sites almost identically reproduced the findings of the prospective phase 2 CALGB and GMALL adult trials.46 In this cohort (median age, 37 years; range, 17-74 years; 73% male, 55% >2 lines of therapy), 36% (43/118) achieved a CR, and 40% (47/118) successfully bridged to allo-HSCT. Patients who received transplantation had the best survival (1-year OS 58% vs 22% among those without transplantation; P < .001) with a notable minority of patients who received transplantation surviving long-term (5-year OS, 38%). Grade 3 to 4 neurologic toxicities were observed in just 8% (9/118) of cases.
In summary, single-agent nelarabine for R/R T-ALL is active, especially for patients in first relapse, with durable remissions achieved in patients who receive allo-HSCT consolidation. Neurotoxicity is the primary toxicity of concern but mostly low-grade, reversible, with perhaps better tolerability in adults receiving every other day dosing, and in patients with less previous exposure to neuro-toxic chemotherapy. Based on the phase 2 data, in 2005 the FDA approved nelarabine in patients with T-ALL/LBL whose disease has not responded to or has relapsed following treatment with at least 2 chemotherapy regimens.47 Hematologic toxicity is minimal, prompting investigators and clinicians to consider combination approaches.
Nelarabine combinations for R/R T-ALL
The Children’s Hospital of Philadelphia reported on 7 children (aged 2-19 years, median 13) with nelarabine in combination with cyclophosphamide (CTX) and etoposide (NECTAR) for R/R T-ALL. Most patients received nelarabine in week 1, followed by CTX and etoposide on 5 days during week 2 (Table 1).48 One patient received all 3 drugs concurrently. Patients with CNS-3 involvement or neurologic abnormalities were excluded. All patients received IT chemotherapy spaced from nelarabine. All patients (5 of 5) with T-ALL achieved CR whereas the 2 patients with T-LBL achieved a PR. In total, 4 patients bridged to allo-HSCT. Of 7 patients, 6 experienced neurotoxicity: grade 2 and 3 sensory and motor neuropathies were most common, each occurring in 4 patients but were mostly transient with only 1 patient requiring nelarabine to be held. The combination therapy was associated with grade 3 to 4 hematologic toxicity with median time to recovery of platelet and neutrophils being 21 and 25 days, respectively.
Later, the University of Pennsylvania group reported on 5 adult patients with NECTAR.49 A CR was achieved in 3 of 5 patients and 2 patients were consolidated with allo-HSCT. One patient died because of neuromuscular weakness, attributed to nelarabine use. Interestingly, this was the only patient who received IT chemotherapy simultaneously with nelarabine (all other patients received IT chemotherapy before or after nelarabine infusions). One additional patient had grade 2 neurotoxicity and 1 patient died because of sepsis.
Subsequently, an investigator-initiated phase 1 trial was conducted to evaluate the safety and efficacy of the NECTAR regimen in children (n = 23; aged 1-18 years; median age, 9.7 years) with T-ALL/LBL in first relapse.50 Three DLs were evaluated with escalating doses of nelarabine and CTX (Table 1); all drugs were given simultaneously on days 1 to 5 in 21-day cycles. DL3 was identified as the MTD (nelarabine 650 mg/m2 per day, CTX 400 mg/m2 per day, etoposide 100 mg/m2 per day) and expanded, enrolling 4 additional patients, before study termination because of slow accrual. The ORR among 21 patients who were evaluable was 38% (8/21); 30% (4/12) in the T-ALL cohort, and 44% (4/9) in the T-LBL cohort. Four patients (17%) experienced grade 3 to 4 motor or sensory neuropathy. Three dose-limiting toxicities were recorded (1 in DL2 and 2 in DL3), all due to neurotoxicity. The authors concluded that response rates of NECTAR were similar to previous phase 2 single-agent nelarabine trials, limiting rationale for further development of the combination. Of note, 4 patients (17%) received prior single-agent nelarabine.
