Key Points
E-cadherin/β-catenin protein expression is conserved in human and rat erythropoiesis and increases during stress erythropoiesis in rat BM.
β-catenin is stabilized by E-cadherin and, upon activation, stimulates erythroblast differentiation.
Abstract
E-cadherin is a crucial regulator of epithelial cell-to-cell adhesion and an established tumor suppressor. Aside epithelia, E-cadherin expression marks the erythroid cell lineage during human but not mouse hematopoiesis. However, the role of E-cadherin in human erythropoiesis remains unknown. Because rat erythropoiesis was postulated to reflect human erythropoiesis more closely than mouse erythropoiesis, we investigated E-cadherin expression in rat erythroid progenitors. E-cadherin expression is conserved within the erythroid lineage between rat and human. In response to anemia, erythroblasts in rat bone marrow (BM) upregulate E-cadherin as well as its binding partner β-catenin. CRISPR/Cas9–mediated knock out of E-cadherin revealed that E-cadherin expression is required to stabilize β-catenin in human and rat erythroblasts. Suppression of β-catenin degradation by glycogen synthase kinase 3β (GSK3β) inhibitor CHIR99021 also enhances β-catenin stability in human erythroblasts but hampers erythroblast differentiation and survival. In contrast, direct activation of β-catenin signaling, using an inducible, stable β-catenin variant, does not perturb maturation or survival of human erythroblasts but rather enhances their differentiation. Although human erythroblasts do not respond to Wnt ligands and direct GSK3β inhibition even reduces their survival, we postulate that β-catenin stability and signaling is mostly controlled by E-cadherin in human and rat erythroblasts. In response to anemia, E-cadherin–driven upregulation and subsequent activation of β-catenin signaling may stimulate erythroblast differentiation to support stress erythropoiesis in the BM. Overall, we uncover E-cadherin/β-catenin expression to mark stress erythropoiesis in rat BM. This may provide further understanding of the underlying molecular regulation of stress erythropoiesis in the BM, which is currently poorly understood.
Introduction
E-cadherin is a highly conserved transmembrane protein and a key component of epithelial cell adherence junctions that critically control tissue homeostasis.1-4 E-cadherin binding of β-catenin additionally links E-cadherin with Wnt/β-catenin signaling.5 In epithelia, E-cadherin is able to stabilize β-catenin by competing with adenomatous polyposis coli (APC) for β-catenin binding.6 APC is a component of the β-catenin destruction complex, which on binding induces phosphorylation and proteasomal degradation of β-catenin.6 Recruitment of β-catenin by E-cadherin may limit Wnt/β-catenin signaling, and was found to render tumor formation upon expression of oncogenic β-catenin mutant variants that are constitutively active.5 However, in response to mechanical tension, E-cadherin/β-catenin signaling promotes cell proliferation by driving β-catenin internalization and transcriptional signaling that can be fortified upon Wnt stimulation.7,8
Regulation of tissue homeostasis by E-cadherin is also evidenced in mouse models with tissue-specific conditional inactivation of Cdh1 (encoding E-cadherin). In mice, conditional E-cadherin deficiency impairs intestinal, skin, and mammary gland formation and promotes tumor development and metastasis.9-13 In humans, CDH1 inactivating mutations or promoter hypermethylation also correlate with tumor progression of gastric, prostate, hepatocellular, breast, and esophageal carcinomas.14-18 In hematopoietic malignancies, such as acute myeloid leukemia, CDH1 promoter hypermethylation is likewise associated with poor clinical outcome.19,20
Within human hematopoietic tissue, we and others identified CDH1 to selectively mark the erythroid lineage.21,22 However, we revealed that hematopoietic E-cadherin is not expressed within the erythroid lineage in mice but instead predominantly marks the basophil lineage.22 Mouse models, therefore, cannot be used to model the role of E-cadherin in erythropoiesis and malignancy formation. Rats, in comparison with mice, were proposed to reflect human erythropoiesis more closely, particularly the erythroid response to anemia (stress erythropoiesis),23 which, similar to humans, occurs primarily in the bone marrow (BM). Therefore, we examined E-cadherin expression in rat BM.
In this work, we reveal E-cadherin and β-catenin to mark erythroblastic islands in rat BM. Furthermore, we show that both E-cadherin and β-catenin protein levels increase in erythroblasts in response to anemia in rat BM. We show that E-cadherin interacts with and stabilizes β-catenin in erythroblasts. Moreover, subsequent activation of β-catenin signaling shows enhanced differentiation in human erythroblasts. Our findings suggest that β-catenin signaling in human and rat erythroblasts is controlled by E-cadherin and that E-cadherin upregulation in response to anemia may promote erythroblast differentiation by stimulating β-catenin signaling. Overall, this underscores that rats more closely mimic the anemic response observed in human BM than mice, the underlying molecular regulation of which is still poorly understood.
Methods
In vitro cell culture
Human material was obtained after informed consent was provided in accordance with the Declaration of Helsinki, the Dutch National ethic board, and the Sanquin internal ethic board (permit medical ethical committee (MEC) 04/042, no. 04.17.370; Amsterdam Medical Centre (AMC), Amsterdam, The Netherlands). Hematopoietic stem and progenitor cells (HSPCs) were isolated from mobilized peripheral blood that was obtained from leukapheresis material. HSPC isolation was performed using CD34 MACS beads (Miltenyi), in accordance with the manufacturer’s protocol. Isolated CD34+ cells were cryopreserved in Iscove modified Dulbecco medium supplemented with 20% fetal calf serum and 5% dimethyl sulfoxide. After thawing, HSPCs were cultured in Cellquin media,24 as described in supplemental Information.
