Key Points
Patients with mantle cell lymphoma have higher relative risks of respiratory, blood, and infectious disease compared with healthy comparators.
Late effects varied very little by treatment with or without transplantation.
Abstract
Studies on late effects in patients with mantle cell lymphoma (MCL) are becoming increasingly important as survival is improving, and novel targeted drugs are being introduced. However, knowledge about late effects is limited. The aim of this population-based study was to describe the magnitude and panorama of late effects among patients treated with or without high-dose chemotherapy with autologous stem cell transplantation (HD-ASCT). The study cohort included all patients with MCL, recorded in the Swedish Lymphoma Register, aged 18 to 69 years, diagnosed between 2000 and 2014 (N = 620; treated with HD-ASCT, n = 247) and 1:10 matched healthy comparators. Patients and comparators were followed up via the National Patient Register and Cause of Death Register, from 12 months after diagnosis or matching to December 2017. Incidence rate ratios of the numbers of outpatient visits, hospitalizations, and bed days were estimated using negative binomial regression models. In relation to the matched comparators, the rate of specialist and hospital visits was significantly higher among patients with MCL. Patients with MCL had especially high relative risks of infectious, respiratory, and blood disorders. Within this observation period, no difference in the rate of these complications, including secondary neoplasms, was observed between patients treated with and without HD-ASCT. Most of the patients died from their lymphoma and not from another cause or treatment complication. Taken together, our results imply that most of the posttreatment health care needs are related to the lymphoma disease itself, thus, indicating the need for more efficient treatment options.
Introduction
Mantle cell lymphoma (MCL) is a rare but aggressive form of lymphoma affecting older individuals in particular. Since 2001, the Nordic MCL2 protocol (rituximab with dose-intensified cyclophosphamide, vincristine, doxorubicin, prednisone [R-CHOP] and high-dose cytarabine followed by high-dose chemotherapy and autologous stem cell transplantation [HD-ASCT]) or other regimens, including high-dose cytarabine, are standard options for younger patients.1-8
In recent trials, novel targeted drugs (such as covalent and noncovalent Bruton tyrosine kinase inhibitors and B-cell lymphoma-2 inhibitors)9 are being introduced even in first line, challenging the current use of chemoimmunotherapy with consolidating HD-ASCT.10,11 The benefit of HD-ASCT in MCL is currently under debate.12 Patients with MCL treated with HD-ASCT have shown better progression-free5,6,13-16 and overall survival17,18 compared with non-HD-ASCT-treated patients but mainly before the era of rituximab maintenance and the role of HD-ASCT in the era of novel targeted drugs is unclear.
The increase in treatment options and improved survival during later years1 calls for updated knowledge about the magnitude and the panorama of late effects in patients with MCL (caused by the cancer itself or by the treatment). Most studies investigating long-term complications and specific causes of death in patients with MCL are based on controlled clinical trials with highly selected patient cohorts. Population-based studies addressing these issues are sparse19,20 and patients treated with HD-ASCT are not always included. Moreover, long-term complications in patients with MCL are rarely compared with the situation among comparators and disentangled from common comorbidities an older population is often facing.
The primary aim of this study was to describe the late effects of MCL in a population-based setting in relation to age- and sex-matched comparators from the general population and to specifically address late events in patients treated with HD-ASCT vs those without. Studies of late effects by different MCL therapies, including the Nordic MCL2 protocol, are of interest as new treatments are introduced and the role of HD-ASCT is questioned21 and may provide a basis for novel treatment strategies and improvements in supportive care and follow-up.
Materials and methods
Study population
Patients with MCL aged 18 to 69 years and diagnosed between 2000 and 2014 were identified from the Swedish Lymphoma Register. This register has a coverage of ∼95% compared with the Swedish Cancer Register22 and includes clinical information such as Ann Arbor stage, primary treatment, and the MCL-specific international prognostic index.23 For each patient, 10 population comparators were selected (matched on birth year, sex, and being alive and lymphoma free at the diagnosis date of the patient) from the Register of the Total Population.24 The full cohort (patients and comparators) was further linked to the Swedish Patient Register (nationwide coverage of hospitalizations since 1987 and specialist outpatient coverage since 2001) and the Swedish Cancer Register for classification of comorbidities according to the Charlson Comorbidity Index (CCI).25 Using the Longitudinal Integrated Database for Health Insurance and Labour Market Studies, information on the highest achieved educational level was additionally linked. The Swedish Cause of Death Register26 was used to retrieve dates and causes of death.
