Key Points
ELN 2022 guidelines improve survival stratification for patients with intermediate and adverse-risk AML treated with induction chemotherapy.
Inclusion of new cytogenetic and molecular events increased the number of patients classified as having intermediate- or adverse-risk AML.
Abstract
Risk stratification in acute myeloid leukemia (AML) remains principle in survival prognostication and treatment selection. The 2022 European LeukemiaNet (ELN) recommendations were recently published, with notable updates to risk group assignment. The complexity of risk stratification and comparative outcomes between the 2022 and 2017 ELN guidelines remains unknown. This comparative analysis evaluated outcomes between the 2017 and 2022 ELN criteria in patients enrolled within the multicenter Beat AML cohort. Five hundred thirteen patients were included. Most patients had 1 or 2 ELN risk–defining abnormalities. In patients with ≥2 ELN risk–defining mutations, 44% (n = 132) had mutations spanning multiple ELN risk categories. Compared with ELN 2017 criteria, the updated ELN 2022 guidelines changed the assigned risk group in 15% of patients, including 10%, 26%, and 6% of patients categorized as being at ELN 2017 favorable–, intermediate–, and adverse–risk, respectively. The median overall survival across ELN 2022 favorable–, intermediate–, and adverse–risk groups was not reached, 16.8, and 9.7 months, respectively. The ELN 2022 guidelines more accurately stratified survival between patients with intermediate- or adverse-risk AML treated with induction chemotherapy compared with ELN 2017 guidelines. The updated ELN 2022 guidelines better stratify survival between patients with intermediate- or adverse-risk AML treated with induction chemotherapy. The increased complexity of risk stratification with inclusion of additional cytogenetic and molecular aberrations necessitates clinical workflows simplifying risk stratification.
Introduction
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy characterized by recurrent cytogenetic and molecular abnormalities resulting in arrested differentiation and proliferation of immature hematopoietic precursors.1,2 Although complete remission (CR) can be attained in ∼60% to 70% of patients treated with intensive induction chemotherapy (IC), relapse remains the predominant cause of treatment failure, with few patients aged >60 years historically experiencing long-term survival.3-5
Identification of cytogenetic subgroups with differing outcomes improved survival prognostication, which was further refined through integration of recurrent somatic mutations.1,6 The most salient cytogenetic and molecular abnormalities are included within current National Comprehensive Cancer Network and European LeukemiaNet (ELN) guidelines.7,8
Classification of prognostic subgroups in AML remains principal in the selection and individualization of induction and consolidation therapy, particularly the use of allogeneic hematopoietic cell transplantation (HCT).9-11 Current guidelines recommend HCT for patients with a relapse risk exceeding 35% to 40% and is considered for patients with intermediate- or adverse-risk disease in the first CR or any risk stratification in the second CR or beyond.9 Although measurable residual disease (MRD) serves as a dynamic marker of treatment response and is an important adjunct enabling therapy adaptation, baseline molecular factors retain a strong prognostic influence.12-14
The 2017 ELN guidelines have recently been updated to aid in the diagnosis and management of AML and include several key changes to prognostic categories.9 In-frame mutations within the bZIP domain of CEBPA are now considered favorable risk, irrespective of the presence of a second-hit CEPBA mutation.15FLT3 internal tandem duplication (ITD) allelic ratio is no longer taken into consideration, with patients who are FLT3 ITD positive being classified as intermediate risk, irrespective of allelic ratio or concurrent mutations in NPM1.15 Patients with mutations in genes associated with myelodysplasia-related (MR) changes (SRSF2, SF3B1, U2AF1, ZRSR2, BCOR, EZH2, and STAG2 in addition to ASXL1 and RUNX1, which were previously included in the 2017 guidelines) are considered to have adverse-risk AML in the absence of a concurrent favorable-risk abnormality.15,16 In addition, 2 new translocations (KAT6A::CEBBP and MECOM(EVI1)-rearranged) are included as adverse-risk cytogenetic events.17,18
Given these updates to the current risk stratification schema in AML, it is imperative to understand how these changes subsequently affect risk group assignment and prognosis, which in turn influence treatment decisions. Herein, we evaluated outcomes using the updated ELN 2022 guidelines within an extensively characterized, large, contemporary cohort of patients with AML.
Methods
Patient selection
All adult patients aged ≥18 years with newly diagnosed AML (N = 513) enrolled in the Beat AML cohort between 1 January 2009 and 31 December 2019, were included for analysis.2,19,20 Patients with relapsed/refractory AML, a diagnosis of acute promyelocytic leukemia, or acute lymphoblastic leukemia were excluded. The Beat AML cohort represents a robust, contemporary, multicenter data set of 942 patient samples from 805 patients with hematologic malignancies and over 59 000 clinical annotations, including diagnosis, clinical laboratory parameters, morphologic and immunophenotypic characterization of leukemia, treatments received, response, and survival outcomes. In addition, the data set includes results of clinical laboratory improvement amendments–approved next-generation sequencing assays targeting recurrently mutated genes in myeloid malignancies and G-banded karyotype analyses, whole-exome sequencing, and bulk RNA sequencing.2,19,20 A full list of frontline treatments received is displayed in supplemental Table 1. Data were collected and analyzed under an institutional review board–approved protocol of the Oregon Health & Science University for prospective sample collection and analysis of patients with hematologic malignancies. The study was conducted in accordance with the Declaration of Helsinki.