Rat CD90+ cells were isolated from flushed rat femur and tibia BM and subsequently positively selected using CD90 microbeads for mouse and rat (Miltenyi). Isolated rat CD90+ cells were cultured in Cellquin medium as described for human CD34+ cells, in which we exchanged human stem cell factor (SCF) for mouse SCF (home produced). The day after isolation, CD90+ cells were edited with CRISPR/CRISPR-associated protein 9 (CRISPR/Cas9), as described in the supplemental Information. Culture medium was replaced with erythroid expansion medium (eg, containing mouse stem cell factor (SCF), erythopoietin (EPO), and dexamethasone) on day 4 after isolation.24
Animal experiments and tissue preparation
All rats were maintained in the animal facility of The Netherlands Cancer Institute. Animal experiments were conducted according to institutional and national guidelines under protocol permit ID 9.4.9961. Phenyl hydrazine (PHZ) was dissolved freshly in phosphate-buffered saline (PBS) on the day of injection. Lewis rats (Janvier) were injected intraperitoneally, 40 mg/kg bodyweight, on 3 consecutive days. Blood was collected in heparin tubes by puncture of the tail vein or by heart puncture on the day of euthanasia. Blood values were measured on a Scil Vet abc Plus machine (Horiba Medical) using the preinstalled “Rat” program. On indicated days, rats were euthanized with CO2. The spleen, femur, lung, intestine, and tibia were collected and fixated in formalin for immunohistochemistry (IHC) or kept in PBS for fluorescence-activated cell sorting (FACS) and subsequent procedures.
Flow cytometry
Cells were incubated with antibodies for 30 minutes at 4°C, as listed in supplemental Table 1. Dead cells were marked by the Fixable near-infrared live/dead staining kit (Thermo Fisher Scientific, L10119). For intracellular staining, Foxp3/Transcription Factor Staining Buffer Set (eBioscience, 00-5523-00) was used, following the manufacturer's specifications. Flow cytometry was performed on a FACS Symphony (BD Biosciences), and data were analyzed using FlowJo version 10 (Treestar) software. Cell sorting was performed on a FACS Aria III (BD Biosciences), and on-site analysis was performed using BD FACSDiva 8.0.1. software.
IHC
Rat BM and spleen were formalin fixed in 10% neutral buffered formalin for 48 hours, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. IHC was performed as previously described.11 All slides were digitally processed using the Aperio ScanScope (Aperio) and captured using ImageScope software version 12.0.0 (Aperio). An overview of antibodies is provided in supplemental Table 1.
Cytospin
In total, 100 000 sorted cells were spun down at 800 rpm on an object glass using a Shandon Cytospin II (Thermo Fisher Scientific), air dried, and fixed in methanol for 5 minutes. Cells were stained with benzidine in combination with the Differential Quick Stain Kit (PolySciences), following the manufacturer’s protocol.
CRISPR/Cas9
Ribonucleoprotein (RNP) formation and nucleofection were performed as previously described by Verhagen et al25 and in the supplemental Information.
Lentiviral production and transduction
Lentiviral production and transduction were performed as described in the supplemental Information.
Immunoprecipitation and western blot
These analyses were performed as described in the supplemental Information.
Immunofluorescent microscopy
Cover slips were coated with 5 μg/mL VCAM-1 in demineralized water per well (R&D Systems) and were allowed to incubate for 2 hours (37°C, 5% CO2). Subsequently, cover slips were washed and placed in a 24-well plate, before 105 erythroblasts per well were seeded in Cellquin medium supplemented with 100 μg/mL streptomycin (Sigma Aldrich), 2 mM L-glutamate (Sigma Aldrich), 10 U/mL EPO, and 700 μg/mL holotransferrin. After a 24-hour incubation (37°C, 5% CO2), immunofluorescence was performed as previously described10 and described in the supplemental Information.
Results
E-cadherin marks erythroblastic islands in rat BM
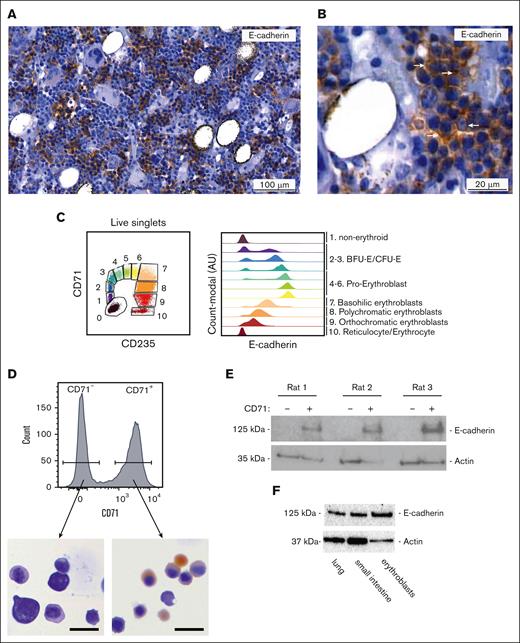
E-cadherin function in human erythropoiesis cannot be modeled in mice, because E-cadherin is not expressed during erythropoiesis in mice.22 Because rats have been postulated to reflect human erythropoiesis more closely, we examined whether E-cadherin is expressed in the erythroid lineage in rat BM. First, we examined E-cadherin protein expression in BM sections derived from the femur and tibia of 8-week-old Lewis rats using immunohistochemistry (IHC). Rat BM displayed a large number of E-cadherin–positive cells that clustered in groups of ∼10 to 20 cells throughout the BM (Figure 1A). E-cadherin mostly accumulated at the peripheral membrane, in line with its role as a cell adhesion protein (Figure 1B). E-cadherin–positive cells were marked by a dense nuclear staining, visualized by hematoxylin staining, in comparison with surrounding E-cadherin–negative cells (Figure 1A-B). The clustered cellular expression pattern, combined with the dense nuclear staining with hematoxylin typically