Key Points
Hydroxyurea adherence increased by 19.8% with a tailored mHealth intervention in people with SCD and adherence <80%.
mHealth boosts hydroxyurea adherence and is associated with reduction in self-reported pain and pain admissions rate in SCD.
Abstract
Hydroxyurea reduces sickle cell disease (SCD) complications, but medication adherence is low. We tested 2 mobile health (mHealth) interventions targeting determinants of low adherence among patients (InCharge Health) and low prescribing among providers (HU Toolbox) in a multi-center, non-randomized trial of individuals with SCD ages 15-45. We compared the percentage of days covered (PDC), labs, healthcare utilization, and self-reported pain over 24 weeks of intervention and 12 weeks post-study with a 24-week preintervention interval. We enrolled 293 patients (51% male; median age 27.5 years, 86.8% HbSS/HbSβ0-thalassemia). The mean change in PDC among 235 evaluable subjects increased (39.7% to 56.0%; P < 0.001) and sustained (39.7% to 51.4%, P < 0.001). Mean HbF increased (10.95% to 12.78%; P = 0.03). Self-reported pain frequency reduced (3.54 to 3.35 events/year; P = 0.041). InCharge Health was used ≥1 day by 199 of 235 participants (84.7% implementation; median usage: 17% study days; IQR: 4.8-45.8%). For individuals with ≥1 baseline admission for pain, admissions per 24 weeks declined from baseline through 24 weeks (1.97 to 1.48 events/patient, P = 0.0045) and weeks 25-36 (1.25 events/patient, P = 0.0015). PDC increased with app use (P < 0.001), with the greatest effect in those with private insurance (P = 0.0078), older subjects (P = 0.033), and those with lower pain interference (P = 0.0012). Of the 89 providers (49 hematologists, 36 advanced care providers, 4 unreported), only 11.2% used HU Toolbox ≥1/month on average. This use did not affect change in PDC. Tailoring mHealth solutions to address barriers to hydroxyurea adherence can potentially improve adherence and provide clinical benefits. A definitive randomized study is warranted. This trial was registered at www.clinicaltrials.gov as #NCT04080167.
Introduction
Hydroxyurea (hydroxycarbamide) is one of only 4 agents approved by the US Food and Drug Administration to treat individuals with sickle cell disease (SCD).1 Hydroxyurea can reduce the frequency of acute events,2 preserve organ function,3 improve quality of life (QoL),4 increase survival,5,6 and reduce health care expenditures.7,8 Adherence to hydroxyurea prescriptions by children and adults living with SCD is low,9-11 commonly <80%,10,12,13 corresponding to less clinical benefit.
Interventions to improve hydroxyurea adherence have failed to provide robust and consistent effects, either because they were not tailored to the barriers of poor adherence or because study designs did not reflect real-world contexts.14 Adherence can be affected by both patient behavior and prescribing practices. Therefore, we conducted a literature synthesis,15-19 population-level claims analysis,10 patient and provider surveys,20,21 semistructured interviews, and focus groups analysis22,23 to identify contributors to poor patient adherence and poor provider prescribing behavior. Cognitive and behavioral factors relevant to improving patient adherence were identified from the empirical and theoretical literature, including psychosocial frameworks, such as the Health Belief Model24 and Social Cognitive Theory.25 Theory-informed factors associated with hydroxyurea use included high perceived disease severity, low motivation to take medications, memory deficit (leading to deficient habituation), low understanding of hydroxyurea benefit (ie, low medication knowledge), and low self-efficacy with taking hydroxyurea. Poor provider prescribing habits were associated with low hydroxyurea prescribing self-efficacy and low hydroxyurea knowledge.
Based on these findings, we designed the following two-level mobile health (mHealth) interventions: the InCharge Health app,22 which targeted the determinants of low hydroxyurea adherence among patients, and the HU Toolbox, which targeted low hydroxyurea prescribing among providers.26 We tested the effectiveness of the combined intervention at improving hydroxyurea adherence in the multicenter SCD Implementation Consortium (SCDIC) study “Integration of mHealth into SCD Care to Increase Hydroxyurea Utilization (meSH).”26 We tested the hypothesis that among patients with SCD aged between 15 and 45 years, a two-level mHealth intervention would promote a 12% mean absolute increase (20% relative increase) in percent days covered (PDC), which is a proxy for medication adherence.27,28
Methods
Study setting and participant selection
The SCDIC was funded by the National Heart Lung and Blood Institute (NHLBI) to develop and evaluate interventions and implementation strategies to bolster patient use and provider adoption of evidence-based therapies for SCD.29 Seven SCDIC sites (4 in the Southeast, 2 in the Midwest, and 1 in the Northeast of the United States) participated. Patients were treated at 1 to 3 academic and community clinics connected to each site, with ∼2200 children and adults with SCD receiving care at these 7 sites.