Stratification of patients based on HD-ASCT
Consolidation with HD-ASCT was identified primarily in the Swedish Lymphoma Register and additionally in the Swedish Patient Register27 (using International Classification of Diseases [ICD] codes, as outlined earlier)28 to assure that all transplantations were captured.29 We used a landmark approach to categorize patients as either HD-ASCT or non-HD-ASCT based on information at 12 months after diagnosis. Patients who had not yet undergone an HD-ASCT at 12 months were regarded as non-HD-ASCT throughout follow-up. Among the patients treated with HD-ASCT, 47% had their transplantation within 6 months of diagnosis and 83% within 12 months, leading us to select a cutoff of 12 months (assuming treatment completion for most patients). Among patients with an HD-ASCT after the landmark, all inpatient visits associated with the transplantation were disregarded. In sensitivity analyses, landmarks at 9, 18, and 24 months after diagnosis were also evaluated because of the occurrence of some late transplantations.
Outcomes
Both short- and long-term complications, defined as health care use or death, were investigated. Fifteen mutually exclusive disease groups were defined based on ICD chapters (supplemental Table 1). Among patients who underwent HD-ASCT, short-term complications were defined as hospitalizations or deaths due to any cause (interpreted as transplant-related mortality) within 60 days of transplantation. Long-term complications were investigated among all patients and comparators and were defined as the first specialist outpatient visit, hospitalization, or death within any of the 15 disease groups that occurred 12 months or later after diagnosis. In addition, to illustrate the total health care burden, all specialist outpatient visits, hospitalizations, and bed days (ie, not only the first), starting from 1 year after diagnosis, were quantified.
Statistical methods
In the landmark analysis, patients were followed up from 12 months after diagnosis (or matching date) until death or 31 December 2017, whichever occurred first. Patients and comparators who died before the start of follow-up did not contribute to these analyses. Incidence rate ratios (IRRs) with 95% confidence intervals (CIs) for the number of outpatient visits, hospitalizations, and bed days during follow-up were estimated using negative binomial regression models, both for the entire follow-up and in intervals (1-5 years, 5-10 years, and >10 years after diagnosis). Hazard ratios with 95% CIs of specific disease groups were estimated using the Cox regression models assuming proportional hazards (the assumption was formally evaluated using Schoenfeld residuals30).
To account for imbalance between the treatment groups, all models were adjusted for the matching variables (age and sex), year of diagnosis, CCI (0, 1, and 2+), and education level (≤9 years, 10-12, and >12 years of schooling). Age at diagnosis and calendar year of diagnosis were modeled as restricted cubic splines.
The cumulative incidence of lymphoma deaths was estimated in the presence of competing causes of death (other malignancy, cardiovascular disease, or remaining causes), stratified by treatment group (HD-ASCT and non-HD-ASCT). The unadjusted nonparametric estimates of the cumulative incidence were complemented by estimates of standardized cumulative incidence from a flexible parametric survival model adjusted for age, sex, calendar year, and CCI. We applied the standsurv package in Stata (Stata Statistical Software version 16.0, College Station, TX) to predict the cumulative incidence function under the assumption that the distribution of adjustment factors was the same for the 2 treatment groups.31
All analyses were based on complete cases and conducted using Stata.
Ethics
The study was approved by the Regional Board of the Ethical Committee in Stockholm, Sweden (2007/1335-31/4, 2010/1624-32).
Results
Demographics
The study cohort comprised 620 patients with MCL and 6200 matched comparators from the general population. The median age at diagnosis or matching was 62 years (range, 22-69) and the median follow-up was 5.3 years (range, 1-17.7). Forty percent of all patients (n = 247) were treated with HD-ASCT within 12 months of diagnosis. These patients were generally younger, had a higher educational level, and a lower comorbidity burden as compared with patients treated without HD-ASCT (Table 1).