resemble erythroblasts in erythroblastic islands, thereby suggesting E-cadherin to mark erythroblasts in rat BM comparable with that in human BM.22 In healthy human BM, E-cadherin can be detected on the cell surface of isolated CD71+CD235a+ erythroblasts using flow cytometry (Figure 1C). However, none of the commercially available tested E-cadherin antibodies (eg, antibody clones: HECD-1, 67A4, DECMA-1, and 36/E-cadherin) detected E-cadherin cell surface expression with flow cytometry on isolated rat BM cells or on the cell surface of rat mammary adenocarcinoma cell line MATBIII that we used as positive control (data not shown). To confirm E-cadherin protein expression in rat erythroblasts, we, therefore, sorted CD71high (transferrin receptor) erythroid precursors and nonerythroid CD71− BM cells from the femurs of 3 rats. Benzidine staining of cytospins indicated that CD71high cells mostly resemble polychromatic erythroblasts that are exclusively present at erythroblastic islands (Figure 1D).26 Western blot analysis showed that CD71high cells expressed E-cadherin, whereas E-cadherin was not observed in CD71− cells (Figure 1E). Specificity of E-cadherin detection by western blot was confirmed using primary rat lung and small intestine as positive control (Figure 1F). Overall, these data reveal that rat CD71high erythroblasts, but not nonerythroid CD71− cells, express E-cadherin similar to that observed for human erythroblasts and, therefore, resemble erythroblastic islands as seen in human BM.27
E-cadherin is expressed in the erythroid lineage in the rat BM. (A) Representative IHC staining for E-cadherin (brown) on healthy rat BM sections. (B) Zoom in of healthy rat BM. IHC staining indicates E-cadherin (brown) cell surface expression. (C) Flow cytometry plot, used to define erythroid differentiation stages (BFU-E; Burst-forming unit-erythroid, CFU-E; Colony-forming unit-erythroid) based on CD71 and CD235a surface expression in BM aspirate of a healthy individual, used to accordingly define E-cadherin cell surface expression. (D) Sorting strategy of rat CD71+ and CD71− rat BM cell population within living singlets. Representative benzidine stained cytospins of sorted CD71+ rat BM cells indicates that the majority of sorted cells represent basophilic erythroblasts (scale bar; 20µm). (E) Western blot of sorted CD71+ and CD71− rat BM cells showing E-cadherin is expressed in CD71+ but not CD71− cells (n = 3). (F) Western blot analysis showing E-cadherin protein expression in rat lung and small intestine material as well as sorted CD71+ rat erythroblasts.
E-cadherin is expressed in the erythroid lineage in the rat BM. (A) Representative IHC staining for E-cadherin (brown) on healthy rat BM sections. (B) Zoom in of healthy rat BM. IHC staining indicates E-cadherin (brown) cell surface expression. (C) Flow cytometry plot, used to define erythroid differentiation stages (BFU-E; Burst-forming unit-erythroid, CFU-E; Colony-forming unit-erythroid) based on CD71 and CD235a surface expression in BM aspirate of a healthy individual, used to accordingly define E-cadherin cell surface expression. (D) Sorting strategy of rat CD71+ and CD71− rat BM cell population within living singlets. Representative benzidine stained cytospins of sorted CD71+ rat BM cells indicates that the majority of sorted cells represent basophilic erythroblasts (scale bar; 20µm). (E) Western blot of sorted CD71+ and CD71− rat BM cells showing E-cadherin is expressed in CD71+ but not CD71− cells (n = 3). (F) Western blot analysis showing E-cadherin protein expression in rat lung and small intestine material as well as sorted CD71+ rat erythroblasts.
Rat erythroblasts upregulate E-cadherin/β-catenin protein levels in response to anemia
In contrast to erythropoiesis in mice that predominantly occurs in the spleen, stress erythropoiesis in rats and human is mostly confined to the BM.24 We, therefore, wondered whether expression of E-cadherin in both human and rat BM-resident erythroblasts may be linked to the regulation of stress erythropoiesis.
To investigate E-cadherin protein expression during stress erythropoiesis, Lewis rats were treated with PHZ to induce anemia, using 40 mg/kg, as previously defined for August Copenhagen Irish (ACI) rats.24 PHZ (or PBS used as control) was delivered by intraperitoneal injections on 3 consecutive days (experimental day −2, −1, and 0; supplemental Figure 1A). Whole blood parameters were measured daily to monitor onset and recovery of anemia (supplemental Figure 2B-E). Hematocrit (supplemental Figure 1B) and mean corpuscular hemoglobin concentration (supplemental Figure 1C) recovered in ∼5 days, whereas red blood cell count (supplemental Figure 1D) and mean corpuscular volume (supplemental Figure 1E) started to recover after day 5 and required ∼18 days to approach control values. Overall, these data revealed PHZ to induce a synchronized anemic response in Lewis rats that resulted in a maximal anemic response at 48 to 96 hours after PHZ treatment (supplemental Figure 1B-C).