Dana-Farber/Harvard Cancer Center investigators conducted a large retrospective analysis to further investigate efficacy and toxicity of nelarabine administered alone or in combination for R/R T-ALL.51 Over an 18-year period, 44 patients were treated (median age, 19 years; range, 2-69 years): 15 with nelarabine monotherapy and 29 with combination therapy, mostly NECTAR (Table 1). Overall, 24 patients (55%) achieved CR, 62% (18/29) with combination therapy and 40% (6/15) with monotherapy. Most responders (21, 88%) pursued allo-HSCT. The median OS of the entire group was 12.8 months (95% confidence interval, 6.9-not reached), which was higher in the combination group compared with the monotherapy group (24-month OS, 53% vs 8%; P = .003). The rate of neurotoxicity was similar between groups (17% vs 27%; P = .46) although grade 3 to 4 cytopenias were more frequent in the combination group. In a multivariate analysis, nelarabine combination therapy and post-nelarabine allo-HSCT consolidation were associated with improved OS (hazard ratio, 0.41; P = .04 and hazard ratio, 0.25; P = .008, respectively).
Nelarabine in upfront treatment
COG first conducted a 2-stage pilot study (AALL00P2) assessing the feasibility and safety of adding nelarabine to an intensive Berlin-Frankfurt-Munich (BFM) regimen in 86 children (aged 1-22 years) with newly diagnosed high-risk (HR) T-ALL (Table 2).52 Patients received 5 or 6 five-day courses of nelarabine. Grade 3 to 4 peripheral neuropathy was reported in 15% of patients whereas 4% experienced nonseizure central neurotoxicity. After analysis of the events, the investigators concluded that only 1 of 72 patients treated with nelarabine experienced a significant neurotoxicity clearly related to nelarabine (Guillain-Barré–like syndrome). The lower rates of toxicity compared with that observed in COG P9673 was attributed to less exposure to other neurotoxic agents including vincristine, high-dose methotrexate, and cranial irradiation. Notably, the integration of nelarabine was not noted to increase myelosuppression or infection.
With the demonstrated safety and feasibility of integrating nelarabine into frontline chemotherapy, COG launched the AALL0434 phase 3 trial. This large trial randomized 1562 patients aged 1 to 31 years to methotrexate plus pegaspargase (Capizzi [C]-MTX) with early whole-brain irradiation or high-dose methotrexate with later whole-brain irradiation. A subset of 659 patients with intermediate risk and HR T-ALL (excluding patient with low risk T-ALL; note HR T-LBL randomization was reported separately53) were additionally randomized to receive or not receive 6 five-day courses of nelarabine in consolidation and maintenance.54-56 Patients with a seizure disorder or preexisting peripheral neuropathy were not randomized. The 5-year disease-free survival (DFS) in the nelarabine arm (n = 323) was superior compared with that of patients randomized to the no-nelarabine arm (n = 336; 88.2% vs 82.1%; P = .029). The best-performing arm of C-MTX plus nelarabine posted a 5-year DFS of 91%. The main driver of benefit appeared to be the impact of nelarabine on reducing CNS relapses, which occurred less frequently in the nelarabine arm (1.3% vs 6.9%; P = .0001). The DFS advantage was not restricted to MTX assignment, age, race, presence of CNS disease at diagnosis, and/or presence of postinduction measurable residual disease (MRD). An increased risk of neurotoxicity was not seen.
The demonstration that nelarabine improved DFS in a HR pediatric population in a large, phase 3 trial was a remarkable achievement, particularly given the historical >90% 5-year DFS survival. Criticism has focused on whether nelarabine added significantly to patients treated with C-MTX, noting similar numbers of relapses, and similar 5-year DFS in the C-MTX (5-year DFS: 87.2%, 11/151) and C-MTX plus nelarabine (5-year DFS: 91.4%, 10/147) groups.57 Although there was no statistical interaction detected between the MTX and nelarabine randomizations, power was diminished in analyzing subsets. Others caution that longer follow-up is needed to confirm that the nelarabine arm maintains a DFS advantage. In addition, the current COG treatment backbone for T-ALL (AALL1231) incorporates dexamethasone, more asparaginase, and omits CNS irradiation, which may mean that the benefit of nelarabine for CNS control may be less, or more, relevant.58 It is important to note that nelarabine was not tested in patients with low risk T-ALL and that it showed no advantage for patients with HR T-LBL.53 The COG0434 study included patients up to aged 31 years but was notably skewed toward younger patients (50% aged <10 years, 33% aged 10-15 years, but only 16% were aged ≥16 years), limiting generalizability to older adolescent and young adults with regard to both toxicity and efficacy. In summary, although the COG AALL0434 demonstrated in a randomized fashion that nelarabine improved DFS by reducing CNS relapse rates, the magnitude of nelarabine contribution in the setting of optimized chemotherapy (ie, with C-MTX and/or dexamethasone) remains uncertain.