For the patients treated with HD-ASCT, the induction regimen was generally R-maxi-CHOP alternating with R-cytarabine given according to the Nordic MCL 2 protocol (Table 2). Consolidative high-dose chemotherapy with BEAM (BCNU, etoposide, cytarabine, and melphalan) or BEAC (BCNU, etoposide, cytarabine, and cyclophosphamide) was used before transplantation. The patients treated without HD-ASCT were mostly treated with R-CHOP/cytarabine, R-CHOP, or R-bendamustine, whereas some patients were treated with chlorambucil alone (mainly before 2005 and none after 2010). A limited number of patients (n = 14 in the patient cohort) received rituximab maintenance.
Short-term complications (within 60 days) in patients treated with HD-ASCT
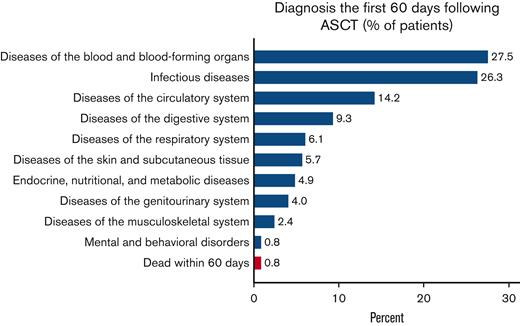
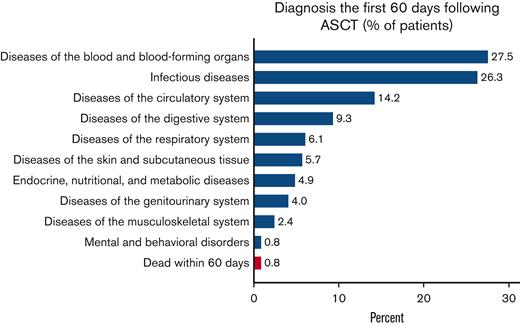
Recorded diagnoses (besides MCL) during these 60 days were mainly blood disorders, infectious diseases, and diseases of the circulatory or digestive system. Respiratory, skin, endocrine, and genitourinary problems were also frequent, whereas musculoskeletal and mental complications were rare (Figure 1). Patients spent a median of 22 days in hospital following the HD-ASCT (supplemental Figure 1). Two (0.8%) patients treated with HD-ASCT died within 60 days after their transplantation (Figure 1).
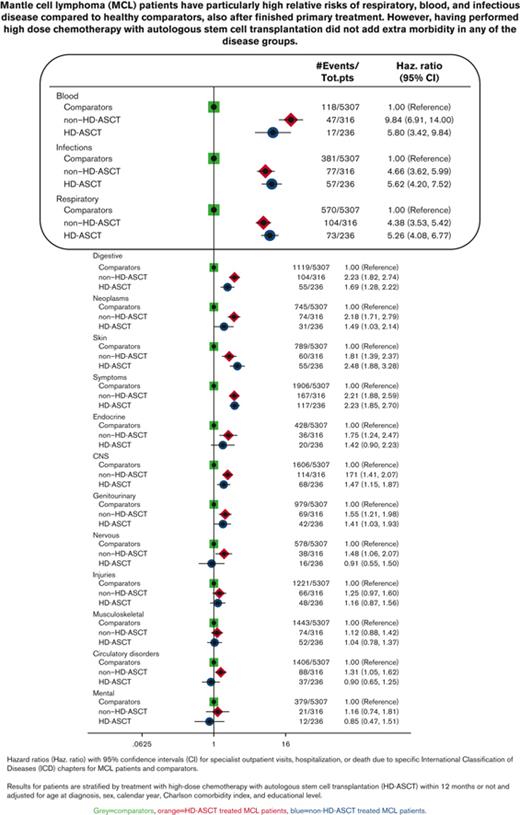
Proportion of patients treated with HD-ASCT with an ICD-specific diagnosis at hospitalization or death within 60 days. Proportion of patients with MCL who were treated with HD-ASCT (within 12 months of diagnosis, n = 247) with an ICD chapter–specific diagnosis at hospitalization (blue) or death due to any cause (red), within the first 60 days of transplantation.
Proportion of patients treated with HD-ASCT with an ICD-specific diagnosis at hospitalization or death within 60 days. Proportion of patients with MCL who were treated with HD-ASCT (within 12 months of diagnosis, n = 247) with an ICD chapter–specific diagnosis at hospitalization (blue) or death due to any cause (red), within the first 60 days of transplantation.