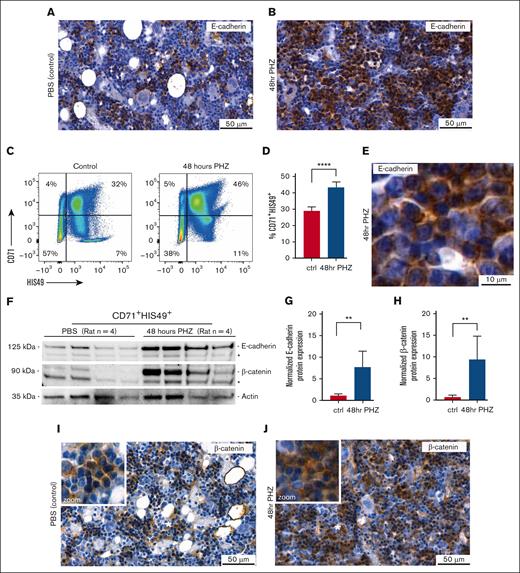
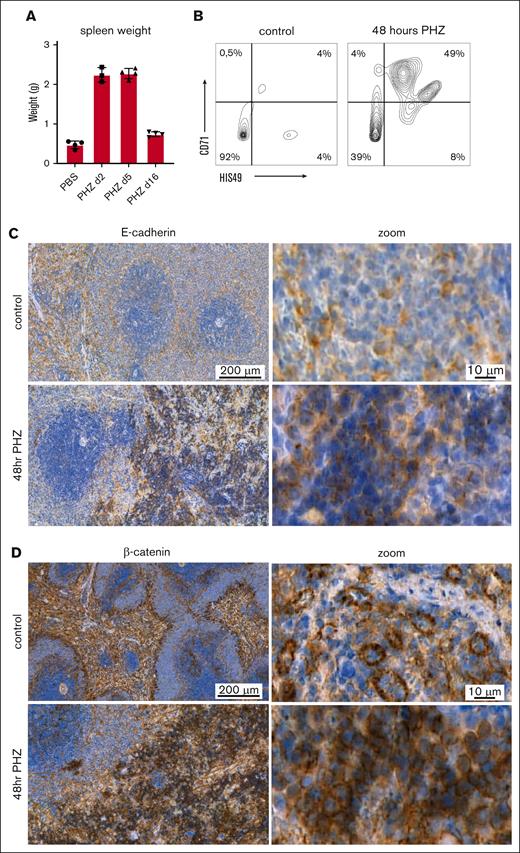
Next, we examined the BM composition and E-cadherin protein expression in the femurs and tibia with IHC 48 hours after PHZ treatment. Anemic BM was characterized by a striking loss of adipocytes, as also previously described for anemic ACI rats (Figure 2A-B).24 Basophilic erythroblasts, defined as CD71+HIS49+, expanded in response to anemia by ∼1.5-fold from ∼28% in the control to 42% in anemic BM (n = 4; Student t test, P = .0004), which correlated with a marked expansion of E-cadherin–positive cells (Figure 2A-D). Moreover, IHC staining for E-cadherin of anemic BM sections indicated E-cadherin protein levels to increase in the cytoplasm and at the plasma membrane in response to anemia (Figure 2A-B,E).
E-cadherin/β-catenin protein levels increase in rat erythroblasts in response to anemia. (A) Representative IHC staining for E-cadherin (brown) on rat BM sections obtained from control (PBS injected) and (B) anemic rats induced with PHZ for 48 hours after intraperitoneal injection. (C) Representative flow cytometry plots, using CD71 and HIS49 cell surface staining to define the percentage of CD71+; HIS49+ basophilic erythroblasts (EBs) in the BM from control (PBS) and anemic (48-hour PHZ) rats. (D) Quantification of flow cytometry analysis reveals CD71+HIS49+ EBs isolated from the rat BM to increase from 28% in control animals to 42% in response to anemia (n = 4; Student t test, ∗∗∗∗P = .0004). (E) Zoom in of representative IHC for E-cadherin on anemic rat BM section (48-hour PHZ), showing E-cadherin (brown) to accumulate at the membrane and cytoplasm. (F) E-cadherin and β-catenin expression defined by western blot in sorted CD71+HIS49+ EBs derived from control (PBS injected) and anemic (48 hours PHZ) rats; background bands are indicated by an asterisk (∗). (G) Revealing E-cadherin and β-catenin protein levels to significantly increase, as normalized to actin input protein levels, by respectively sevenfold (n = 4; Student t test, ∗∗P = .0122) for E-cadherin (H) and ninefold (n = 4; Student t test, ∗∗P = .0181) for β-catenin expression in CD71+HIS49+ sorted rat EBs in response to anemia. (I) Representative IHC staining for β-catenin (brown) on rat BM sections obtained from control (PBS injected) and (J) anemic rats as induced with PHZ for 48 hours after intraperitoneal injection. ctrl, control; hr, hour.
E-cadherin/β-catenin protein levels increase in rat erythroblasts in response to anemia. (A) Representative IHC staining for E-cadherin (brown) on rat BM sections obtained from control (PBS injected) and (B) anemic rats induced with PHZ for 48 hours after intraperitoneal injection. (C) Representative flow cytometry plots, using CD71 and HIS49 cell surface staining to define the percentage of CD71+; HIS49+ basophilic erythroblasts (EBs) in the BM from control (PBS) and anemic (48-hour PHZ) rats. (D) Quantification of flow cytometry analysis reveals CD71+HIS49+ EBs isolated from the rat BM to increase from 28% in control animals to 42% in response to anemia (n = 4; Student t test, ∗∗∗∗P = .0004). (E) Zoom in of representative IHC for E-cadherin on anemic rat BM section (48-hour PHZ), showing E-cadherin (brown) to accumulate at the membrane and cytoplasm. (F) E-cadherin and β-catenin expression defined by western blot in sorted CD71+HIS49+ EBs derived from control (PBS injected) and anemic (48 hours PHZ) rats; background bands are indicated by an asterisk (∗). (G) Revealing E-cadherin and β-catenin protein levels to significantly increase, as normalized to actin input protein levels, by respectively sevenfold (n = 4; Student t test, ∗∗P = .0122) for E-cadherin (H) and ninefold (n = 4; Student t test, ∗∗P = .0181) for β-catenin expression in CD71+HIS49+ sorted rat EBs in response to anemia. (I) Representative IHC staining for β-catenin (brown) on rat BM sections obtained from control (PBS injected) and (J) anemic rats as induced with PHZ for 48 hours after intraperitoneal injection. ctrl, control; hr, hour.