ELN variant calling
ELN risk–defining mutations were identified according to consensus classification using a computed internal filtering pipeline of whole-exome sequencing results in conjunction with the manual review of available clinical laboratory improvement amendments–certified targeted next-generation sequencing assays. Cytogenetic analysis was performed using standard G-banded metaphase chromosomes. Gene fusions were identified using clinically available fluorescent in situ hybridization and/or RNA-sequencing data. After manual review, patients were assigned to respective ELN 2022 risk groups.9
Response definitions
CR and CR with incomplete hematologic recovery (CRi) were recorded in accordance with the revised international working group criteria for AML.21 Overall survival (OS) was calculated from the time of AML diagnosis to the date of death or last follow-up. Survival was not censored at the time of HCT.
Statistical considerations
Categorical variables were compared using Fisher exact or χ2 tests, whereas continuous variables were compared using the Wilcoxon or Kruskal-Wallis test, as appropriate. Survival outcomes were assessed using the log-rank method, with Cox proportional hazard modeling, as appropriate. Adjustments for multiple comparisons were undertaken using the false discovery rate method, as described by Benjamini and Hochberg.22
Results
In total, 513 adult patients were included in the final analysis. Median patient age was 62 years (range, 18-88 years). A portion of the cohort had a clinical diagnosis of secondary AML (sAML; 16% [n = 82]) progressing from myelodysplastic syndromes (8.6% [n = 44]), myeloproliferative neoplasms (4.7% [n = 24]), or MDS/MPN (2.7% [n = 14]). Most patients with available race data (87% [n = 322 of 369]) self-identified as White, with a slight male predominance (56% [n = 288]). ELN 2022 risk was favorable, intermediate, and adverse in 29% (n = 147 of 513), 34% (n = 121 of 513), and 48% (n = 245 of 513), respectively. Additional baseline demographics based on ELN 2022 risk group are displayed in Table 1.
Patients with ELN 2022 adverse–risk disease were older (median age, 66 years; [range, 21-88 years]) compared with patients with favorable- (56 years; [range, 18-83 years]) or intermediate-risk AML (59 years; [range, 18-87 years]) (P value < .001), more likely to have a clinical diagnosis of sAML (favorable, 2%, [n = 3 of 147]; intermediate, 5% [n = 6 of 121]; and adverse, 30% [n = 73 of 245]; P value < .001) and receive lower-intensity/hypomethylating agent (HMA)–based therapy (favorable, 12%, [n = 16 of 136]; intermediate, 9.4%, [n = 10 of 106]; and adverse, 30%, [n = 64 of 214]; P value < .001).
Distribution of ELN molecular and cytogenetic abnormalities
At diagnosis, most patients had 1 (36%, [n = 183]) or 2 (31%, [n = 159]) ELN risk–defining abnormalities (out of a possible 31 unique cytogenetic events and/or mutations included in the ELN 2022 guidelines) Figure 1B. Six percent (n = 33) of patients had no identifiable ELN risk–defining mutation or cytogenetic event; within this group, other recurrent mutations identified in AML (ie, DNMT3A, IDH1, etc) but not currently included in the ELN 2022 guidelines were present in 76% (n = 25 of 33) of patients.
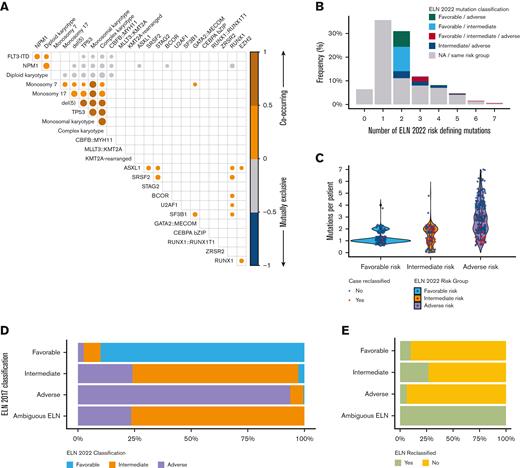
Distribution of ELN risk defining mutations within the Beat AML Cohort. (A) Correlation plot demonstrating relationship of ELN risk-defining mutations. NPM1 frequently associated with FLT3 ITD mutations, both of which were associated with diploid karyotypes. Conversely, adverse-risk cytogenetics and TP53 mutations tended to correlate with each other. Size of circles indicates the frequency (larger circles indicate increasing and smaller circles indicate decreasing frequency) of co-occurrence. Only correlations with P < .001 are displayed. (B) Percentage of patients with no identifiable, 1 identifiable, or multiple identifiable ELN risk–defining mutations. Colored bars indicate percentage of patients with multiple ELN risk–defining mutations spanning different (ie, favorable and adverse) risk groups. (C) Number of ELN risk–defining mutations identified per patient across ELN 2022 risk groups. Red circles indicate cases that were reclassified based on the updated ELN 2022 guidelines. (D-E) Bar plot demonstrating the percentage of patients reclassified according to ELN 2022 risk group (D) and overall (E). Patients listed as ambiguous ELN include 17 patients with co-occurring NPM1 and FLT3 ITD mutations in whom the FLT3 ITD allelic ratio was unknown.