The Japanese group published a modified pediatric BFM–based regimen incorporating nelarabine in patients with HR and very HR disease and reported good outcomes, although the relative contribution of nelarabine was not defined.59 Grade 3 motor and sensory neuropathy was seen in 11 (3%) and 6 (2%) patients, respectively. Dose adjustment of nelarabine was performed in 18 patients.
The first reported effort to incorporate nelarabine into an adult ALL regimen was a large, single-institution (MD Anderson Cancer Center) phase 2 trial integrating nelarabine into the CTX, vincristine sulfate, and doxorubicin hydrochloride (hyper-CVAD)60 regimen. Nelarabine was initially given after hyper-CVAD completion and before 6-mercaptopurine + vincristine + MTX + prednisone (POMP) therapy; this was amended after 30 patients, to introduce nelarabine earlier after cycles 4 and 5 and then again during the POMP maintenance phase. The 3-year OS (65%) of 67 patients who were treated was comparable with historical patients treated with hyperCVAD (3-year OS of 64%).61,62 Neurotoxicity was reported in 72%, mostly grade 1 to 2, with only 5 grade ≥3 neurotoxicity events. The investigators have further updated their regimen incorporating pegaspargase, 2 additional cycles of nelarabine as late intensification instead of cycles 18 and 19 of POMP, and venetoclax, with improved results, suggesting potential benefit of pegaspargase addition63 but myelosuppression associated with venetoclax.64 Regarding the efficacy of hyper-CVAD plus nelarabine in different subgroups, a retrospective study in 171 adults (66 with nelarabine) found that nelarabine addition improved survival only among patients with non-ETP T-ALL (5-year OS 83% vs 38% with hyperCVAD plus nelarabine vs hyper-CVAD, P = .003), whereas no improvement was seen in patients with ETP T-ALL or T-LBL.65
The first randomized phase 3 investigation of nelarabine in the adult upfront setting is the UKALL14 trial of 175 patients (median age, 38 years; range, 25-65 years) randomized to receive or not receive 1.5 g/m2 nelarabine on days 1, 3, and 5 after the second induction.66 The first results were recently presented and were notable for no increase in neurotoxicity in the standard of care (SOC) + nelarabine arm vs SOC arm (7 vs 6 events, respectively). The 3-year Event free-survival (EFS), OS, and relapse rate among responders were comparable between the SOC and SOC plus nelarabine arms: 57% vs 62%, P = .61; 62% vs 66%, P = .73; and 29% vs 28%, P = .91. Of note, only 71% of patients received nelarabine because of protocol-dictated exclusions, and only 1 course nelarabine was administered in this study, which may have led to negative results.
Another approach to incorporating nelarabine into initial therapy is to use nelarabine in a “risk-adapted” fashion. The GMALL 08/2013 trial recruited 281 patients with T-ALL and defined patients at HR as having early or mature immunophenotype or those with failure to achieve CR after induction 1 or with molecular failure (MRD of >10−4) later in treatment.67 Patients with HR T-ALL were offered single-agent nelarabine after consolidation 1 as a bridge to allo-HSCT. At the latest report, only 14 patients received nelarabine monotherapy for persistent MRD. Disappointingly, of the 13 patients who were evaluable for molecular response, only 1 achieved a molecular CR and 2 achieved a molecular response. Patients at standard risk received nelarabine in combination with CTX later in consolidation: 123 received 1 cycle and 96 received 2 cycles of nelarabine-based therapy for a total of 219 cycles. Nelarabine consolidation was well tolerated with just 1 grade 3 to 4 neurologic toxicity reported (Guillain-Barré syndrome); and despite combination with CTX, grade 3 to 4 cytopenias were only seen in 15% to 20% of patients.
In summary, the data on benefit of nelarabine as part of initial treatment of adults are limited and this continues to be studied. Toxicity appears manageable, with most toxicities being of grade 1 to 2. Various theories for lack of benefit include offering nelarabine at insufficient doses (UKALL14) or too late in treatment (hyper-CVAD), or it may be that the studies have not been powered sufficiently to detect a relatively modest benefit. Disappointingly, nelarabine has not been demonstrated to be successful in eradicating persistent MRD, suggesting that alternative approaches are needed.
