Key Points
Atezo–R-CHOP appeared to improve CR rates compared with historical controls but not so much as to warrant further study.
The atezo–R-CHOP combination introduced immune-related AEs; however, these did not interfere with the delivery of R-CHOP.
Abstract
Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the current standard therapy for patients with diffuse large B-cell lymphoma (DLBCL) and is curative in ∼60% of patients. Atezolizumab is a humanized immunoglobulin G1 monoclonal antibody that targets programmed death–ligand 1 and has previously shown antitumor activity in several tumor types. In a phase 1b/2 trial (NCT02596971), we evaluated the safety and efficacy of atezolizumab in combination with R-CHOP (atezo–R-CHOP; for 6-8 cycles) in patients with previously untreated DLBCL. Patients achieving a complete response (CR) at the end of induction received consolidation therapy with atezolizumab on day 1 of each 21-day cycle for an additional 17 cycles. Overall, 42 patients with DLBCL were included in this analysis. The primary endpoint, CR rate at the end of induction, as assessed by an independent review committee (modified Lugano 2014 criteria), was 77.5% (95% confidence interval [CI], 64.0-87.7; n = 40). Investigator-assessed progression-free survival and overall survival at 3 years were 77.4% (95% CI, 59.7-88.0) and 87.2% (95% CI, 71.9-94.5), respectively. All treated patients experienced ≥1 adverse event (AE; 32 patients [76.2%] had grade 3-4 AE). One patient had a fatal AE (unconfirmed progressive multifocal leukoencephalopathy) that was considered related to atezolizumab and rituximab, and 17 patients (40.5%) experienced atezolizumab-related AEs of special interest. In previously untreated patients with DLBCL, atezo–R-CHOP demonstrated encouraging clinical efficacy and a safety profile consistent with the known toxicities of the individual drugs. This trial was registered at www.clinicaltrials.gov as #NCT02596971.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive non-Hodgkin lymphoma.1 Rituximab, an anti-CD20 monoclonal antibody, in combination with chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]) is the current standard front-line therapy for advanced-stage DLBCL, significantly improving survival compared with chemotherapy alone.2,3 Although standard R-CHOP therapy is curative in ∼60% of patients with DLBCL, the remainder of patients experience refractory or relapsed disease due to R-CHOP resistance, and poor outcomes are observed in these patients.4 An international study (SCHOLAR-1) reported the median overall survival (OS) to be 6.3 months for patients with DLBCL refractory to first-line treatment.5
R-CHOP remains the standard of care for patients with previously untreated DLBCL.3,6 The addition of the type 2 anti-CD20 antibody, obinutuzumab, to chemotherapy has not improved progression-free survival (PFS) compared with the standard rituximab-based chemoimmunotherapy.6,7 Various attempts have been made to improve outcomes in patients with DLBCL; in recent years, multiple novel targeted agents have been added to R-CHOP, including lenalidomide, bortezomib, and ibrutinib.4,8-10 However, until recently, none of these combination therapies had improved outcomes in randomized phase 2/3 trials compared with R-CHOP alone. Recent data from a phase 3 trial (NCT03274492) evaluating polatuzumab vedotin plus R-cyclophosphamide, doxorubicin, and prednisone demonstrated a prolongation of PFS compared with R-CHOP (hazard ratio, 0.73; 95% confidence interval [CI], 0.57-0.95; P = .02; 2-year PFS, 76.7% vs 70.2%), with no OS benefit.11,12
Atezolizumab, a humanized immunoglobulin G1 monoclonal antibody that targets programmed death–ligand 1 (PD-L1), prevents the interaction between PD-L1 and its receptor, programmed cell death protein 1 (PD-1). Atezolizumab was engineered to eliminate the Fc-effector function via a single amino acid substitution at position 298 on the heavy chain, resulting in a nonglycosylated antibody that has minimal binding to Fc receptors and consequently eliminates detectable Fc-effector function. By eliminating the Fc-effector function and antibody-dependent cell-mediated cytotoxicity, the antibody-mediated clearance of activated effector T cells is also eliminated.13 Atezolizumab has previously shown promising antitumor activity in several tumor types and hematologic malignancies.14-17
Furthermore, preclinical studies and phase 1 clinical trials have demonstrated that the combination of targeted therapies and PD-1 inhibition can lead to durable responses not achieved with either agent alone, thereby potentially enhancing therapeutic activity.18,19 A phase 1 trial (NCT02541565) with the anti–PD-1 antibody pembrolizumab in combination with R-CHOP demonstrated a 2-year PFS rate of 83% in patients with previously untreated DLBCL.20 We hypothesized that the addition of atezolizumab to R-CHOP (atezo–R-CHOP) may enhance antitumor immune activation, leading to robust and long-lasting antitumor responses, with the potential to improve patient outcomes.