Distribution of ELN risk defining mutations within the Beat AML Cohort. (A) Correlation plot demonstrating relationship of ELN risk-defining mutations. NPM1 frequently associated with FLT3 ITD mutations, both of which were associated with diploid karyotypes. Conversely, adverse-risk cytogenetics and TP53 mutations tended to correlate with each other. Size of circles indicates the frequency (larger circles indicate increasing and smaller circles indicate decreasing frequency) of co-occurrence. Only correlations with P < .001 are displayed. (B) Percentage of patients with no identifiable, 1 identifiable, or multiple identifiable ELN risk–defining mutations. Colored bars indicate percentage of patients with multiple ELN risk–defining mutations spanning different (ie, favorable and adverse) risk groups. (C) Number of ELN risk–defining mutations identified per patient across ELN 2022 risk groups. Red circles indicate cases that were reclassified based on the updated ELN 2022 guidelines. (D-E) Bar plot demonstrating the percentage of patients reclassified according to ELN 2022 risk group (D) and overall (E). Patients listed as ambiguous ELN include 17 patients with co-occurring NPM1 and FLT3 ITD mutations in whom the FLT3 ITD allelic ratio was unknown.
Newly included MR-gene mutations (SRSF2, SF3B1, U2AF1, ZRSR2, BCOR, EZH2, and STAG2) were identified in 33% (n = 171 of 513) of patients and were enriched in patients with a clinical diagnosis of secondary vs de novo AML (68% [n = 56 of 82] vs 27% [n = 115 of 431]; P value < .001). SRSF2 mutations were most common (13% [n = 65]), followed by STAG2 (8% [n = 43]), BCOR (6% [n = 30]), U2AF1 (6% [n = 30]), SF3B1 (4% [n = 22]), EZH2 (4% [n = 18]), and ZRSR2 (2% [n = 11]). These MR mutations frequently co-occurred with RUNX1 and/or ASXL1 mutations (46% [n = 79 of 171]) or other ELN 2022 adverse–risk abnormalities without ASXL1 or RUNX1 (18% [n = 31 of 171]). In patients classified as ELN 2022 favorable risk, it was 14% (n = 23 of 171).
In-frame mutations within the bZIP domain of CEBPA were identified in 4% (n = 18) of patients; 78% (n = 14 of 18) occurring within the context of biallelic mutations in CEBPA. Newly included translocations were infrequent (both <1%); KAT6A::CREBBP and MECOM-rearranged karyotypes were identified in 3 and 2 patients, respectively.
Consistent with prior analyses, certain ELN defining mutations and cytogenetic abnormalities were significantly correlated with one another (ie, NPM1 and FLT3-ITD; TP53 with adverse-risk cytogenetic events) Figure 1A. Patients with STAG2 mutations commonly had comutations in SRSF2 (44% [n = 19 of 43]), and patients with GATA2::MECOM-rearranged AML commonly had comutations in SF3B1 (40% [n = 4 of 10]).
In patients with ≥2 ELN risk–defining mutations (58% [n = 297]), 44% (n = 132; representing 26% of the entire cohort) had co-occurring mutations spanning ELN categories including 41% (n = 54 of 133) with favorable- and intermediate-risk mutations, 27% (n = 36 of 133) with favorable- and adverse-risk mutations, 22% (n = 29 of 133) with intermediate- and adverse-risk mutations, and 10% (n = 13 of 133) with mutations spanning all 3 risk categories Figure 1B. Most cases with mutations spanning multiple ELN categories were results of co-occurring mutations in NPM1, FLT3-ITD, or adverse-risk gene mutations supplemental Table 2.
Patients with ELN 2022 adverse–risk AML had more risk-defining mutations per patient (median 3; [range, 1-7]) compared with patients classified as favorable (median 1; [range, 1-4]) or intermediate risk (median 1; range, 0-5; P value < .001). However, the median number of risk-defining mutations in patients reclassified between the 2017 and 2022 guidelines was 2 (range, 1-3), highlighting the relative abundance of adverse-risk markers and a tendency for these to co-occur Figure 1C.