Nelarabine neurotoxicity: impressions and recommendations
Nelarabine is a well-tolerated chemotherapy but neurotoxicity remains a particular concern. In the phase 1 trial of nelarabine, neurotoxicity was reported as occurring extremely frequently (72%) and was ultimately dose limiting.42 The subsequent phase 2 pediatric trial (COG P9693) of single-agent nelarabine for R/R T-ALL also reported a high rate of grade ≥3 neurologic adverse events (18%).43 In contrast, grade ≥3 neurotoxicity is less frequently reported (<10%) in adults receiving single-agent nelarabine for R/R ALL (CALGB 19801, 10%; GMALL, 7%; and Italian real-world cohort, 8%).44-46 Although the reason for differences is not clear, it may be related, in part, to the intermittent dosing of nelarabine given in adults vs the continuous administration in children. Moreover, compared with children, adult patients may have received less cumulative exposure to other neurotoxic agents, which are core components of pediatric ALL regimens (including vincristine, IT chemotherapy, radiation, and other purine nucleosides) and may be less vulnerable developmentally. Notably, children treated with nelarabine as part of frontline therapy (COG AALL00P2, AALL0434) have not had trouble with neurotoxicity, which suggests a role of previous treatment in increasing risk of nelarabine-related neurotoxicity.52,54
It is important to recognize that the patients treated in the phase 2 studies for relapsed disease were carefully selected to be at less risk for neurotoxicity. For instance, the CALGB 19801 and GMALL studies excluded patients with CNS leukemia that would require IT or CNS radiation therapy, as well as patients with history of seizures, prior grade ≥3 neurologic toxicity. The CALGB study excluded patients with neuropathy of grade ≥2 at the time of registration regardless of causality. Thus, caution must be used when considering nelarabine treatment in patients with any of these features.
To date, the mechanism of nelarabine-induced neurologic toxicity is not well understood although other chemotherapeutic agents including IT and IV methotrexate can cause similar toxicity, thus, the toxicity may be a class effect of antimetabolites. It has been noted that brain and nerve tissues express high levels of deoxyguanosine kinase activity, possibly leading to high concentrations of ara-GTP in the CNS but this mechanism is speculative.43
Additional therapeutic strategies for R/R T-ALL/LBL
Nelarabine is only 1 option for improving clinical outcomes of patients with T-ALL. Other strategies are also being explored to better the outcomes of this population. In the COG ALL1231 study, the proteosome inhibitor bortezomib was integrated into the chemotherapy backbone and children randomized to receive bortezomib demonstrated an improved EFS and OS.68 Bortezomib has also demonstrated activity in the R/R setting.69,70 Another strategy, supported by preclinical studies, involves treating patients with R/R ALL with the B-cell lymphoma 2 and the B-cell lymphoma XL protein inhibitors, venetoclax and navitoclax, in combination with chemotherapy.71,72 In a pilot study that included children and adults, the CR rate in the R/R T-ALL/LBL cohort (n = 47) was 52.6% with a median OS of 6.6 months, which is encouraging in a heavily pretreated population.73 Venetoclax is also being studied in combination with hyper-CVAD for treatment of R/R T-ALL in adults, with a reported CR rate of 60% in a single-institution study.74 Another approach involves targeting CD38, either with the monoclonal anti-CD38 antibody daratumumab or with a bispecific T-cell engager (CD38-CD3), both of which are strategies currently being investigated.75-79 Chimeric antigen receptor T-cell therapy has lagged behind for T-ALL but results from preclinical studies and early clinical results are also now being reported for CD5- and CD7-specific chimeric antigen receptor T-cell therapies as a bridging therapy to alloSCT.80-83 Finally, NOTCH pathway inhibitors continue to be investigated for NOTCH-mutated T-ALL although results have been disappointing to date.84,85 Whether any of these therapies, once developed, should replace or add to nelarabine will require further study. T-ALL is an uncommon disease and the challenge of conducting clinical trials for this rare population should be recognized with specific support provided to help clinical sites accomplish these important investigational protocols.