To evaluate this hypothesis, we performed a phase 1b/2 clinical trial (NCT02596971) to assess the safety and efficacy of induction therapy with atezo–R-CHOP followed by consolidation with single-agent atezolizumab in patients with previously untreated DLBCL. The safety and efficacy of atezolizumab and obinutuzumab plus bendamustine in patients with follicular lymphoma were also evaluated in this trial; the results of this analysis will be published separately.
Methods
Trial design
This open-label, nonrandomized, phase 1b/2 trial in patients with previously untreated follicular lymphoma or DLBCL included an initial safety run-in phase with intensive safety monitoring before the main enrollment (expansion phase). Here, we report the data from patients with previously untreated DLBCL who were enrolled in the expansion phase.
The trial was conducted in accordance with the declaration of Helsinki and Good Clinical Practice. The trial protocol was approved by participating center ethics committees. All patients provided written informed consent.
Patients and treatment
Patients aged ≥18 years with previously untreated advanced DLBCL (defined as stage III or IV, with an International Prognostic Index (IPI) ≥ 2, or stage II with bulky disease) and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2 were eligible to participate. All details of the inclusion and exclusion criteria are outlined in supplemental Methods.
Induction treatment consisted of R-CHOP therapy during cycle 1, followed by atezo–R-CHOP from cycle 2 onwards: atezolizumab 1200 mg intravenously (IV) every 3 weeks; rituximab 375 mg/m2 IV for cycles 1 to 8; plus 6 or 8 cycles of standard-dose CHOP. Eligible patients achieving complete response (CR) at the end of induction (EOI) received consolidation therapy consisting of atezolizumab 1200 mg IV on day 1 of each 21-day cycle for an additional 17 cycles. Atezolizumab was added to rituximab starting with cycle 2 to mitigate the risk of increased infusion-related reactions (IRRs) during the first infusion of rituximab. The incidence and severity of IRRs related to rituximab decreased substantially with the second and subsequent infusions.
Study endpoints and assessments
The primary efficacy endpoint was a CR rate at EOI (by positron emission tomography–computed tomography [PET-CT]) determined by an independent review committee (IRC) using modified Lugano 2014 criteria, whereby the designation of a partial response (PR) per PET-CT required the response to meet the criteria for a PR or CR per CT scan, and if there was bone marrow involvement at baseline, a CR had to be confirmed by a negative bone marrow biopsy at EOI.21
Secondary efficacy endpoints included an investigator-assessed CR rate at EOI using the Lugano 2014 criteria, CR rate at EOI by the IRC and investigator (modified Cheson 2007 criteria),22 and an objective response rate (ORR) at EOI by the IRC and investigator (Lugano 2014 and modified Cheson 2007 criteria).
The exploratory efficacy endpoints included OS, PFS, and minimal residual disease (MRD) (centrally assessed using next-generation sequencing). MRD as an exploratory endpoint was centrally assessed by the next-generation sequencing of the B-cell receptor’s variable, diversity, and joining region (Adaptive Biotechnologies Corp, Seattle, WA), as previously described.23 In brief, clones were identified from a baseline tissue using the ClonoSEQ assay (version 2; Adaptive Technologies).24 The test for sequence accuracy assessed ∼442.5 million nucleotides for sequence agreement between the original calibrating clonotype sequence and the sequences identified in the MRD assessment. The overall observed sequence error rate was ∼3.5 parts per 100 000.
The relationship between the EOI response and PD-L1 or CD8 expression (the median cutoff was used to study the association between expression and response), measured using immunohistochemistry (IHC), was evaluated post hoc as an exploratory endpoint. IHC assays were performed on pretreated, formalin-fixed, paraffin-embedded tissue samples for PD-L1 using clones SP142 and SP263 and for CD8 using clone SP57. PD-L1 was scored based on the tissue area occupied by PD-L1+ cells using the following algorithm: IHC 0 ≤ 1%, IHC 1 = 1%–5%, IHC 2 = 5%–10%, and IHC 3 ≥10%. CD8 staining was scored based on the tumor area occupied by CD8+ cells (scored as the percentage of CD8+ cells in the tumor area).