Reclassification of ELN categories
The ELN 2022 guidelines revised risk group classification in 15% (n = 75) of patients, resulting in reclassification of 10% of patients with ELN 2017 favorable (n = 16 of 160), 26% of patients with ELN 2017 intermediate (n = 29 of 112), and 6% (n = 13 of 224) of patients with ELN 2017 adverse risk Figure 1D-E. Risk classification was able to be accurately assigned to 17 patients with ambiguous (ie, favorable or intermediate and intermediate or adverse) ELN 2017 classification because of the removal of FLT3-ITD allelic ratio requirements from the updated guidelines. Most patients were reclassified into ELN 2022 intermediate– (51% [n = 38]) or adverse–risk groups (45% [n = 34]).
Reasons for changes in risk group included reclassification of patients with FLT3 ITD mutations (53% [n = 40]), presence of a MR mutation (33% [n = 25]), reclassification of CEBPA (n = 4), identification of a newly included KAT6A::CREBBP fusion (n = 1), TP53 variant allele frequency of <10% (n = 1), change in hyperdiploidy classification (n = 1), or multiple changes (n = 3; ie, FLT3-ITD reclassification and identification of a newly included MR mutation) supplemental Figure 1A.
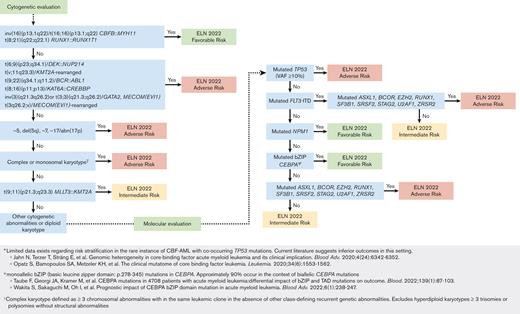
Given the high frequency of competing ELN risk–defining mutations spanning multiple risk categories identified per patient and the progressively complex nature of AML risk stratification, a workflow was created to simplify prognostic risk assignment based on the work of Döhner et al Figure 2.9
Proposed workflow using hierarchal classification of ELN risk–defining abnormalities. Notably, instances in which a favorable-risk and adverse-risk mutation co-occurred (ie, core binding factor AML with mutated TP53) without clear risk group assignment were infrequent.
Proposed workflow using hierarchal classification of ELN risk–defining abnormalities. Notably, instances in which a favorable-risk and adverse-risk mutation co-occurred (ie, core binding factor AML with mutated TP53) without clear risk group assignment were infrequent.
Response outcomes according to ELN 2022 criteria
Eighty-nine percent (n = 456) of patients received treatment (IC, n = 345; HMA-based, n = 90 [including 14 patients treated with HMA+venetoclax combinations]; targeted therapy, n = 21). Patients receiving IC were younger (median age, 57 years; [range, 18-79 years]) than those receiving HMA-based (median age, 72 years; [range, 43-87 years]) or targeted therapy (median age, 73 years; [range, 24-87 years]) (P value < .001). Thirty-six percent (n = 165) of patients underwent consolidative HCT (74% [n = 123] in CR/CRi) after a median of 4.1 months (range, 0-36 months).
CR/CRi rates across ELN 2022 favorable–, intermediate–, and adverse–risk groups were 79% (n = 108 of 136), 54% (n = 57 of 106), and 38% (n = 82 of 214), respectively (P value < .001). In patients treated with IC, corresponding CR/CRi rates across favorable-, intermediate-, and adverse risk–groups were 86% (n = 100 of 117); 59% (n = 54 of 92) and 49% (n = 67 of 136), respectively (P value < .001).
No significant difference in response was observed between ELN risk groups in patients treated with HMA-based therapies, which were uniformly suboptimal (favorable, 38% [n = 6 of 16]; intermediate, 30% [n = 3 of 10]; and adverse, 19% [n = 12 of 64]; P value = .20).
Survival outcomes according to ELN 2022 criteria
After a median follow-up of 36 months (95% confidence interval [CI], 33.6-44.2), median OS of all patients that had received treatment was 16.6 months (95% CI, 13.8-20.7). The median OS across ELN 2022 favorable–, intermediate–, and adverse–risk groups was not reached (estimated 5-year OS, 53% [standard error (SE), 0.06%]), 16.8 (95% CI, 11-48), and 9.7 (95% CI, 8.3-10.8) months, respectively. OS in all patients that had received treatment, and in those treated with IC based on ELN 2017 vs ELN 2022 criteria is displayed in supplemental Figure 2.
Overall, in comparison with the ELN 2017, the ELN 2022 stratification more accurately stratified survival in patients with intermediate- or adverse-risk AML. Using the ELN 2022 criteria, compared with patients with intermediate-risk AML, patients with favorable-risk AML (hazard ratio [HR], 0.44; 95% CI, 0.30-0.65; P value < .001) had significantly improved survival, whereas patients with adverse-risk AML had significantly inferior survival (HR, 1.62; 95% CI, 1.20-2.20; P value = .002) Figure 3A-B. Using ELN 2017 criteria, compared with patients with intermediate-risk AML, patients with favorable-risk AML had significantly improved survival (HR, 0.43; 95% CI, 0.30-0.63; P value < .001), whereas only a trend for inferior survival was observed in patients with adverse-risk AML (HR, 1.31; 95% CI, 0.96-1.79; P value = .09).