Long-term follow-up (ie, after 12 months)
Patients with MCL had a twofold increased incidence rate of outpatient visits during follow-up compared with the general population comparators (IRR, 2.0; 95% CI, 1.8-2.2) (Table 3) and a sevenfold to eightfold increased rate of inpatient visits and number of bed days (hospital visits, IRR, 7.2; 95% CI, 6.3-8.3 and bed days, IRR, 8.3; 95% CI, 6.8-10.1). Patients treated with HD-ASCT had a slightly higher rate of outpatient visits during the first 5 years after diagnosis and lower rates of inpatient visits beyond 5 years after diagnosis compared with patients treated without HD-ASCT. The rate of bed days was similar in both groups (Table 3).
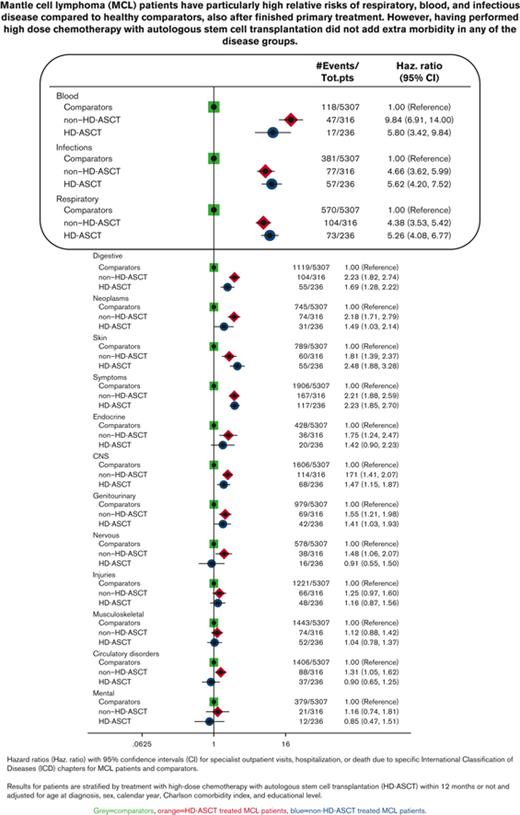
In relation to the matched comparators, patients with MCL had the most pronounced relative risks (Figure 2) and health care burden (supplemental Table 2) for diseases of the blood and blood-forming organs, infectious diseases, and diseases of the respiratory system. Similar rates were seen irrespective of treatment with or without HD-ASCT. The same pattern was observed in the sensitivity analysis with follow-up starting 9, 18, or 24 months after diagnosis. Among respiratory disorders, upper respiratory infections, influenza, and pneumonia were dominant (supplemental Figure 2A). Among infectious diseases, bacterial infections were most common, but patients treated with HD-ASCT were diagnosed with slightly more viral infections than those treated without HD-ASCT (supplemental Figure 2B). Diseases of blood and blood-forming organs were dominated by anemia, idiopathic thrombocyte platelet deficiency, immunodeficiency, and other diseases of blood-forming organs (supplemental Figure 2C). Frequencies of neoplasms other than MCL are presented in supplemental Figure 2D, the most frequent being melanoma and neoplasms of the skin, prostate cancer, and malignant neoplasm of the urinary tract. Patients treated with HD-ASCT were not diagnosed with more secondary malignancies than those treated without HD-ASCT (Figure 2; supplemental Figure 2D; Table 2). To illustrate when the different complications occurred during follow-up, the mean number of outpatient visits, hospitalizations, and bed days for different disease chapters and time windows are shown in supplemental Figure 3. Patients treated with HD-ASCT did not have more outpatient visits or hospitalizations for neoplasm in the time span after 10 years of follow-up (supplemental Figure 3).
Comparisons of rates of different ICD-chapters between MCL patients (by HD-ASCT treatment) and comparators. Hazard ratios with 95% CIs of first ICD chapter–specific diagnosis (specialist outpatient visits or hospitalization) or death among patients with MCL (by HD-ASCT [blue circles] or non-HD-ASCT [red dimonds]) and comparators (green squares). All models were adjusted for age at diagnosis, sex, calendar year, CCI, and educational level. CNS, central nervous system.