We, therefore, defined E-cadherin protein levels in sorted CD71+HIS49+ erythroblasts from control and anemic BM (n = 4) by western blot. In line with the more intense immunohistochemical staining for E-cadherin, erythroblasts isolated from anemic BM displayed an approximate sevenfold upregulation of E-cadherin protein (n = 4; Student t test, P = .0122), as normalized to actin input (Figure 2F-G). Intriguingly, protein levels of β-catenin, a prominent binding partner of E-cadherin, which on binding protects its proteasomal degradation, similarly increased (n = 4; Student t test, P = .0181) in response to anemia (Figure 2F,H).26 Of note, CD71 cell surface expression after PHZ treatment did not alter (supplemental Figure 2). Comparable with E-cadherin staining (Figure 2A-B), immunohistochemical analysis also revealed β-catenin to stain clusters of cells throughout the rat BM, which prominently increased in response to PHZ (Figure 2I-J). Besides its accumulation at the peripheral cell membrane, β-catenin was also detected in the cytoplasmic/nuclear regions of erythroblasts in response to PHZ (Figure 2J, zoom). These results reveal that protein levels of both E-cadherin and its binding partner β-catenin increase in rat erythroblasts in response to anemia.
E-cadherin/β-catenin expression marks extramedullary erythropoiesis in the rat spleen
In more severe cases of anemia, humans and rats upregulate extramedullary hematopoiesis in the spleen, to support BM erythropoiesis.23 Spleen weight was assessed during the onset and recovery to PHZ-induced anemia (Figure 3A). From 48 hours to 5 days after PHZ induced anemia, the spleen weight increased approximately fourfold, from 0.5 to 2 g (Figure 3A). In the control spleen, erythroblasts (CD71+HIS49+) were barely detected, but they strongly expanded in response to anemia, representing 49% of isolated cells in the anemic spleen (Figure 3B). We accordingly assessed E-cadherin protein expression with IHC in the spleen during steady state and anemic conditions. Weak-positive staining for E-cadherin was observed in splenic sections derived from control rats, marking cells that scattered throughout the red pulp and marginal zone (Figure 3C; supplemental Figure 3). Based on their frequency and cellular morphology, these cells likely reflect splenic macrophages. In contrast, the red pulp of the anemic spleen harbored large numbers of erythroblasts, as recognized by the clustered cellular and dense nuclear staining that were not seen in control conditions, as also in line with the flow cytometry data (Figure 3C). Similarly, as observed in the BM, erythroblasts in the anemic spleen displayed membranous staining for E-cadherin (Figure 3C, zoom).
E-cadherin and β-catenin mark EBs that arise in the rat anemic spleen. (A) Rat spleen weight defined after isolation from control (PBS; n = 4) and anemic animals (2, 5, and 16 days after final injection with PHZ; n = 4) reveals spleen weight increase by fourfold from ∼0.5 g to 2 g within 48 hours after PHZ treatment. (B) Representative flow cytometry plots of isolated splenocytes stained for CD71 and HIS49, revealing that splenocytes from control (PBS) rats barely contain CD71+HIS49+ EBs (∼3%), which increase up to 49% in response to anemia (PHZ 48 hours). (C) Representative IHC staining for E-cadherin (brown) on rat spleen sections obtained from control (PBS) and anemic (PHZ 48 hours) rats revealing E-cadherin to mark macrophage-typed cells in the red pulp in control conditions and to mark EB clusters that develop in the red pulp in response to anemia. (D) Representative IHC staining for β-catenin (brown) in the rat spleen, 48 hours after PBS (control) or PHZ treatment, revealing β-catenin protein expression to mostly mark endothelial cells in control conditions while marking EBs that arise in the red pulp in response to anemia.
E-cadherin and β-catenin mark EBs that arise in the rat anemic spleen. (A) Rat spleen weight defined after isolation from control (PBS; n = 4) and anemic animals (2, 5, and 16 days after final injection with PHZ; n = 4) reveals spleen weight increase by fourfold from ∼0.5 g to 2 g within 48 hours after PHZ treatment. (B) Representative flow cytometry plots of isolated splenocytes stained for CD71 and HIS49, revealing that splenocytes from control (PBS) rats barely contain CD71+HIS49+ EBs (∼3%), which increase up to 49% in response to anemia (PHZ 48 hours). (C) Representative IHC staining for E-cadherin (brown) on rat spleen sections obtained from control (PBS) and anemic (PHZ 48 hours) rats revealing E-cadherin to mark macrophage-typed cells in the red pulp in control conditions and to mark EB clusters that develop in the red pulp in response to anemia. (D) Representative IHC staining for β-catenin (brown) in the rat spleen, 48 hours after PBS (control) or PHZ treatment, revealing β-catenin protein expression to mostly mark endothelial cells in control conditions while marking EBs that arise in the red pulp in response to anemia.