OS of patients based on 2017 and 2022 ELN classifications for all treatments and IC treatment subgroup. (A-B) OS based on ELN 2017 (A) and ELN 2022 (B) criteria in all treated patients. Although both criteria appropriately identified patients with favorable-risk AML, the 2022 criteria more effectively stratified survival between patients with intermediate and adverse-risk AML. (C-D) Similar findings were observed when limited to the population of patients treated with intensive chemotherapy, in which the ELN guidelines are most well validated.
OS of patients based on 2017 and 2022 ELN classifications for all treatments and IC treatment subgroup. (A-B) OS based on ELN 2017 (A) and ELN 2022 (B) criteria in all treated patients. Although both criteria appropriately identified patients with favorable-risk AML, the 2022 criteria more effectively stratified survival between patients with intermediate and adverse-risk AML. (C-D) Similar findings were observed when limited to the population of patients treated with intensive chemotherapy, in which the ELN guidelines are most well validated.
The ELN guidelines are predominantly validated in patients receiving IC. A sensitivity analysis performed in this population (n = 345) yielded similar results with respect to survival stratification. Patients with favorable-risk AML had improved survival (HR, 0.38; 95% CI, 0.24-0.59; P value < .001), whereas patients with adverse-risk AML had inferior survival (HR, 1.58; 95% CI, 1.12-2.25; P value = 0.01) compared with patients with intermediate-risk AML treated with IC Figure 3C-D.
No significant survival difference was observed in patients treated with HMA-based therapies based on ELN 2022 risk group, with a median OS of 8.8 (95% CI, 3; not estimated [NE]), 9.4 (95% CI, 2.0; NE), and 9.4 (95% CI, 4.9-11.7) months across favorable-, intermediate-, and adverse-risk groups, respectively.
Survival with HCT
Survival across ELN 2022 risk groups based on the receipt of HCT is displayed in supplemental Figure 3A. A landmark analysis performed at the median time to HCT in patients with intermediate- or adverse-risk AML undergoing HCT compared with patients in CR/CRi not undergoing HCT demonstrated a survival benefit from HCT during remission within these subgroups supplemental Figure 3C. No survival benefit was observed with HCT in patients classified as favorable risk using ELN 2022 criteria.
Comparison of ELN 2017 and ELN 2022 in patients having received intensive treatment, and in all patients that had received treatment
After multivariate modelling adjusting for age group (≥60 years vs <60 years), ELN 2022 risk, and the receipt of HCT, patients with favorable-risk AML continued to demonstrate improved survival (HR, 0.26; 95% CI, 0.16-0.41; P value < .001), and patients with adverse-risk AML demonstrated inferior survival (HR, 1.47; 95% CI, 1.03-2.11; P value = .035) compared with patients with intermediate-risk AML when treated with IC; no significant difference was observed based on age group (HR, 1.22; 95% CI, 0.89-1.67; P value = .21). When comparing the model using ELN 2022 vs 2017 risk classification in patients treated with IC, inclusion of the ELN 2022 criteria demonstrated a modest improvement in goodness of fit (concordance index, 0.68 [SE, 0.02] vs 0.66 [SE, 0.02]).
After adjusting to include all treatments, receipt of venetoclax, age group, and ELN 2022 risk, ELN favorable risk (HR, 0.45; 95% CI, 0.30-0.66; P value < .001) was associated with improved survival, whereas no significant survival difference was observed in patients with ELN adverse-risk compared with patients with intermediate-risk AML (HR, 1.27; 95% CI, 0.92-1.74; P value = .11). Age ≥60 years was associated with inferior survival compared with those aged <60 years (HR, 1.44; 95% CI, 1.07-1.93; P value = .017). Compared with HMA-based therapy, the receipt of IC (HR, 0.56; 95%CI, 0.41-0.79; P value < .001) was associated with improved survival, whereas no difference was observed in those receiving targeted therapy regimens (HR, 0.95; 95% CI, 0.53-1.69; P value = .86). Receipt of venetoclax-based therapy was associated with a nonsignificant trend toward improved OS, similar to that observed with IC (HR, 0.51; 95% CI, 0.24-1.05; P value = .069).
Survival outcomes according to ELN 2022 criteria in patients treated with HMA+venetoclax combinations
As a minority of included patients (n = 14) in the Beat AML cohort received HMA+venetoclax combinations, an additional institutional cohort of 41 patients treated with HMA+venetoclax were identified to evaluate outcomes using ELN criteria in this expanded patient population (N = 55).