Physicians, nurse practitioners, and physician assistants who treated at least 1 patient with SCD for an anticipated minimum of 12 months after study enrollment were eligible.26 Patient participants (all nested within clinics where provider participants worked) had to have a documented SCD diagnosis, aged between 15 and 45, treated at a participating SCDIC site, English-speaking, and owned a mobile phone. They also needed to have at least 1 written hydroxyurea prescription (regardless of whether it is filled) in the 3 months before enrollment. Hydroxyurea initiation at baseline was allowed but excluded subjects from primary outcome analysis. All sites obtained study institutional review board approval, and all participants provided consent or assent before study participation.
Study design and intervention
meSH was a non-nonrandomized, closed cohort hybrid-effectiveness trial (ClinicalTrials.gov NCT04080167).28 The 7 sites were split into 3 groups which started the study at 6-month intervals. To allow time for training on study procedures, providers started using HU Toolbox 3 months before their patients were enrolled and used it for 9 months, whereas patients used InCharge Health app for 6 months. Study enrollment occurred from September 2019 to September 2021.
The InCharge Health intervention is a smartphone health app that directly targets determinants of low hydroxyurea adherence. It offers (1) daily customizable reminders; (2) daily recording of hydroxyurea adherence (click “yes” or “no” to taking hydroxyurea) and pain intensity scores; (3) pain severity vs adherence tracking; (4) communication with health care providers (via the patient portal) and other individuals with SCD; (5) educational resources about SCD and hydroxyurea; and (6) a patient-appointed accountability partner who receives daily notifications if the user has not documented the use of hydroxyurea for >4 hours.22 The HU Toolbox is a decision-support tool that contains NHLBI guidelines adapted for pediatric and adult providers, an artificial intelligence algorithm guiding clinicians on hydroxyurea prescribing and side effects monitoring through a chatbot, and a built-in feature to consult SCD experts.26 All sites’ research and clinical staff were trained to download and use InCharge Health and HU Toolbox. Participants were instructed on how to use their designated apps only during enrollment. After enrollment, study visits occurred at weeks 12 and 24 for patients and week 36 for providers. Providers were aware of the study interventions that their patients received.
Outcomes
The primary outcome was a change in PDC. We compared PDC over a 24-week baseline interval that ended the day before enrollment with PDC over 24 weeks of intervention. PDC is a process measure of adherence used by the Centers for Medicare & Medicaid Services reflective of real-world settings.28,30 Baseline PDC and change in PDC were calculated only for subjects with at least 1 prescription filled before starting the baseline period. In some, but not all cases, the prebaseline prescription covered the start of the baseline period. Study coordinators collected refill data from the pharmacies where patient participants reported having filled their prescriptions or from the electronic health record.
Secondary patient outcomes included the change in mean acute care use (emergency department, ED, and hospitalization encounters) and laboratory markers of hydroxyurea effect (fetal hemoglobin [HbF], hemoglobin [Hb], mean corpuscular volume [MCV], reticulocyte percent count, absolute neutrophil count [ANC], and platelets) between the baseline period and the 24 weeks of the intervention. Validated patient-reported outcome (PRO) measures were investigated as predictors of adherence change, including the Adult Sickle Cell QoL Measurement Information System social functioning, pain impact, pain frequency, pain severity,31-33 the Patient-Reported Outcomes Measurement Information System pain quality,34 self-efficacy,35 and Neuro-QoL measures of cognitive and attention problems.36 Pain PROs were compared between baseline and week 24. Secondary provider outcomes included a change in hydroxyurea prescribing knowledge (5-question survey about appropriate dosing and toxicity management) and self-efficacy in prescribing hydroxyurea (4-question survey confidence in prescribing) between baseline and week 36.
The RE-AIM framework evaluated the robustness of the intervention.37 RE-AIM domains include (1) reach: proportion and representativeness of the eligible target patient population enrolled, (2) effectiveness: adherence improvement among patients and hydroxyurea prescribing knowledge and self-efficacy among providers, (3) adoption: proportion of clinics and providers approached that enrolled, (4) implementation: level of app engagement by patients and providers, (5) maintenance: continuation of effectiveness and app engagement beyond the study period (ie, through week 36).