IHC for β-catenin revealed β-catenin to mainly stain vessels within the red pulp of the control spleen, reflecting β-catenin expression by the endothelium (Figure 3D; supplemental Figure 4). In response to anemia, the accumulated erythroblasts displayed a strong membranous as well as a cytoplasmic/nuclear staining for β-catenin (Figure 3D, zoom). These findings indicate E-cadherin and β-catenin to mark erythroblasts both in the spleen and BM of anemic rats.
E-cadherin binds and stabilizes β-catenin in human erythroblasts
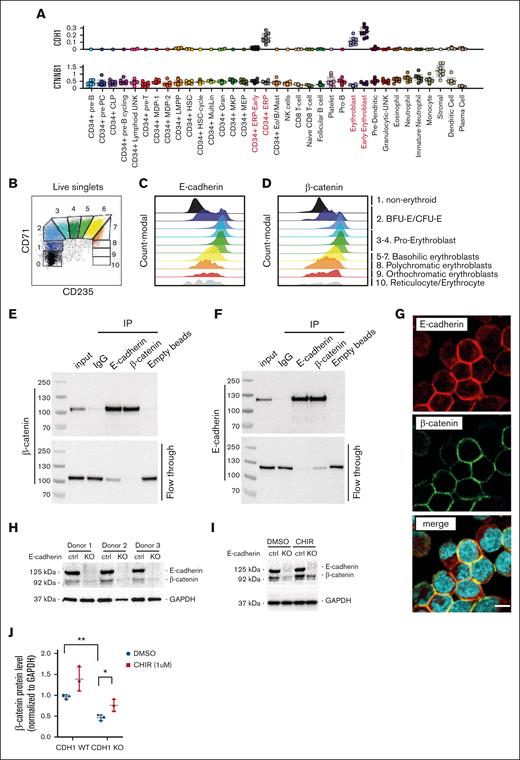
To define the similarities between human and rat erythropoiesis, we then examined CTNNB1 and CDH1 RNA (co)expression within the human hematopoietic cell lineage. We mined a publicly available, single-cell RNA-sequencing database comprising hematopoietic and stromal cell lineages derived from BM aspirates from 8 healthy human individuals.28 Within the hematopoietic cell lineage, CDH1 selectively marked erythroid cell populations, including early erythroid progenitors (CD34+), early erythroblasts, and erythroblasts (Figure 4A). The BM stroma, however, did not express detectable expression levels of CDH1. In contrast, CTNNB1 expression was detected throughout the hematopoietic cell lineages as well as for the stromal compartment in the BM (Figure 4A).
E-cadherin binds and stabilizes β-catenin in human EBs. (A) CDH1 (encoding E-cadherin) and CTNNB1 (encoding β-catenin) expression in hematopoietic and stromal cell lineages, derived from a publicly available single-cell RNA-sequencing database.28 (B) Representative flow cytometry plot, used to define erythroid differentiation stages based on CD71 and CD235a expression, of cultured human EBs derived from CD34+ mobilized HSPCs, revealing both (C) E-cadherin and (D) β-catenin protein expression to increase upon EB formation. (E-F) Western blot analysis of the association of E-cadherin with β-catenin in cultured human EB by immunoprecipitation of endogenous E-cadherin and β-catenin, using isotype immunoglobulin G (IgG) antibody and empty beads as negative controls, as also defined by western blot analysis of the flow through. (G) Representative immunofluorescent staining of cultured EBs on VCAM-1–coated slides, revealing E-cadherin (red) and β-catenin (green) to colocalize at the plasma membrane and to be absent in the nucleus (turquoise, visualized with Hoechst 33342). (H) Western blot analysis of E-cadherin and β-catenin protein expression in control and E-cadherin KO human EBs, as generated by CRISPR/Cas9 editing of mobilized CD34+ HSPCs from 3 donors. (I) Representative western blot revealing E-cadherin and β-catenin protein expression in control and E-cadherin KO EBs treated with CHIR99021, a selective GSK3β inhibitor, or dimethyl sulfoxide (DMSO) used as control. (J) Quantification of β-catenin protein expression defined by western blot (n = 3), as normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used as input, in control and E-cadherin KO EBs that are treated with DMSO (used as control) or selective GSK3β inhibitor CHIR99021 (n = 3; Student t test; DMSO KO vs CHIR KO: ∗∗P = .002; DMSO WT vs DMSO KO: ∗P = 0.02). IP, intraperitoneal; WT, wild-type.
E-cadherin binds and stabilizes β-catenin in human EBs. (A) CDH1 (encoding E-cadherin) and CTNNB1 (encoding β-catenin) expression in hematopoietic and stromal cell lineages, derived from a publicly available single-cell RNA-sequencing database.28 (B) Representative flow cytometry plot, used to define erythroid differentiation stages based on CD71 and CD235a expression, of cultured human EBs derived from CD34+ mobilized HSPCs, revealing both (C) E-cadherin and (D) β-catenin protein expression to increase upon EB formation. (E-F) Western blot analysis of the association of E-cadherin with β-catenin in cultured human EB by immunoprecipitation of endogenous E-cadherin and β-catenin, using isotype immunoglobulin G (IgG) antibody and empty beads as negative controls, as also defined by western blot analysis of the flow through. (G) Representative immunofluorescent staining of cultured EBs on VCAM-1–coated slides, revealing E-cadherin (red) and β-catenin (green) to colocalize at the plasma membrane and to be absent in the nucleus (turquoise, visualized with Hoechst 33342). (H) Western blot analysis of E-cadherin and β-catenin protein expression in control and E-cadherin KO human EBs, as generated by CRISPR/Cas9 editing of mobilized CD34+ HSPCs from 3 donors. (I) Representative western blot revealing E-cadherin and β-catenin protein expression in control and E-cadherin KO EBs treated with CHIR99021, a selective GSK3β inhibitor, or dimethyl sulfoxide (DMSO) used as control. (J) Quantification of β-catenin protein expression defined by western blot (n = 3), as normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used as input, in control and E-cadherin KO EBs that are treated with DMSO (used as control) or selective GSK3β inhibitor CHIR99021 (n = 3; Student t test; DMSO KO vs CHIR KO: ∗∗P = .002; DMSO WT vs DMSO KO: ∗P = 0.02). IP, intraperitoneal; WT, wild-type.