Gene expression profiling was carried out using the NanoString nCounter analysis system (NanoString Technologies, Inc., Seattle, WA) for cell-of-origin analysis. The quality control of RNA samples, analysis of samples, and provision of raw data files were carried out by NanoString Technologies, Inc, who performed the data analysis and provided the final cell-of-origin results.
Safety and tolerability were assessed, including documentation of adverse events (AEs), serious AEs (SAEs), and AEs of special interest (AESIs; related to atezolizumab). AESIs related to atezolizumab were defined as pneumonitis, colitis, endocrinopathies, hepatitis, systemic lupus erythematosus, neurological disorders, hypersensitivity reactions, nephritis, ocular toxicities, myositis, myopathies, vasculitis, and grade ≥2 cardiac disorders.
Statistical analysis
The first patient was enrolled on 28 December 2015, and the final data cutoff date for the results presented here was 8 May 2020.
Up to 40 patients with DLBCL were planned to be enrolled and receive atezo–R-CHOP treatment. Assuming an observed PET-CT–defined CR rate of 72% was achieved, the sample size was deemed to be sufficient for providing adequate precision for the CR rate and for the lower limit of the 90% CI to rule out a clinically uninteresting CR rate of <59%, a value selected based on the CR rate for R-CHOP reported in the GOYA study.6
All PET-evaluable patients who received at least 1 dose of atezolizumab were included in the efficacy population. All patients who received any study drug were analyzed for safety. The proportion of patients achieving CR at EOI and the two-sided 90% Clopper–Pearson exact CI were calculated. Patients without a postbaseline tumor assessment were considered nonresponders. Kaplan-Meier analysis was used to assess PFS and OS, with a Cox proportional hazards model used to calculate hazard ratios and 95% CIs.
Results
Patients
A total of 42 patients with previously untreated DLBCL were enrolled and received treatment with R-CHOP. Two patients were withdrawn from the study before starting atezolizumab treatment (withdrawn consent, n = 1; physician decision, n = 1). As shown in Table 1, 52% of the patients were aged ≥65 years and 62% were male. Most patients had advanced-stage disease at diagnosis (Ann Arbor stage III/IV: 95%), 69% were in the high-intermediate/high (3-5) IPI risk group, and 74% had extranodal involvement.
Seven patients discontinued the study treatment before EOI (AE, n = 4; progressive disease [PD], n = 1; other, n = 2); 4 additional patients (9.5%) discontinued at EOI (PD, n = 2; PR, n = 2) (supplemental Figure 1). Of the 31 patients with a CR-initiated consolidation treatment, 16 discontinued treatment during the consolidation phase (AE, n = 8; PD, n = 3; physician decision, n = 1; withdrawal by subject, n = 4).