Power calculation
With minimal available data regarding the variance of baseline PDC within sites, site-to-site variation in mean baseline PDC, and treatment response, simulations were based on conservative assumptions regarding variability in baseline PDC and change in PDC. We determined the sample size required to detect a mean absolute increase of 12% in PDC (20% relative increase) after the intervention (∼1 additional day of treatment weekly).26 Initial simulations indicated that 276 subjects would provide 90% power to reject the null hypothesis of no change in PDC when the average increase in PDC was 12%. The sample size was inflated to 368 subjects to account for an expected 25% loss during the study. Actual variance components during the study were lower than initially assumed, resulting in >90% power to detect an absolute increase of 12% in PDC with 235 evaluable subjects.
Analysis plan
The primary outcome, mean change in PDC, was evaluated using a 2-sided 1-sample t-test. A sensitivity analysis was conducted to account for missing follow-up data in which missing follow-up PDCs were conservatively set at zero.
Predictors of change in PDC included baseline PDC, site or clinic, frequency of InCharge Health app use, demographics, and baseline PROs (supplemental Table 1). The frequency of InCharge Health app use was defined as the percentage of 168 days on which the app was accessed at least once. App use was treated as continuous in 1 analysis and as a 5-level categorical variable in another, with cut points producing following 5 groups of similar size: ≥50.1%, ≥21.5% but <50.1%, ≥7.2% but <21.5%, ≥0.6% but <7.2%, and <0.6%. Predictors were evaluated using linear models. Because we expected a negative correlation between baseline PDC and change in PDC, baseline PDC was included in all models. First, predictors were evaluated individually. Least square means (referred to as adjusted mean changes) were calculated to adjust for variation in the distribution of baseline PDC among levels of predictors, with the predictor treated as categorical and baseline PDC as continuous. Next, predictors that were statistically significant at the first step, plus those that were not, were considered in 2 multivariable models. Clinic was nested within the site, creating linear dependence, so 1 model included the clinic and the other site. Backward elimination reduced the models to the subset of statistically significant predictors (P < .05), retaining baseline PDC and site or clinic at all steps to reduce heterogeneity. No adjustment for multiple comparisons was made, because results should be considered exploratory.
The proportions with PDC >80% at baseline and follow-up were compared using McNemar test. One-sample t-tests were used to examine changes in laboratory measures. Changes in counts of ED visits and hospitalizations were assessed using Poisson regression with generalized estimating equations to account for the repeated observations on the same subjects. Associations between changes in PDC and changes in number of ED visits and hospitalizations were investigated using linear models. We used SAS software (copyright 2012-20, SAS Institute Inc) for all analyses.
Results
Participant characteristics and study participation
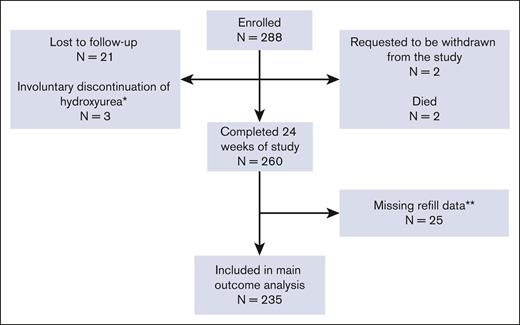
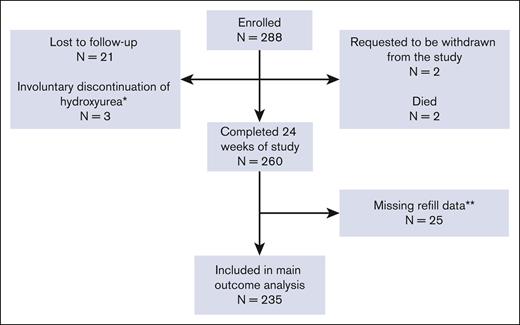
We enrolled 293 individuals with SCD. Five individuals started hydroxyurea at baseline and did not contribute to the main outcome analysis. Of the remaining 288, 21 were lost to follow-up, 3 involuntarily discontinued hydroxyurea (2 pregnancies and 1 loss of health coverage precluding financial access to medication), 2 withdrew, and 2 died (1 violent death and 1 central line–associated sepsis and multiorgan failure). The remaining 260 completed the 24 weeks of the study, yielding a study retention of 90.3%. Of those completing the 24 weeks, 11 had incomplete baseline prescription refill data (no prescription before the enrollment date), 14 changed the dispensing pharmacy during the study, and the new pharmacy information was unavailable, preventing the calculation of the 24-week PDC value. The remaining 235 had baseline and 24-week PDC data and were included in the primary analysis (Figure 1). Most patient participants were non-Hispanic African Americans with HbSS or HbSβ0-thalassemia (Table 1). A balance of males and females participated. Most had at least a high school degree and household incomes <$25 000 per year. Evaluable patient participants had higher baseline PDC than unevaluable ones, and they tended to be older, female, and had slightly higher income (Table 2; supplemental Table 2).