β-catenin protein expression is mostly posttranscriptionally regulated at the level of protein stability.29 To examine E-cadherin and β-catenin protein expression during erythropoiesis, we cultured erythroid progenitors from peripheral blood mobilized cell–derived CD34+ HSPCs, as previously described.24 E-cadherin cell surface protein expression was determined by flow cytometry, which allows for the assessment the differentiation stage of erythroblasts, because the cells first gain CD71 expression, then gain CD235 (glycophorin A (GPA)), and ultimately lose CD71 again. E-cadherin cell surface expression peaked during formation of CD71+CD235a+ basophilic erythroblasts (Figure 4B-C). BM-derived primary erythroid progenitors and erythroblasts, obtained from BM aspirates, similarly revealed a peak of E-cadherin cell surface expression within the transition between proerythroblast and basophilic erythroblast cell stages (Figure 1C). Whereas CTNNB1 expression levels did not increase during erythroid commitment; β-catenin protein levels, like E-cadherin, peaked during formation of CD71+CD235+ erythroblasts (Figure 4D). The concurrent increase of β-catenin with E-cadherin during erythroblast formation may be driven through its binding with E-cadherin, because this is known to prevent β-catenin degradation.6 Immunoprecipitation of endogenous E-cadherin or β-catenin, using cultured human (pro)erythroblasts, revealed both proteins to mostly associate with each other (Figure 4E-F). In line with this notion, E-cadherin and β-catenin colocalized at the peripheral cell membrane of CD71+CD235adim sorted (pro)erythroblasts (population 3-4 in Figure 4B) that were adhered onto VCAM-1–coated cover slips (Figure 4G). Overall, these data reveal β-catenin protein levels to increase in parallel with E-cadherin levels during human erythroblast formation, which may be driven by the protective binding of E-cadherin.
To test whether β-catenin is predominantly stabilized in human erythroblasts by E-cadherin, we deleted E-cadherin using CRISPR/Cas9 and guide RNA targeting CDH1 in mobilized CD34+ HSPCs of 3 different donors. Loss of E-cadherin decreased β-catenin protein levels by approximately twofold as compared with CRISPR/Cas9-control treated erythroblast (Figure 4H-J). However, E-cadherin deficiency did not affect erythroblast differentiation using our erythroid suspension culture conditions (supplemental Figure 5). Notably, inhibition of β-catenin degradation using 1 μM CHIR99021, a selective glycogen synthase kinase 3β (GSK3β) inhibitor, rescued β-catenin expression in E-cadherin knockout (KO) erythroblasts (Figure 4I-J). Although epithelial E-cadherin promotes cell-to-cell adhesion, its expression did not induce spontaneous clustering of human erythroblasts in suspension as examined with microscopy of generated erythroblast during expansion and/or differentiation (supplemental Figure 6). Overall, these data implicate E-cadherin to stabilize β-catenin within the human erythroid cell lineage.
E-cadherin stabilizes β-catenin in cultured rat erythroblasts
To define the control of E-cadherin on β-catenin protein stability in rat erythroblasts, we developed a protocol to generate and culture rat erythroblasts from HSPCs obtained from the rat BM. Antibodies used for characterization of human (eg, CD34) or mouse (eg, Spinocerebellar ataxia type 1 (SCA1)) HSPCs are unfortunately not available for rat cells. CD90+ cells partially mark human HSPCs and can be detected and enriched for with commercial and validated antibodies.30,31 We therefore tested whether CD90+ cells from the rat BM can be used to generate rat erythroblasts in vitro. The rat BM displays ∼22% CD90+ cells (Figure 5A; supplemental Figure 7). Characterization of rat BM–derived CD90+ cells, which we enriched for with microbeads, revealed these cells to lack erythroid (CD71 and HIS49), myeloid (CD11b), and lymphoid (CD3) hematopoietic linage markers (Figure 5A-E). However, a large population of CD90+ BM rat cells coexpress CD45R (B220) that mostly seems to characterize lymphoid precursor cells, as previously described (Figure 5F).30 We, however, reasoned that our erythroid-defined serum-free culture medium containing interleukin-3, EPO, and SCF would not permit survival or outgrowth of potential lymphoid progenitors but may mostly promote survival and differentiation of noncommitted and/or erythroid-defined CD90-marked progenitors. CD90+ cells were isolated from rat BM, purified with microbeads, and cultured in serum-free erythroid-defined cell expansion culture medium (containing EPO, SCF, and dexamethasone; Figure 5G). Seven days after taking the purified CD90+ in culture, a homogenous erythroblast cell culture was obtained, as characterized by high cell surface expression of CD71 and HIS49 (Figure 5H-I). To test whether E-cadherin also stabilizes β-catenin in rat erythroblasts, we deleted E-cadherin using CRISPR/Cas9 and guide RNA targeting rat Cdh1 in BM-derived CD90+ cells of 3 different Lewis rats. Loss of E-cadherin did not perturb rat erythroblast formation, as also found for human E-cadherin KO erythroblasts (Figure 5H-I). Similarly, as seen for human erythroblasts, the loss of E-cadherin in rat erythroblasts also reduced β-catenin protein expression, as evident by a ∼70% KO of E-cadherin that correlated with a ∼30% reduction in β-catenin protein expression (Figure 5J-K). These findings, thereby, underscore the conserved control of E-cadherin on β-catenin protein stability in both human and rat erythroblasts.