Comparisons of rates of different ICD-chapters between MCL patients (by HD-ASCT treatment) and comparators. Hazard ratios with 95% CIs of first ICD chapter–specific diagnosis (specialist outpatient visits or hospitalization) or death among patients with MCL (by HD-ASCT [blue circles] or non-HD-ASCT [red dimonds]) and comparators (green squares). All models were adjusted for age at diagnosis, sex, calendar year, CCI, and educational level. CNS, central nervous system.
Causes of death
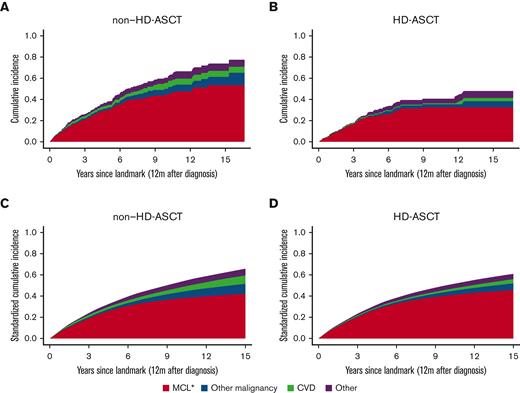
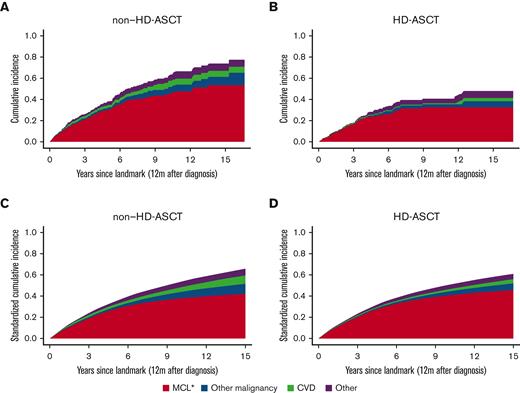
Deaths (≥12 months) due to causes other than MCL were rare in both patients treated with and without HD-ASCT (Figure 3). The 5-year cumulative probability of MCL-specific death in patients treated with HD-ASCT was 23% (95% CI, 18%-30%) and 32% (95% CI, 26%-38%) in patients treated without HD-ASCT. When eliminating potential differences in age, sex, CCI, and education level between the 2 treatment intensity groups (standardized analysis), there was no evidence of a difference in cumulative probabilities of MCL-specific death between the groups. As a reference, causes of death for the matched comparators can be found in supplemental Figure 4.
Crude and standardized cumulative probabilities of death due to different causes by HD-ASCT. Cumulative probabilities of death portioned into MCL, other malignancies, cardiovascular disease (CVD), and other causes, within 12 months from diagnosis among patients with MCL, by without HD-ASCT (A,C) and with HD-ASCT (B,D). Crude estimates (A,B) and standardized estimates (C,D) over age, sex, CCI, and education level are shown. ∗Include ICD-10: C83-C91.
Crude and standardized cumulative probabilities of death due to different causes by HD-ASCT. Cumulative probabilities of death portioned into MCL, other malignancies, cardiovascular disease (CVD), and other causes, within 12 months from diagnosis among patients with MCL, by without HD-ASCT (A,C) and with HD-ASCT (B,D). Crude estimates (A,B) and standardized estimates (C,D) over age, sex, CCI, and education level are shown. ∗Include ICD-10: C83-C91.