CONSORT diagram of patient participants. ∗Involuntary discontinuation of hydroxyurea; reasons included pregnancy or lack of drug access. ∗∗Participants were missing baseline refill data (11 patients) or 24-week refill data (14 patients) due to the inability to locate a dispensing pharmacy.
CONSORT diagram of patient participants. ∗Involuntary discontinuation of hydroxyurea; reasons included pregnancy or lack of drug access. ∗∗Participants were missing baseline refill data (11 patients) or 24-week refill data (14 patients) due to the inability to locate a dispensing pharmacy.
Of the 89 providers, 49 (57.7%) were physicians (most hematologists or with substantial SCD experience), and the remaining were nurse practitioners (n = 27), physician assistants (n = 9), primarily working in hematology clinics, or preferred not to report their position (n = 4; Table 1). Most providers were female non-Hispanic White, or Asian, aged between 30 and 60 years. Among the 89 enrolled providers, 6 relocated to other institutions and were removed, and 11 had incomplete baseline activities (ie, did not download the HU Toolbox or complete the study surveys) or withdrew participation. Therefore, provider retention was 81%.
mHealth app RE-AIM outcomes
The study acceptance rate was 85%, and approximately half of all eligible patients were enrolled (Table 2). Approximately 85% of patients used the app >1 day during the study period (Table 2). Overall, the patients reported that the app was easy to navigate and understand. Among evaluable participants, use-days of the InCharge Health app ranged from 1 to 156 of 168 days (0.6%-92.9%; median, 17.0%; interquartile range, 4.8%-45.8%) and was greater among patients aged between 25 and 45 vs those between 15 and 24 years (44.02±44.7 vs 27.63 study days, P = .0032). All 7 sites actively participated in the study, and 85.6% of providers agreed to participate. Implementation of HU Toolbox was low; 58 of 89 providers (65.2%) downloaded and used the HU Toolbox app at least once, but only 10 (11.2%) used the app at least 6 days over 168 days (maximum, 18 days). Most use occurred in the first 6 months, with 117 (54.4%) of 215 days on which the HU Toolbox app occurred in the first month, and only 4 (1.9%) occurred in month 6. Patients continued app use at a slower pace (50.2% >1 day) after the study (supplemental Table 3). Providers had low poststudy use (11.2% >1 day). More detailed results of the RE-AIM evaluation are presented in Table 2.
Change in hydroxyurea adherence
Mean PDC increased from 39.7% (±28.0% standard deviation [SD]) at baseline to 56.0% (±30.1% SD) during the 24-week follow-up interval (P < .0001), a relative increase of 29.1% and an absolute increase of 16.3% (median absolute increase, 17.8%). The null hypothesis was rejected, and the study end point was met.
PDC over the 24-week follow-up period could not be calculated for 14 participants because of missing 24-week prescription data. Adding these participants to the 235 participants in the analytical cohort, with zeros replacing the missing values, reduced the mean absolute change in PDC to 14.8%, which remained significantly greater than zero (P < .0001).
Adherence ≥80% is optimal to increase the likelihood of the best clinical benefits from medication.7,12,13 PDC increased from <80% at baseline to ≥80% at follow-up for 50 of 235 subjects and decreased from ≥80% at baseline to <80% at follow-up for 12 subjects, resulting in a net change from 20 (8.5%) subjects with PDC >80% at baseline to 56 (23.8%) with PDC ≥80% at follow-up (P < .0001). The highest mean increases occurred among the 215 participants with baseline PDC <80%, from 34.9% (±23.8% SD) to 54.6% (±29.9% SD), an absolute increase of 19.8%; P = .0001. Individuals whose PDC increased from <80% at baseline to ≥80% at follow-up trended toward using the app more often than all others (mean, 48 ± 48.8 vs 34.8 ± 39.5 days; P = .08). Baseline PDC did not predict app use (r = 0.0066; P = .91).
The 36-week PDC was available for 232 of 235 subjects. PDC remained higher at 51.4% (29.4% SD) in the 12 weeks after study completion relative to that at baseline (mean difference, 11.65%; P < .001).