Clinical activity
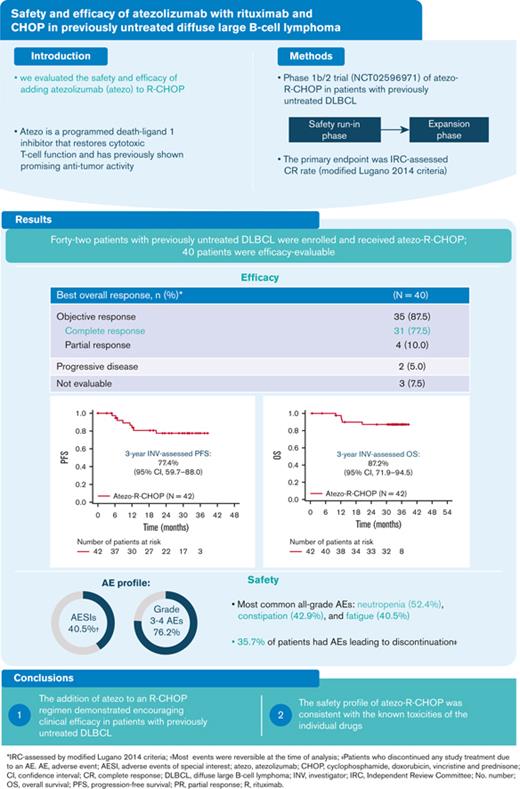
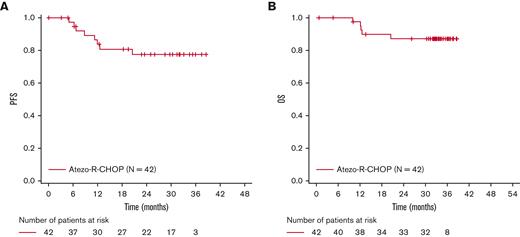
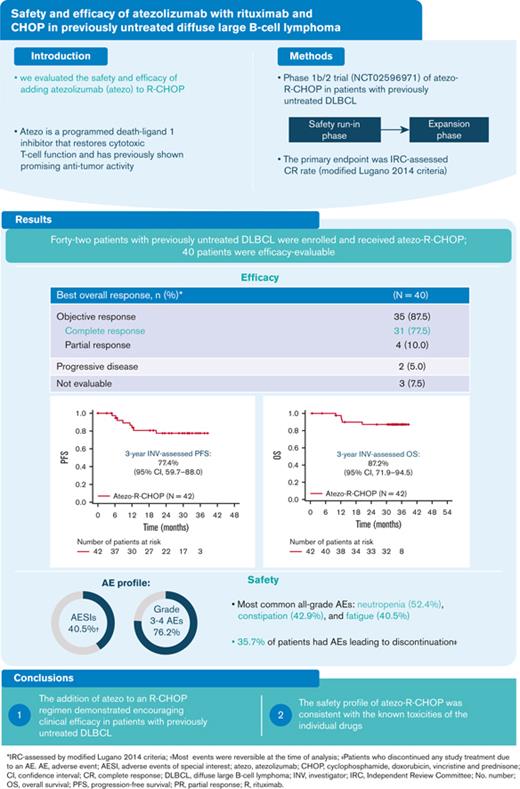
At the time of data cutoff (8 May 2020), the median observation time in the study population was 32.3 months (range, 0.7-38.6 months), and the median length of time on treatment was 11.1 months (range, 0.0-20.4 months). The median time from the initial diagnosis to treatment was 0.74 months (range, 0.1-2.4 months). Patients who received at least 1 dose of atezolizumab were evaluated for efficacy at EOI (N = 40). The clinical response rates for EOI are outlined in Table 2. The IRC-assessed CR rate (primary endpoint) by PET-CT at EOI (modified Lugano 2014 criteria) was 77.5% (95% CI, 64.0-87.7), and 10.0% of the patients had a PR (Table 2). The IRC-assessed CR rates at EOI, as assessed using the Lugano 2014 and modified Cheson 2007 criteria (secondary endpoint), were 80.0% and 77.5%, respectively. The investigator-assessed CR rates were 77.5% (Lugano 2014 criteria) and 75.0% (modified Cheson 2007 criteria). The corresponding ORR at EOI ranged from 87.5% to 90.0% (Table 2). At 3 years, investigator-assessed PFS and OS rates were 77.4% (95% CI, 59.7-88.0) and 87.2% (95% CI, 71.9-94.5), respectively (Figure 1).
Exploratory analyses
Overall, 14 of 40 patients were MRD evaluable at EOI (ie, tested positive at baseline and had an available sample at EOI); MRD negativity at baseline was the most common reason for patients not being MRD evaluable at EOI (supplemental Figure 2). Of the patients who were MRD evaluable, 13 were MRD negative at 10−5 sensitivity at EOI, of whom 11 (84.6%) were in CR and 1 (7.7%) was in PR for both the investigator and IRC assessments; 1 sample was not available at the time of analysis (Table 3). These response rates were similar to those of the total efficacy-evaluable population. One patient was MRD positive at EOI and achieved a PR; this patient did not experience PD or death at the time of data cutoff. Two patients who were MRD negative at EOI later relapsed.
PD-L1 expression levels were measured in 26 patients with available tissue samples. High PD-L1 expression (IHC 2-3), measured using the monoclonal antibody SP263, was not associated with a significantly improved CR rate at EOI in this study. There was a trend toward greater response rates in the germinal center B-cell-like patient subtype at EOI (supplemental Figure 3).