Summary of approach
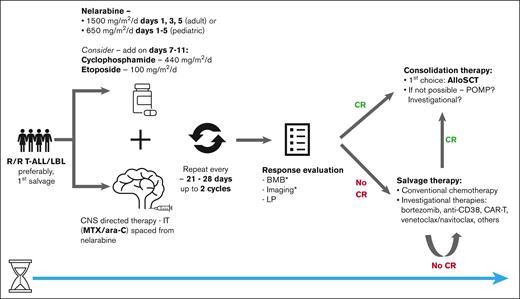
Our approach in patients with R/R T-ALL/LBL is summarized in Figure 1. In patients who are refractory to initial therapy or relapsed, we recommend treating with nelarabine in earlier lines of therapy (ie, first relapse/primary refractory) in whom it may be more effective and less toxic.43 However, there may still be responses in later lines of therapy, because nelarabine has a unique mechanism of action.44 If CR is achieved, we recommend consolidating with allo-HSCT, because durability of response is limited and long-term remission without allo-HSCT is rare.44,51 If allo-HSCT is not possible, we recommend consolidating with additional therapy, although there is no data to support any particular approach. Although some studies showed a lower response/worse outcome with T-LBL,43,65 this is inconsistent across trials and patients with T-LBL should not be excluded from nelarabine salvage therapy. Patients who do not respond after 2 cycles should be referred for alternative salvage therapy, because most patients respond after 1 or 2 course of treatment.45,51 Based on retrospective data, there may be additive value of nelarabine in combination therapy and we recommend combination approaches with CTX and etoposide in patients who are eligible.48,49,51 Patients should receive CNS prophylaxis but IT chemotherapy should not be administered concurrently with nelarabine.
Suggested approach for nelarabine treatment in patients with R/R T-ALL/LBL. ∗Bone marrow biopsy and imaging should be completed in patients with marrow and/or extramedullary involvement, respectively. ara-C, cytarabine; BMB, bone marrow biopsy; LP, lumbar puncture; alloSCT, allogeneic stem cell transplantation.
Suggested approach for nelarabine treatment in patients with R/R T-ALL/LBL. ∗Bone marrow biopsy and imaging should be completed in patients with marrow and/or extramedullary involvement, respectively. ara-C, cytarabine; BMB, bone marrow biopsy; LP, lumbar puncture; alloSCT, allogeneic stem cell transplantation.
There are randomized data supporting the use of nelarabine in the upfront setting in children with T-ALL, but not in those with T-LBL, in which setting it may be used in the context of the COG BFM–based backbone.56 In contrast, there are less data to support the routine use of nelarabine in the upfront setting in adults aged >30 years outside the context of a clinical trial and we do not routinely do so.62,66,67 It may be that incorporation of nelarabine earlier in the treatment course or with additional doses may improve outcomes although this remains to be demonstrated and enrollment in clinical trials is encouraged.
Finally, neurotoxicity adverse events are usually grade 1 to 2 and self-limited.44,45,51,62 We recommend avoiding concurrent administration of nelarabine with IT chemotherapy and patients should be monitored closely for neurotoxicity. Patients with a significant history of central or peripheral nervous system conditions should not receive nelarabine because safety in this population is not demonstrated.
Conclusions
In summary, nelarabine represents a valuable agent for the treatment of T-ALL. The strongest evidence supports the use of nelarabine for the treatment of early relapse, with retrospective data suggesting improved chance of response when used in combination with other chemotherapy such as CTX and etoposide. Durable clinical benefit requires consolidation with allo-HSCT. The role of nelarabine in the initial treatment of T-ALL is less established, although supported for treatment of young patients with HR T-ALL in combination with the COG 0434 backbone with benefit related to reduction in CNS relapse. The benefit of nelarabine in other contexts including in combination with other frontline adult and pediatric regimens, and for treatment of persistent MRD, is not established. For those treated with nelarabine, neurotoxicity appears to be a modest and manageable risk in appropriately selected patients but vigilance is required.
Acknowledgment
This work was supported by the Foley Family Fellowship Fund.
Authorship
Contribution: M.R.L, D.J.D, and S.S designed the review; S.S. and M.R.L created the first draft; D.J.D. reviewed and completed revisions and corrections; and all coauthors reviewed and agreed on the final version.
Conflict-of-interest disclosure: D.J.D. has served as a consultant for Amgen, Autolos, Agios, Blueprint, Forty-Seven, Gilead, Incyte, Jazz, Novartis, Pfizer, Servier, and Takeda; and declares research funding from AbbVie, Glycomimetics, Novartis, and Blueprint Pharmaceuticals; M.R.L. receives research support from AbbVie and Novartis. S.S. declares no competing financial interests.
Correspondence: Marlise R. Luskin, Division of Leukemia, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana 2056, Boston, MA 02215; email: marlise_luskin@dfci.harvard.edu.