Predictors of adherence change
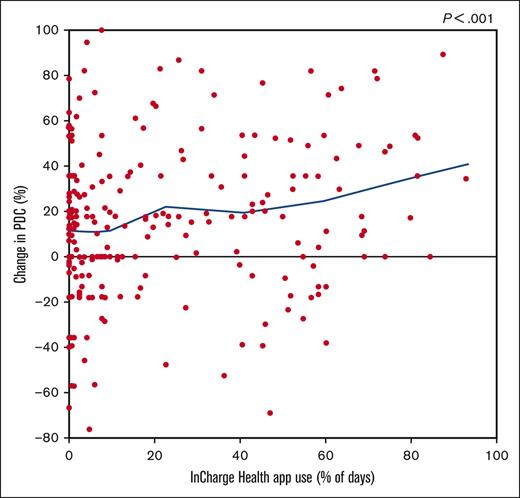
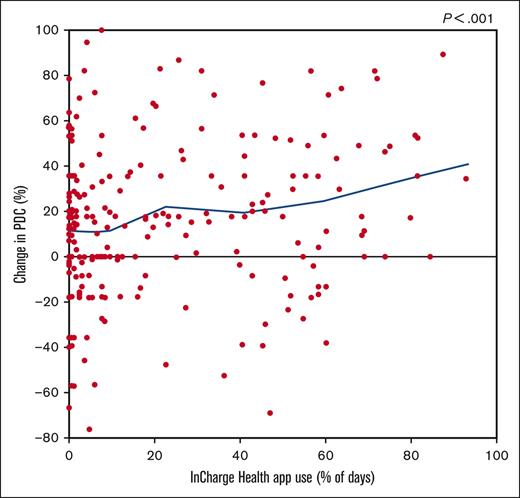
Frequency of app use, health insurance plan type, frequency of pain in the past 12 months, and severe pain frequency and interference in the past 6 months, with the 3 pain measures all reported at enrollment, were all associated with greater change in PDC (supplemental Table 1). Although as little as 13 days of app use was associated with significant PDC increases, patients who used the app for >50% of the duration of the study (≥85 days) saw the greatest mean increase in PDC (26.9%) (Table 3).
Patients with private insurance had a larger adjusted mean increase in PDC, followed by those with government insurance (supplemental Table 4). Adjusted mean change in PDC varied inversely with severe pain frequency in the last 6 months, the frequency of pain interference in the last 6 months, and the frequency of pain episodes in the previous 12 months (Table 4). Larger adjusted increase in PDC was seen in patients aged between 35 and 39 and between 40 and 45 years compared with that in younger patients (supplemental Table 4).
The number of provider participants per clinic ranged from 1 to 36 (median = 14 providers). Mean change in PDC per clinic, controlling for mean baseline PDC per clinic, was not associated with mean HU Toolbox use (P = .98).
In multivariable analysis, controlling for baseline PDC and site, greater app use, older age, having private insurance, and experiencing less pain interference in the previous 6 months were significantly associated with higher PDC increases after backward elimination. When clinic replaced site in the multivariable model, results were similar, except that pain frequency in the past 12 months replaced pain interference (supplemental Table 5).
Acute care use, laboratory markers, and PRO measures
The number of ED visits for pain declined with increasing PDC in both the baseline and follow-up intervals (baseline, P = .0013; follow-up, P < .0001) as did the number of admissions for pain (baseline, P = .0318; follow-up, P = .0003). However, changes in PDC among intervals were not associated with changes in either ED visits or admissions for pain (ED visits, r = –0.03; P = .62; admissions, r = 0.0648; P = .32). Although the rate of ED visits increased (1.16 to 1.51 visits per patient), it reflected a small number of subjects with large changes in ED visits rather than a general trend. Excluding the extreme values, mean change was only 0.036 visits per patient. Individuals who had at least 1 admission during the baseline period experienced a 25% reduction in hospitalizations for pain, with the rate dropping from 1.97 to 1.48 events per 24 weeks per patient (P = .0045; supplemental Table 6). Moreover, these individuals maintained lower hospitalization rates after the study’s completion, decreasing from 1.97 to 1.25 events per 24 weeks per patient (35% reduction from baseline; P = .015; supplemental Table 6). Patients without admissions in the baseline period did not exhibit any noticeable difference in hospitalization rates.
After 24 weeks of intervention, mean HbF increased, whereas ANC and count of reticulocytes declined, compared with values at baseline. No other laboratory changes were found (supplemental Table 6). PDC change was associated with a change in HbF (r = 0.21; P = .02). During the poststudy period, reticulocytes remained lower than the baseline. There were no other significant changes in laboratories (supplemental Table 6).
Patient-Reported Outcomes Measurement Information System and Adult Sickle Cell Quality of Life Measurement Information System PROs were analyzed for pain frequency, quality, severity, and interference data at baseline and at week 24. The mean frequency of self-reported pain in the past 12 months declined from 3.54 events per patient at baseline to 3.35 events per patients at follow-up (P = .0412). No other changes in PROs were detected (supplemental Table 6).