Discussion
In this population-based study, we found that patients with MCL overall, as expected, had a higher rate of outpatient visits, hospitalizations, and bed days than general population comparators of the same age and sex. Also, most patients with MCL died from their lymphoma and not from a comorbidity or a treatment complication. Importantly, intensive first-line treatment with the Nordic MCL2 protocol including HD-ASCT was not associated with higher rates of the measured late effects compared with less-intensive treatment (non–HD-ASCT). In a previous study on the same cohort, selection for treatment with HD-ASCT was also associated with a better overall survival.29
Patients with MCL had particularly higher rates of blood disorders, infections, and diseases of the respiratory system than the comparators. Similar complications were reported in a previous study but in older patients with lymphoma who received transplantation.32 The category of blood disorders reflected visits that could be seen as associated with the underlying lymphoma, such as anemia, idiopathic thrombocyte platelet deficiency, eosinophilia, and immunosuppression. No codes for transformed lymphoma or myelodysplastic syndromes were seen. Infectious complications, known to be associated with both the lymphoma and given treatment,20 including viral as well as bacterial infections, were observed at higher rates after the end of first-line treatment (≥12 months of diagnosis) among patients compared with comparators. This could be regarded as a late effect of primary treatment or the lymphoma disease itself but not due to rituximab maintenance because very few patients in this cohort were treated with rituximab maintenance (n = 14). Regarding secondary neoplasms, a wide range of different neoplasms were seen, as indicated in the supplement, with a higher frequency among patients than in comparators. No difference in the rate of secondary neoplasms was observed between the patients treated with and without HD-ASCT. This indicates that the underlying disease and first-line chemotherapy could be a driver of secondary neoplasms, rather than consolidating HD-ASCT, at least in the absence of total body irradiation as induction (as in the Nordic protocol). However, as the observation period is still relatively short, we cannot rule out that a higher incidence of secondary neoplasms may appear with prolonged follow-up.
Observational comparisons of late effects among patients administered with different treatments can be challenging, as the groups are rarely comparable with regard to baseline characteristics. Patients selected for HD-ASCT treatment in this study were younger and had less comorbidity compared with patients in the non-HD-ASCT group, for which we tried to accommodate in the adjusted models. The long-term health care burden that we observed mainly reflected disorders related to the underlying MCL and potential relapses. In addition, MCL was the main underlying cause of death in the cohort. This is in line with data from a randomized study comparing health-related quality of life after different treatments, concluding that the underlying MCL caused most of the later complications.33 If this interpretation holds in the novel targeted treatment era remains to be seen. In a pooled analysis of 4 randomized controlled studies of the Bruton tyrosine kinase inhibitor ibrutinib vs chemotherapy in chronic lymphocytic leukemia and patients with MCL, a favorable benefit-risk profile of ibrutinib treatment was shown compared with that of other standard treatments, despite prolonged ibrutinib therapy.34
A strength of this study is the generalizability of the population-based setting compared with selected cohorts in randomized trials, the long follow-up, and the detailed outcome analysis. The advantage of comparing late effects in patients vs comparators is the ability to disentangle excess health care use due to the disorder and its treatment from normal morbidity of an older population. Although the register-based setting allows for the study of an unselected group of patients, information on patients’ own reported adverse events was not captured, and we were not able to capture milder comorbidities or outcomes, typically treated in nonspecialized outpatient care,26 as this is not included in the National Patient Register. In addition, misclassification of exposure due to transplantations occurring after the landmark of 12 months could have biased the results, but in a sensitivity analysis using other landmarks (9, 18, and 24 months), the overall results were in line with those from the 12-month landmark approach, leading us to interpret this bias as minor.
In most clinical situations, the aim of MCL treatment is not to cure, but rather to prolong the patient’s life and maximize quality of life.19 Studies comprehensively evaluating both MCL-related symptoms and late adverse effects are thus crucial in the decision making of the care of patients with MCL. Although several review articles have discussed which patients with MCL should undergo transplantation, these have mostly focused on survival and patient eligibility for transplantation (based on comorbidity burden and tumor biological characteristics11,35,36) and not on adverse effects and future quality of life. As our results show, long-term monitoring of these patients is needed and there is room for potential preventive measures. Patients with MCL in Sweden are not routinely considered for infectious prophylaxis during follow-up, which could be considered. In diffuse large B-cell lymphoma survivors, a high rate of infections has also been seen and preventive measures such as infectious prophylaxis have been discussed.37
Conclusions
We have shown that patients with MCL, irrespective of treatment intensity with or without HD-ASCT, have higher hospitalization rates and particularly higher rates of respiratory disease, blood disorders, and infectious diseases, compared with matched comparators. Avoiding efficient MCL treatment because it is more demanding and possibly cause late effects may seem reasonable in the short term, but our results indicate that most of the long-term health care needs in patients aged up to 70 years are related to the lymphoma per se. This calls for continued efforts to improve treatment efficacy in MCL.