Safety
In the safety population (N = 42), all patients experienced at least 1 AE, and 32 patients (76.2%) experienced at least 1 grade 3-4 AE. The most common grade 3/4 AEs were neutropenia (45.2%) and febrile neutropenia (11.9%) during the induction phase (Table 4). The most common all-grade AEs were neutropenia (52.4%), constipation (42.9%), and fatigue (40.5%; Table 5). Fifteen patients (35.7%) discontinued treatment due to AEs, including 6 patients (14.3%) who discontinued treatment during the induction phase (Table 4). The most frequently (>5%) reported AEs that led to discontinuation of any study treatment were neutropenia and increased lipase (7.1% for each).
Atezolizumab-related AESIs were observed in 17 patients (40.5%), and the majority of events occurred during consolidation treatment (28.6%). The most frequent AESIs related to atezolizumab were increased lipase (9.5%), increased amylase (7.1%), hypothyroidism (7.1%), and pancreatitis (4.8%); of the 17 AESIs reported, most events (65%), including lipase and amylase increase, were reversible at the time of analysis (Table 6).
The most common SAE was febrile neutropenia (14.3%). The most frequent treatment-related SAEs were febrile neutropenia (7.1%), pneumonia (7.1%), and IRRs (4.8%). A total of 5 deaths (11.9%) were reported. Four deaths (9.5%) were recorded because of PD, and 1 grade 5 event (2.4%) was recorded as death due to an AE (unconfirmed progressive multifocal leukoencephalopathy considered to be related to atezolizumab and rituximab; this patient was in PR when the AE occurred). This grade 5 event was reported during the follow-up phase and required prolonged inpatient hospitalization.
Overall, 23 patients (54.8%) experienced AEs that led to dose interruptions of any study treatment. By system organ class, the most frequently (≥20%) reported category was blood and lymphatic system disorders (23.8%). The most frequently (≥10%) reported AEs by preferred term that led to dose interruption were neutropenia (16.7%) and peripheral sensory neuropathy (11.9%). The mean dose intensities for atezolizumab and rituximab were 98.3% (range, 60%-100%) and 99.4% (range, 88%-104%), respectively, and the number of dose reductions was not evaluable. In addition, there was no effect on the dose intensity of R-CHOP because of AEs related to atezolizumab. There were no delays in R-CHOP treatment due to atezolizumab immune-related AEs.
Discussion
In patients with previously untreated DLBCL, atezo–R-CHOP combination therapy resulted in a high PET-CR rate of 77.5% and ORR of 87.5% at EOI. The overall safety profile of the atezo–R-CHOP combination was manageable. However, the combination of atezo–R-CHOP seemed to introduce AEs not typically associated with R-CHOP, but which were consistent with the known safety profile of atezolizumab, including elevated amylase and lipase levels, pancreatitis, and hypothyroidism.
In recent years, several randomized clinical trials have investigated the potential of adding targeted agents to R-CHOP; however, at the time of this analysis, these novel agent combinations had failed to demonstrate an improved OS over R-CHOP alone.8-10 Results from a phase 2 trial with the anti–PD-1 inhibitor, durvalumab, in combination with R-CHOP, demonstrated no greater benefit compared with R-CHOP alone in patients with previously untreated, high-risk DLBCL.25 In this study, atezo–R-CHOP achieved encouraging efficacy. The 3-year PFS and OS rates were 77.4% (95% CI, 59.7-88.0) and 87.2% (95% CI, 71.9-94.5), respectively. The PET-CR at EOI and PFS rates at 18 months were 77.5% and 80.6% (95% CI, 63.5-90.3), respectively. In the R-CHOP arm of GOYA, 58% of patients were in the low/low-intermediate IPI risk groups (IPI scores 1-2), and 43% of patients were in the high-intermediate/high IPI risk groups (IPI scores 3-5), whereas herein 26% of patients were in the low-intermediate risk group and 69% were in the high-intermediate/high IPI risk groups. In addition, 74% of the patients in this trial had extranodal disease, whereas 66% of the patients in the R-CHOP arm of GOYA presented with extranodal involvement. Although the patients in our study had higher-risk baseline characteristics than those in the randomized phase 3 GOYA study (NCT02541565), the PET-CR at EOI (59.1%) and the PFS rate (72.0%) at 18 months in the R-CHOP arm of GOYA26 were not superior to the 18-month results reported herein with atezo–R-CHOP. Certainly, other confounding factors can skew cross-trial comparisons, and these should be taken into consideration. Moreover, immunosuppression due to concurrent chemotherapy might have inadvertently suppressed atezolizumab-mediated activation of the immune system, resulting in a lack of long-term additive benefits of the atezo–R-CHOP combination.