Providers knowledge and self-efficacy
Throughout the study, providers demonstrated consistently high levels of knowledge and self-efficacy in prescribing hydroxyurea, with mean scores of 4.6 of 5 (baseline) and 4.7 of 5 (follow-up) for knowledge, and 9.6 of 12 (baseline) and 9.5 of 12 (follow-up) for self-efficacy. However, providers who initially exhibited lower levels of hydroxyurea knowledge and self-efficacy experienced significant improvement during the study (Table 2). No significant differences in scores were appreciated between physicians and advanced practice providers (ie, nurse practitioners and physician assistants).
Discussion
To our knowledge, in the first study of its kind, we tested the effectiveness and implementation of a multilevel intervention to act on the behavioral determinants of low hydroxyurea adherence in patients with SCD and their providers, leveraging mHealth to deliver both interventions. In a multicenter proof-of-concept nonrandomized prospective clinical trial, we found a significant absolute mean increase of 16.3% in adherence, which sustained and promoted a 25% reduction in admissions for pain. This effect size is equivalent to using hydroxyurea slightly over 1 day per week. Our findings mirrored those of previous studies on mHealth adherence in chronic conditions, such as cardiovascular disease, asthma, and hypertension, which have demonstrated similar effect sizes ranging from 5% to 32%.38-42 Our data indicate that the use of mHealth by patients (but not providers) was associated with better hydroxyurea adherence and potential clinical benefit.
We did not observe MCV rise, but other laboratory markers of hydroxyurea effect (HbF, ANC, and reticulocytes) reflected improved adherence. The slight increase in HbF (1.9%) may not be clinically meaningful and could reflect stress erythropoiesis owing to hypoxia adaptation.43 The lack of overall reduction in acute care use and MCV rise likely stems from the very low overall baseline adherence level (<40%) and because only a quarter of patients crossed the 80% threshold for optimal adherence. Traditional efficacy clinical trials invariably select the most adherent patients for participation, which does not reflect real-world experiences. Our study represents a signal of effect that could be refined for future and more definitive implementation studies that will raise adherence >80%.
The COVID-19 pandemic had possible negative effects on the study. Clinic shutdowns, suspension of non–COVID-19 research activities, and staff resignation, all reduced hydroxyurea prescribing practices, curtailed app use, and created barriers to enrollment.44 The magnitude of the findings of this study may have been diminished owing to this global emergency. Because this study occurred during the COVID-19 pandemic, when care use fluctuated, these findings must be replicated.
Compared with older adults, adolescents and young adults used the app less and had less of an increase in medication adherence. This finding challenges the assumption that adolescents and young adults most benefit from modern technology interventions. Despite widespread acceptance of mobile devices and phone applications, younger patients may require additional support to sustain good self-management practices, given their developmental stage and maturity level. This is particularly relevant, because hydroxyurea adherence declines during adult care transition,10 thus threatening its long-term benefits, including organ protection. To achieve a higher number of patients with optimal medication adherence (ie, PDC ≥80%) and potentially engage younger patients, additional evidence-based interventions tailored to the determinants of low adherence should be tested concurrently with mHealth to boost and sustain adherence. For example, motivational interviewing is an evidence–based behavioral intervention successfully used to improve medication adherence in young45 and older46 adults with chronic diseases. It could be combined with mHealth interventions to improve hydroxyurea adherence and app engagement.
Reduced adherence to medication was observed in cases where baseline pain severity, frequency, and interference were higher. Sometimes, it is uncertain whether pain contributes to low adherence or vice versa. Thus, addressing the burden of pain is crucial to improving medication adherence. Furthermore, the type of health coverage seemed to predict intervention response, with private insurance associated with higher adherence. Health inequities are a product of social opportunities linked to economic barriers. As such, individuals with public insurance may also experience poverty, lower levels of digital literacy, and less social support. Any intervention focused on improving adherence must effectively address socio determinants of health to achieve the best results.
InCharge Health app implementation was high (∼85% downloaded and used the app at least once). However, app engagement was relatively low (∼17% of patients used the app >50% of the days in the study) and declined after the study. Other mHealth studies in SCD showed similar difficulty engaging with patients and waned use over time.47,48 Engagement with digital interventions involves investing affective, cognitive, and physical energies. Thus, low affective investment in the digital tool and low momentary motivation are common reasons for app disengagement.49 We are still in the infancy of understanding and measuring what is a meaningful amount and pattern of app engagement to promote effective and sustained behavior changes.50 Thus, understanding the factors predicting app engagement, user’s experience, how often and how apps are used is critical, and the next steps in our research.