Patients treated with HMA+venetoclax were comparable to the Beat AML HMA population with respect to age (HMA+venetoclax median age, 71 years [range, 19-87 years] vs HMA, 73 years [43-87 years]; P value = .6), and ELN risk group (P value = .13). HMA+venetoclax treatment improved CR/CRi rates (51% vs 17%; P value < .001), and median OS (12 vs 9 months; HR, 0.61; 95% CI, 0.39-0.96; P value = .031) after adjusting for ELN risk.
Similar to patients treated with HMA, ELN 2022 risk classification did not result in significant survival stratification between patients with favorable- (HR, 0.51; 95% CI, 0.14-1.92; P value = .32) or adverse-risk AML (HR, 0.91; 95% CI, 0.34-2.50; P value = .86) compared with patients with intermediate-risk AML treated with HMA+venetoclax. Notably, patients with adverse-risk AML demonstrated a median OS of 12 months and 32 months if attaining CR/CRi supplemental Figure 4A-D.
Survival outcomes with reclassified ELN 2022 risk groups
Supporting the updated risk stratification, survival was significantly improved in patients who had received treatment who were reclassified as ELN 2022 intermediate risk (n = 30) vs adverse risk (n = 35) (median OS, 16.8 months; 95% CI, 10.8, NE vs 9.5 months, 95% CI, 4.2-15.2; HR, 2.03; 95% CI, 1.08-3.8; P value = .027).
In patients with mutations spanning multiple risk groups, patients with co-occurring favorable- and adverse-risk mutations had similar survival (P value = .22), whereas patients with mutations spanning ELN favorable-intermediate (P value < .001), favorable-intermediate-adverse (P value = .007), and intermediate-adverse risk (P value < .001) had inferior survival compared to patients with ELN favorable risk AML (Figure 4).
OS of patients with co-occurring mutations spanning multiple risk groups compared with patients with mutation(s) in a single ELN risk group. (A-D) Patients with mutations in ELN favorable and adverse-risk categories (B) had survival similar to that of patients classified as ELN favorable-risk, whereas patients with other combinations of mutations, such as ELN favorable– and intermediate–risk (predominantly comprised of patients with co-occurring NPM1 and FLT3 ITD mutations) (A); favorable-, intermediate-, and adverse-risk (C); and intermediate- and adverse-risk (D) had inferior outcomes.
OS of patients with co-occurring mutations spanning multiple risk groups compared with patients with mutation(s) in a single ELN risk group. (A-D) Patients with mutations in ELN favorable and adverse-risk categories (B) had survival similar to that of patients classified as ELN favorable-risk, whereas patients with other combinations of mutations, such as ELN favorable– and intermediate–risk (predominantly comprised of patients with co-occurring NPM1 and FLT3 ITD mutations) (A); favorable-, intermediate-, and adverse-risk (C); and intermediate- and adverse-risk (D) had inferior outcomes.
Co-occurring NPM1 and FLT3 ITD mutations represented the largest molecular subgroup with mutations spanning multiple ELN risk groups (n = 52). Compared with other patients at intermediate risk, no significant difference in OS was observed in patients with co-occurring NPM1 and FLT3 ITD mutations (HR, 1.34; 95% CI, 0.79-2.27; P value = .27), supporting recent guideline changes, supplemental Figure 5. No survival difference was observed in patients with low vs high FLT3 ITD allelic ratio. A minority of patients with mutated FLT3 ITD received FLT3 inhibitors (41% [n = 41 of 100]), resulting in a nonsignificant improvement in median OS (23 months; 95% CI, 9-NE vs 14 months; 95% CI, 11-23, P value = .27). Of patients with FLT3 ITD–mutated AML, 53% proceeded to HCT, correlating with a median OS of 48 months (95% CI, 14.4, NE) (supplemental Figure 3D).
Within the ELN 2022 adverse-risk group, mutations in ZRSR2 (HR, 2.20; 95% CI, 1.08-4.49; P value = .031) or U2AF1 (HR, 1.77; 95% CI, 1.11-2.80; P value = .016) correlated with inferior survival, similar to patients with TP53-mutated AML (HR, 2.75; 95% CI, 1.90-3.99; P value < .001) (supplemental Figure 6A-D). Only 5 patients had KAT6A::CREBBP (n = 3) or MECOM-rearranged (n = 2) fusions; median OS for these 2 groups were 4.5 (95% CI, 0.8, NE) and 8.3 (95% CI, 8.3, NE) months, respectively. Given the small sample size of certain molecular and cytogenetic subgroups, these outcomes should be considered exploratory in nature.
Isolated MR mutations (ie, ASXL1, RUNX1, SRSF2, SF3B1, U2AF1, ZRSR2, BCOR, EZH2, and STAG2) were identified in 19% (n = 25 of 134) of patients with de novo AML and a co-occurring favorable-risk abnormality who had received treatment. Within this setting, the negative prognostic impact of MR mutations appeared abrogated (supplemental Figure 1B-C).