Seventeen patients had no clones identifiable for MRD assessment or were MRD negative at baseline. In addition, only 14 of the 40 efficacy-evaluable patients were MRD evaluable at EOI. Patients who were MRD negative at EOI achieved a high CR rate (84.6%); of these, 2 patients had PD. The low rate of MRD evaluable samples suggests that the ClonoSEQ assay may have limited widespread use as a monitoring test for MRD detection in patients with DLBCL. However, the ClonoSEQ assay may not accurately reflect the potential of MRD testing in DLBCL, and other assays may have prognostic or predictive values. Two patients with MRD negativity at EOI relapsed, indicating that MRD negativity did not accurately predict cure.
Studies in patients with non–small cell lung cancer, metastatic triple-negative breast cancer, and melanoma have shown a correlation between improved survival with atezolizumab therapy and high PD-L1 and CD-8 IHC expression levels on tumor cells, suggesting that biomarker expression is predictive of clinical benefit.14,27,28 In contrast, the prognostic ability of PD-L1 could not be confirmed in this study. This might have been because of the small number of available biomarker-evaluable samples, and a larger sample size would be required to confirm the prognostic ability of biomarkers and establish a strong association between PD-L1/biomarker expression and response. Alternatively, this may also be because of intrinsic differences in innate immune tumor tolerance between solid and hematologic cancer models.
The overall safety profile of atezo–R-CHOP seemed manageable, and most AEs were consistent with the known safety profile of atezolizumab. Overall, 15 patients (35.7%) discontinued treatment because of an AE, including 6 patients (14.3%) who discontinued treatment during the induction phase. Fewer than half of the patients experienced an SAE (42.9%), of which the most frequently reported was febrile neutropenia (14.3%). AESIs related to atezolizumab were reported in 17 patients, most commonly, increased lipase (n = 4), increased amylase (n = 3), hypothyroidism (n = 3), and pancreatitis (n = 2). The events of lipase increase were mostly reversible and not serious. Immune-related AEs, including pneumonitis, hepatitis, colitis, endocrinopathies, and infections, are documented side effects of checkpoint inhibitors.29,30 However, given the known tolerability profile of R-CHOP alone, the increased toxicity observed with atezolizumab should be considered in the context of overall clinical benefit.
To our knowledge, this is the first report to demonstrate the long-term clinical activity and safety of the atezo–R-CHOP combination in patients with DLBCL. It is limited by its single-arm study design, preventing direct comparisons with standard DLBCL treatment. A comparative study would be needed to ascertain whether the atezo–R-CHOP combination provides significant benefits vs R-CHOP alone, but the available data suggest that the efficacy differences between the 2 treatments are likely to be modest, and the additional AEs introduced by atezolizumab should be taken into consideration. Therefore, this combination will not be investigated further. Regardless, these analyses provide useful insights into the efficacy and safety of atezolizumab in the treatment of DLBCL and have the potential to guide further clinical development.
In conclusion, this study in patients with previously untreated DLBCL demonstrated that the atezo–R-CHOP combination provided durable clinical activity, and the safety profile was consistent with the known toxicities of the individual drugs.
Acknowledgments
The authors thank the patients and their families, study investigators, study coordinators, and nurses involved.
This study was sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance under the direction of A.Y. was provided by Aisling Lynch and Louise Profit of Ashfield MedComms, an Inizio Company, and was funded by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: A.Y. and J.P.S. contributed to study design; G.S., J.B., B.D.C., E.H., C.D., I.L., U.H., J.P.S., M.T., U.V., and C.K. conducted the study; J.B., B.D.C., E.H., C.D., I.L., S.Y., U.H., G.M., J.P.S., M.T., U.V., and C.K. contributed to recruitment and follow-up of patients; G.S., M.S., E.H., C.D., U.H., A.R., J.P.S., M.T., S.F., and C.K. collected the data; G.S., T.N., M.S., J.B., I.L., A.R., and J.P.S. analyzed the data; G.S., T.N., M.S., J.B., B.D.C., E.H., C.D., I.L., A.R., J.P.S., M.T., U.V., and S.F. interpreted the data.