Although adoption of HU Toolbox among providers was relatively high (65% agreed to use it), only 11% meaningfully used the app during the study. Our study included mainly academic sites and clinics, with access to providers who consider themselves SCD specialists.51 It is possible that participating providers did not see the need to use the app, because most were from academic sites or practiced at SCD specialty clinics. Because we firmly believe that improvement in hydroxyurea prescribing practices is needed in the United States, modifications to the provider app are planned to bolster its usability for future testing among non-SCD experts. We will, however, omit this intervention in future studies of experienced SCD clinicians.
Limitations of the study included a lack of a control group, short poststudy follow-up, and missing follow-up prescription data in 9.6% of patients. Although to a lesser extent than high app users, low app users also saw an increase in PDC, likely a limitation of the study design. As with many nonrandomized intervention studies, we cannot eliminate all threats to internal validity, including potential compensatory behaviors that may affect the extent and timing of the intervention’s effects.52 However, we point to the following to highlight the plausibility that the app was responsible for the rise in PDC: (1) baseline PDC did not predict app use, (2) research staff and patient contact was brief and constrained to only 3 study visits, (3) higher than observed PDC change would have been expected should the patients who could not be evaluated had been included, given their lower baseline PDC and the negative correlation between baseline PDC and change in PDC, and (4) persistence of treatment response beyond the intervention period is not predicted by compensatory behaviors. Despite the limitations of the study design, we observed some benefits of mHealth intervention.
Although patients present themselves as taking the medication at study entry, upon reviewing pharmacy records, they often do not. This scenario reflects the challenges of conducting effectiveness studies, that is, outside the artificial setting of clinical trials. Given our implementation-effectiveness study design, we intentionally did not use a direct measure of adherence but inferred it from medication refills. This is both a strength for reflecting real-world practices and a limitation, because there was no way of knowing if drug possession equated to ingestion. Although cognitive function difficulties affect hydroxyurea adherence,53 our study did not measure if the app could have mitigated the effects of cognitive dysfunction on medication adherence. Future studies should include cognitive assessments and how they may change during the mHealth intervention. Furthermore, patients with high baseline adherence participated, which may have diminished effectiveness, because a PDC of 90% can only increase it by an additional 10%. Restricting the intervention to individuals with low adherence is planned in future studies.
In conclusion, in a multicenter prospective intervention study, an mHealth intervention tailored to the determinants of low hydroxyurea adherence among patients with SCD promoted a 29% relative increase in adherence. Concomitant provider use of mHealth to support hydroxyurea prescribing did not help in increasing adherence in this study of primary academic sites. The use of the patient mHealth intervention to increase hydroxyurea adherence promoted improvement of some hematologic markers of hydroxyurea effect, reduced pain admissions, and self-reported frequency of pain. Although not definitive, the results from this large mHealth study are encouraging and support a randomized clinical trial with a longer poststudy observation period to increase hydroxyurea adherence further and improve health outcomes.
Appendix
The Sickle Cell Disease Implementation Consortium (SCDIC) participating site members are:
ST. JUDE CHILDREN’S RESEARCH HOSPITAL
Jane S. Hankins, Jason Hodges, Yvonne Carroll, Lisa Klesges, Hamda Khan, Matthew Smeltzer, Chinonyelum Nwosu, James Gurney, Jerlym Porter, Nicole Alberts, Reginald French, Sherif Badawy, Michael DeBaun, Guolian Kang, Jeremie Estepp, Winfred Wang, Curtis Owens, Margaret Debon, Ray Osarogiagbon, Marquita Nelson
UNIVERSITY OF CALIFORNIA IN SAN FRANCISCO
Marsha Treadwell, Elliott Vichinsky, Ted Wun, Michael Potter, Danielle Hessler, Ward Hagar, Anne Marsh, Lynne Neumayr
MEDICAL UNIVERSITY OF SOUTH CAROLINA
Cathy Melvin, Julie Kanter, Shannon Phillips, Robert Adams, Martina Mueller, Tina Abrams, Nathalia Davia