Discussion
AML is a heterogeneous disease characterized by numerous mutations requiring individualization of treatment based on clinical and molecular risk stratification. Within a large population of patients with AML, the updated ELN 2022 guidelines resulted in risk reclassification in 15% of the study population and better stratified survival in patients classified as intermediate and adverse risk treated with IC, in comparison with the ELN 2017 guidelines. This analysis highlights the progressively complex process of risk stratification as the number of included gene abnormalities and cytogenetic events increases, and the role of risk stratification in a varied treatment landscape.
The ELN 2022 guidelines incorporate only a fraction of the mutations identified in patients with AML.1,2 Strikingly, using only this limited panel of molecular drivers, over half of the included patients had >1 ELN risk–defining abnormality (58% [n = 297]). Of these patients, 44% (n = 132; representing 26% of entire study population) had mutations spanning multiple ELN risk groups (most commonly NPM1 and FLT3 ITD). Clinicians may frequently encounter conflicting risk–defining abnormalities within the same patient that may affect management. Given the importance of proper risk stratification, a proposed flow diagram simplifying risk assignment for clinicians and highlighting areas devoid of substantive literature has been provided.
Notable updates to the ELN 2022 guidelines include removal of FLT3 ITD allelic ratio and NPM1 comutation status with subsequent allocation of FLT3 ITD into the intermediate-risk group and inclusion of additional adverse-risk MR-gene mutations in conjunction with RUNX1 and ASXL1. These changes were responsible for the largest shifts in risk reclassification, accounting for 53% (n = 40) and 33% (n = 25) of patients, respectively. Mutations in MR genes often co-occurred with mutations in RUNX1 or ASXL1 (46%, n = 79 of 171) or other ELN 2022 adverse-risk mutations (18%; n = 31 of 171). Nonetheless, inclusion of these additional MR mutations resulted in 5% of the study population being allocated to the adverse-risk group.
Overall, the updated guidelines resulted in 96% of reclassified patients being allocated to the intermediate- or adverse-risk groups and better stratified survival in patients with intermediate- or adverse-risk AML treated with IC compared with the ELN 2017 guidelines. Patients with ELN favorable-risk AML experienced long-term survival irrespective of consolidative HCT. However, the median OS in patients with intermediate- or adverse-risk AML remained suboptimal (16.8 vs 9.7 months). Within the entire study cohort, older age also remained an independent predictor of inferior survival (HR, 1.44; 95% CI, 1.07-1.93; P value = .017).
Given the inferior long-term survival observed in patients with ELN intermediate- or adverse-risk AML, investigations of regimens combining cytotoxic chemotherapy with targeted therapies, such as venetoclax and the routine incorporation of consolidative HCT (which was notably used in only 36% of the present study population) for eligible patients should be prioritized given the demonstrable improvements in MRD eradication and survival observed with this approach, particularly within these risk groups.10,23-25
Decreased survival is also observed in older adults with AML compared with younger adults, including those with ELN favorable-risk mutations (ie, core-binding factor or NPM1-mutated AML) in which HCT in first remission is not currently standard.26,27 Older adults may also be more likely to receive noncurative, lower-intensity therapies. Within this population, the use of dynamic markers of disease assessment, such as MRD, to guide management and the consideration of curative HCT irrespective of ELN risk classification in select patients remains an attractive approach.7,12-14,28,29
Survival outcomes of reclassified patients support recent changes to the guidelines. Patients with in-frame bZIP mutations in CEBPA had survival comparable with other favorable-risk patient subgroups (ie, core binding factor AML or NPM1-mutated AML), whereas patients with FLT3 ITD mutations (of whom only 41% received a FLT3 inhibitor, however 53% proceeded with HCT) experienced survival comparable with other patients at intermediate-risk. Neither comutations in NPM1 nor the FLT3 ITD allelic ratio influenced outcomes, supporting reclassification of these patients as intermediate risk.
Inferior survival was observed in the few patients with KAT6A::CREBBP or MECOM rearrangements, and patients with mutations in ZRSR2 or U2AF1 (both previously associated with inferior survival in patients treated with IC).30,31 In the present analysis, survival in patients harboring these MR mutations was similar to patients with mutated TP53, identifying subgroups with particularly poor outcomes.
In patients with de novo AML, isolated MR mutations were associated with inferior survival. However, no detrimental survival impact was observed when occurring in patients classified as ELN favorable risk. It is plausible some MR mutations represent a background preleukemic clone in this latter setting, with the later acquisition of a favorable-risk mutation driving leukemogenesis.31
The ELN 2022 risk stratification guidelines represent an improvement compared with the 2017 guidelines and should be commended. A potential limitation that may prohibit broad applicability is the predominant inclusion of studies using IC regimens.30-33 Although the ELN criteria appropriately prognosticated survival in patients treated with IC, survival prognostication was less clear when including patients receiving alternative therapies. In the subset of included patients treated with HMA-based therapies without venetoclax in this study cohort, ELN 2022 risk classification did not differentiate outcomes, which were uniformly poor. Improved outcomes were observed in patients treated with HMA+venetoclax combinations despite comprising a minority of included patients; however, ELN criteria similarly did not result in significant survival prognostication in this patient population.