Conflict-of-interest disclosure: A.Y. is employed by and has stock and other ownership interests in AstraZeneca; has received honoraria from Merck, F. Hoffmann-La Roche Ltd, Takeda, Janssen, AbbVie, Curis, and Epizyme; has a consulting or advisory role with BioPath Holdings Inc, Xynomic Pharma, Epizyme, F. Hoffmann-La Roche Ltd, Celgene, and HCM; has received research funding from Janssen, Curis, F. Hoffmann-La Roche Ltd, Genentech, Inc, Merck, Bristol Myers Squibb, Syndax; and other relationship with AstraZeneca. J.M.B. reports an advisory board role for Gilead, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, Bayer, AstraZeneca, AbbVie, Verastem, MorphoSys, Adaptive Biotechnologies, Epizyme, Kura, and Seattle Genetics, and has served on speaker's bureau for Seattle Genetics and Beigene. B.D.C. reports other fees from F. Hoffmann-La Roche Ltd, Genentech, Inc, and personal fees from Celgene, AbbVie, AstraZeneca, TG Therapeutics, Epizyme, Beigene, Karyopharm, MorphoSys, Symbios, Merck, Kite, GlaxoSmithKline, Janssen/Pharmacyclics, and Lilly, outside the submitted work. C.S.D. reports an advisory board/consultancy role for Celgene, F. Hoffmann-La Roche Ltd, Genentech, Inc, Bristol Myers Squibb, Merck, Seattle Genetics, Incyte, MorphoSys, and MEI Pharma. U.H.H. reports grants from F. Hoffmann-La Roche Ltd. E.A.H. reports research funding from F. Hoffmann-La Roche Ltd, Bristol Myers Squibb/Celgene, Merck KGaA, AstraZeneca; has served on advisory boards for F. Hoffmann-La Roche Ltd, Bristol Myers Squibb, Gilead, Novartis, AstraZeneca, Merck Sharp & Dohme, Janssen, Antigene, Specialised Therapeutics, and Beigene; and has served on the speaker's bureau for F. Hoffmann-La Roche Ltd, AstraZeneca, and Janssen. C.K. has served on speaker's bureau for Genentech, Inc, AstraZeneca, AbbVie, Beigene, Incyte, Kite, ADC Therapeutics, Karyopharm, and Seattle Genetics. I.S.L. has served on advisory boards for Seattle Genetics, Janssen Scientific, and Verastem, Inc. G.M. reports advisory board/consultancy fees from F. Hoffmann-La Roche Ltd, Incyte, and Janssen. U.V. has served on an advisory board for Janssen, Gilead, Celgene, Juno Therapeutics, Incyte, and Genmab, and received personal fees from F. Hoffmann-La Roche Ltd, Janssen, Gilead, and AbbVie. S.Y. has received travel assistance from Seattle Genetics. A.R. reports previous employment with Genentech, Inc and F. Hoffmann-La Roche Ltd. M.S. is employed by F. Hoffmann-La Roche Ltd. T.N. is employed by and holds equity in F. Hoffmann-La Roche Ltd. G.S. is employed by and owns nonvoting shares in F. Hoffmann-La Roche Ltd. J.P.S. reports a consultancy role with AbbVie, AstraZeneca, Beigene, Bristol Myers Squibb, Pfizer, Genentech, Inc. The remaining authors declare no competing financial interests.
The current affiliation for B.D.C. is Lymphoma Research Foundation, New York, NY.
The current affiliation for M.S. is Clinical Biomarkers, Translational Science Department, Arcus Biosciences, Hayward, CA.
Correspondence: Anas Younes, Department of Medicine, Memorial Sloan Kettering Cancer Center, 40 E 78 Street, New York, NY 10075; e-mail: anas_younes@mac.com.
References
Author notes
Qualified researchers may request access to individual patient-level data through the clinical study data request platform available at https://vivli.org/ourmember/roche/.
Further details on Roche's Global Policy on the Sharing of Clinical Information are available at https://go.roche.com/data_sharing.
Anonymized records for individual patients across more than one data source external to Roche cannot and should not be linked because of the potential increase of patient re-identification.
The full-text version of this article contains a data supplement.