DUKE UNIVERSITY
Nirmish Shah, Paula Tanabe, Hayden Bosworth, George Jackson, Fred Johnson, Rachel Richesson, Janet Prvu-Bettger
WASHINGTON UNIVERSITY
Allison King, Ana Baumann, Cecilia Calhoun
AUGUSTA UNIVERSITY
Abdullah Kutlar, Robert Gibson, Angie Snyder, Maria Fernandez, Richard Lottenberg
MT SINAI
Lynne D. Richardson, Jeffrey Glassberg, Jena Simon, Nicholas G. Genes, George T. Loo, Jason S. Shapiro, Kimberly Souffront, Cindy Clesca, Elizabeth Linton, Gery Ryan
RTI INTERNATIONAL
Barbara L. Kroner, Tabitha Hendershot, Lisa DiMartino, Sara Jacobs, Whitney Battestilli, Donald Brambilla, Lisa Cox, Liliana Preiss, Norma Pugh, Sophie Li, Annie VonLehmden
NHLBI
Sharon M. Smith, William P. Tonkins, Marlene Peters-Lawrence, Cheryl Boyce, Whitney Barfield
LURIE CHILDREN’S
Alexis Thompson
UNIVERSITY OF ILLINOIS AT CHICAGO
Principal Investigators
Victor Gordeuk, a
Melissa Gutierrez, i, j
Jana Hirschtick, i, j, Lewis Hsu, b, Jerry Krishnan, c
Nadew Sebro, j
Larissa Verda, j
Abe Wandersman, k
Co-Investigators
Michael Berbaum, f
Kishore Bobba, j
Joe Colla, g
Kim Erwin, d
Andrea Lamont, k
Molly Martin, b
Sarah Norell, d
Ananta Pandit, j
Kay Saving, h
Robin Shannon, e
Robert Winn, c
Leslie Zun, j
Research Staff
Taif Hassan, a
Patricia Lasley, j
Kristin Monnard, i, j
Judith Nocek, a
Pamela Roesch, i, j
Affiliation
a. University of Illinois at Chicago, Division of Hematology and Oncology
b. University of Illinois at Chicago, Department of Pediatrics
c. University of Illinois at Chicago, Division of Pulmonary, Critical Care, Sleep and Allergy, Associate Vice Chancellor for Population Health Sciences
d. Program for Healthcare Delivery Design, Population Health Sciences Program, Office of the Vice Chancellor for Health Affairs
e. University of Illinois at Chicago, College of Nursing
f. University of Illinois at Chicago, Department of Epidemiology and Biostatistics
g. University of Illinois Health, Department of Emergency Medicine
h. University of Illinois College of Medicine, Peoria
i. Sinai Urban Health Institute
j. Sinai Health System
k. University of South Carolina
Acknowledgments
The authors thank all the study coordinators, research personnel, and patients and their families who participated in this research study. The authors also thank Keith Perry and Jeff Frey for their support with the design of the InCharge Health app. The InCharge Health app was developed by Agency 39a, and the HU Toolbox by SickleSoft.
The Sickle Cell Disease Implementation Consortium was supported by US Federal Government cooperative agreements U24HL133948, U01HL133964, U01HL133990, U01HL133996, U01HL133994, U01HL133997, U01HL134004, U01HL134007, and U01HL134042 from the National Heart Lung and Blood Institute and the National Institute on Minority Health and Health Disparities (Bethesda, MD). This work was also funded by the American Society of Hematology Bridge Grant. S.M.B. received research support from K23HL150232 during the conduct of this study. L.M.K. received research support from the St. Jude Children's Research Hospital–Washington University St. Louis Implementation Sciences Collaborative.
Authorship
Contribution: J.S.H. and N.S. contributed in study concept, enrollment of participants, and manuscript writing; M.B.P., A.K., R.G., V.R.G., J.S., R.L., L.D., S.J., M.E.F., H.B.B., and L.M.K. contributed in study concept, enrollment of participants, and manuscript editing; A.A.K., A.A.B., C.M., L.L.H., C.N., J.S.P., N.M.A., S.M.B., and J.A.G. enrolled participants and revised the manuscript; and D.B. conducted the statistical analysis.
Conflict-of-interest disclosure: J.S.H. receives consultancy fees from Forma Therapeutics and Global Blood Therapeutics (GBT). N.S. receives consultancy fees from GBT, Forma, Agios, Bausch, and bluebird bio, and speaks for GBT, Emmaus Pharmaceuticals, and Alexion. L.L.H. received consultancy fees from Hilton Publishing HPC, Forma Therapeutic, Emmaus, Novartis, and Aruvant, and research funding from Asklepion, Pfizer, CRISPR/Vertex, and Bayer.
The current affiliation for L.D. is The University of Texas Southwestern Medical Center, Dallas, TX.
A complete list of the members of the Sickle Cell Disease Implementation Consortium appears in “Appendix.”
Correspondence: Jane S. Hankins, Department of Global Pediatric Medicine, St. Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: jane.hankins@stjude.org.
References
Author notes
Original data are available in BioLINCC. For deidentified participant data access, please refer to the instructions at https://biolincc.nhlbi.nih.gov/home/.
The full-text version of this article contains a data supplement.