Azacitidine with venetoclax is now the standard of care in older patients unfit for IC therapy, and differing sensitivity to venetoclax has been observed across molecular subgroups and AML subtypes.19,20,34 Given the near ubiquitous use of azacitidine and venetoclax and the potential for differing outcomes in this setting, development of treatment-specific risk stratification schemas akin to the ELN 2022 guidelines incorporating patients receiving venetoclax-based regimens are eagerly awaited.35-39
Whether the current ELN criteria are readily applicable to patients receiving molecularly targeted therapies (ie, FLT3, IDH1, or IDH2 inhibitors) or oral azacitidine maintenance therapy (which recently demonstrated improved relapse-free and OS irrespective of MRD, NPM1, and FLT3 mutation status) also remains unknown.40,41 The relatively recent approval of these agents requires additional follow-up to understand how such therapies influence prognosis within certain molecular and cytogenetic subgroups.
Further adding to the complexity of risk stratification, recent revisions to both the international consensus classification and the World Health Organization guidelines for myeloid neoplasms now recognize numerous genetically defined AML subtypes with a myeloblast threshold of ≥10%.42,43 Variation exists between guidelines with respect to specifics of included mutations (ie, CEBPA) and disease-defining subgroups with a broader blast enumeration (the international consensus classification now classifies 10%-20% blasts as MDS/AML).42,43
Certain molecular subgroups (ie, TP53) indeed have similar outcomes irrespective of blast enumeration.44 These changes to disease classification may also alter ELN survival prognostication as it pertains to other ELN risk–defining molecular aberrations. Because the present study included patients using the prior classification schema, future investigations are needed to address risk stratification in patients with ≥10% myeloblasts categorized as MDS/AML or AML with a defining genetic abnormality.
In conclusion, the updated ELN 2022 guidelines improve survival stratification in patients with AML treated with IC. Risk stratification is becoming increasingly complex as our understanding of disease heterogeneity expands. Future guideline iterations will need to account for recent changes in AML classification, the increasing use of targeted therapies, and venetoclax-based combination therapies, which will continue to improve outcomes in patients with AML.
Authorship
Contribution: C.A.L., N.L., J.W.T., E.T., J.S., A.G., L.F.N., B.H.-L., R.T.M., J.L., D.B., S.M., J.D., R.P., G.M., R.S., R.J.C., and B.J.D. were responsible for data collection and analysis, drafting of the manuscript, and review of the final manuscript.
Conflict-of-interest disclosure: A.G. serves on the advisory boards for Gamida Cell, Talaris Therapeutics. J.W.T. has received research support from Acerta, Agios, Aptose, Array, AstraZeneca, Constellation, Genentech, Gilead, Incyte, Janssen, Kronos, Meryx, Petra, Schrodinger, Seattle Genetics, Syros, Takeda, and Tolero and serves on the advisory board for Recludix Pharma. B.J.D. reports serving on the advisory boards for Adela Bio, Aileron Therapeutics, Therapy Architects (ALLCRON), Cepheid, Celgene, DNA SEQ, Nemucore Medical Innovations, Novartis, RUNX1 Research Program, and Vivid Biosciences (inactive); the advisory board and stock interests in Aptose Biosciences, Blueprint Medicines, Enliven Therapeutics, Iterion Therapeutics, GRAIL, and Recludix Pharma; the board of directors and stock interests in Amgen and Vincerx Pharma; the board of directors of Burroughs Wellcome Fund and CureOne; the joint steering committee for Beat AML LLS; advisory committee for Multicancer Early Detection Consortium; being the founder of VB Therapeutics; having a sponsored research agreement with Enliven Therapeutics and Recludix Pharma; receiving clinical trial funding from Novartis and AstraZeneca and royalties from patent 6958335 (Novartis exclusive license), OHSU, and Dana-Farber Cancer Institute (1 Merck exclusive license, 1 CytoImage, Inc exclusive license, and 1 Sun Pharma Advanced Research Company nonexclusive license); and holding US patents 4326534, 6958335, 7416873, 7592142, 10473667, 10664967, and 11049247. R.S. serves as a consultant for COTA HealthCare. J.L. reports consulting for Takeda, Adaptive, Pfizer, Amgen, AbbVie, and KiTE; and receiving honoraria from Adaptive. E.T. has served on advisory boards for Astellas, AbbVie, Daiichi-Sankyo, and Servier and receives research funding from Incyte, Schrodinger, and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Curtis Lachowiez, Department of Hematology/Medical Oncology, Knight Cancer Institute, Oregon Health & Science University, Portland, OR 97239; e-mail: lachowie@ohsu.edu.
References
Author notes
∗C.A.L. and N.L. are joint first authors.
Original data are available on request from the corresponding author, Curtis A. Lachowiez (lachowie@ohsu.edu).
The full-text version of this article contains a data supplement.