Acknowledgment
This work was supported by the Swedish Cancer Society CAN 19 0123 Pj 01 H.
Authorship
Contribution: I.G., S.E., and C.E.W. designed the study and interpreted the study results; S.E. provided statistical analysis and figures; I.G. and S.E. drafted the manuscript; I.G., K.E.S., and M.J. performed the collection of data and verification of underlying data; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: M.J. received honoraria from Janssen, Gilead, Celgene, Roche, and Acerta and research support from Janssen, Roche, Celgene, AbbVie, and Gilead. K.E.S. received honoraria from Celgene and research support from Janssen. I.G. received honoraria from Janssen. All authors participate in a public-private real-world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals NV.
Correspondence: Ingrid Glimelius, Uppsala University Hospital, Oncology Clinic, Entrance 78, 751 85 Uppsala, Sweden; e-mail: ingrid.glimelius@igp.uu.se.
References
Author notes
The data underlying this study are available at the National Board of Health and Welfare, Sweden, and Statistics Sweden for investigators with the appropriate approvals, but restrictions apply. However, data can be made available from the authors upon reasonable request for meta-analyses and with the appropriate approvals of the Swedish Ethical Review Authority (https://etikprovningsmyndigheten.se).
The full-text version of this article contains a data supplement.
C.E.W. and I.G. contributed equally to this study.

![Comparisons of rates of different ICD-chapters between MCL patients (by HD-ASCT treatment) and comparators. Hazard ratios with 95% CIs of first ICD chapter–specific diagnosis (specialist outpatient visits or hospitalization) or death among patients with MCL (by HD-ASCT [blue circles] or non-HD-ASCT [red dimonds]) and comparators (green squares). All models were adjusted for age at diagnosis, sex, calendar year, CCI, and educational level. CNS, central nervous system.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/5/10.1182_bloodadvances.2022007241/6/m_blooda_adv-2022-007241-gr2.jpeg?Expires=1767963422&Signature=zF9SsIiy9K7edJuMsw53JjSglczhlG-qINvn88iEpJp7px45tL8bTvaF7fKtmkqi3l7a8HBGjfiOJ3ZdP2sidG59or~Sgq~89gpWk2mjCb5LZYIpkAEu9u7b6ai3xlMvx9PbyrfSB0L~E9Mh-eQrI0XFoG9hW7MuQMXXfLU7GCeit565ckcEcU~lLQFmw5DTYk7WwJgWTt97U634rfYYwxBCSG7-aM6NGdLxTAJz0lfygjabfwqLQWu2h8roM9Jii43d2~1AEhsxYq2Nj5NVol7bEFPfJyU~01A7jdRiRL9OZ56oldiopBAuqa7y4GyS6IBfAqXIK22Hsax~UXwD-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Comparisons of rates of different ICD-chapters between MCL patients (by HD-ASCT treatment) and comparators. Hazard ratios with 95% CIs of first ICD chapter–specific diagnosis (specialist outpatient visits or hospitalization) or death among patients with MCL (by HD-ASCT [blue circles] or non-HD-ASCT [red dimonds]) and comparators (green squares). All models were adjusted for age at diagnosis, sex, calendar year, CCI, and educational level. CNS, central nervous system.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/5/10.1182_bloodadvances.2022007241/6/m_blooda_adv-2022-007241-gr2.jpeg?Expires=1767963423&Signature=a0Gpb4Toc9bpQl5H8rEK~JRQv6x9MuH~9KcxsCcGPqssbZ9gSEjoV3roXa57ODJ2h7bvYWGxLOmsjOqTd5ulPZa4cB1Kqb7X0bM3E2gh9cNO~0EUK1j0z3pGtmqQv4JqoSTWyurXIfl1WoX2vVofqxv1KmYokwMmWT8Cgd2l1v5BvMWHj6Big7tPwQsI4qLMmhT0Ly4osElhWhc74kF0DlrdrdXFP0NjbOBjGVYVMCFwfaSXTbXHFTpb9av0LOcVbbhpDOemBWgsbpItMGK8OHNPH-DjsI~Ytyiz66fLk7y01pv~q1TD7mF9SBS-yTDoBD3FkVx4X7LsA-DgyXaVVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)