E-cadherin also stabilizes β-protein expression in rat EBs. (A) Representative flow cytometry plot showing CD90 expression in whole rat BM and rat BM–derived CD90+ cells enriched using microbeads. (B-F) Flow cytometry plots showing His49 (B) CD71 (C), CD11b (D), CD3 (E), and CD45R (F) in rat BM–derived CD90 microbead enriched cells (blue histogram); compared rat whole BM cells positive for these markers used as a positive control (red histogram). (G) Representative microscopy picture of cultured rat CRISPR-edited EBs on day 7 after isolation from the rat BM. Cells were edited either using CRISPR with Cas9 only (left) or with Cas9 and RNPs targeting Cdh1 (right). (H) Flow cytometry plot showing erythroid differentiation, based on His49 and CD71 surface staining, of rat EBs edited using CRISPR with either only Cas9 or Cas9 + Cdh1 guides on day 7 of culturing. (I) Western blot showing E-cadherin and β-catenin protein expression of control (ctrl) EBs or EBs edited using CRISPR for Cdh1 in 3 different rats. Actin was used as a loading control. (J) Quantification of E-cadherin and β-catenin protein expression defined by western blot, as normalized to actin used as input, in rat EBs edited using CRISPR with Cas9 only (ctrl) or Cas9 + Cdh1 guides (n = 3; two-way analysis of variance [ANOVA], E-cadherin ctrl vs Cas9 Cdh1: ∗∗∗∗P < .0001; β-catenin ctrl vs Cas9 Cdh1: ∗P = .0116).
E-cadherin also stabilizes β-protein expression in rat EBs. (A) Representative flow cytometry plot showing CD90 expression in whole rat BM and rat BM–derived CD90+ cells enriched using microbeads. (B-F) Flow cytometry plots showing His49 (B) CD71 (C), CD11b (D), CD3 (E), and CD45R (F) in rat BM–derived CD90 microbead enriched cells (blue histogram); compared rat whole BM cells positive for these markers used as a positive control (red histogram). (G) Representative microscopy picture of cultured rat CRISPR-edited EBs on day 7 after isolation from the rat BM. Cells were edited either using CRISPR with Cas9 only (left) or with Cas9 and RNPs targeting Cdh1 (right). (H) Flow cytometry plot showing erythroid differentiation, based on His49 and CD71 surface staining, of rat EBs edited using CRISPR with either only Cas9 or Cas9 + Cdh1 guides on day 7 of culturing. (I) Western blot showing E-cadherin and β-catenin protein expression of control (ctrl) EBs or EBs edited using CRISPR for Cdh1 in 3 different rats. Actin was used as a loading control. (J) Quantification of E-cadherin and β-catenin protein expression defined by western blot, as normalized to actin used as input, in rat EBs edited using CRISPR with Cas9 only (ctrl) or Cas9 + Cdh1 guides (n = 3; two-way analysis of variance [ANOVA], E-cadherin ctrl vs Cas9 Cdh1: ∗∗∗∗P < .0001; β-catenin ctrl vs Cas9 Cdh1: ∗P = .0116).
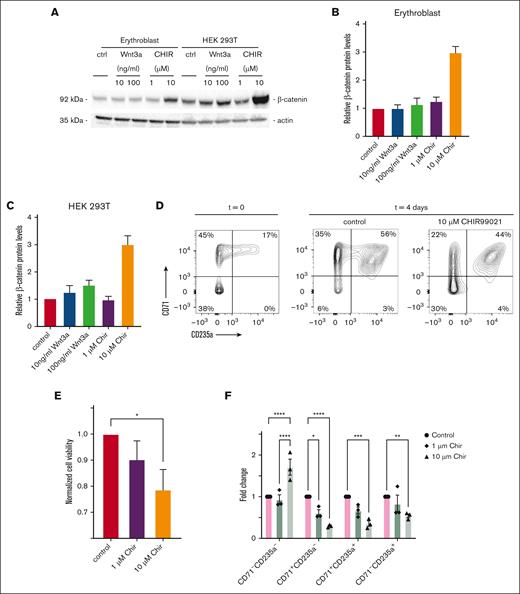
CHIR99021 hampers expansion and differentiation of cultured human erythroblasts
In general, β-catenin stabilization is induced by canonical Wnt signaling.32,33 However, stimulation of human erythroblasts with recombinant Wnt3a (10-100 ng/mL) did not increase β-catenin protein levels (Figure 6A-B). In comparison, Wnt3a-stimulated HEK293T cells, which were used as positive control, displayed a dose-dependent increase in β-catenin protein expression levels (Figure 6A,C). Although direct inhibition of GSK3β activity with 10 μM CHIR99021 in human erythroblasts did stabilize β-catenin protein expression with an approximate threefold increase (Figure 6B), CHIR99021 treatment of human proerythroblast cultures, reduced cell formation and viability of CD71+CD235a+ erythroblasts in a concentration-dependent fashion, as defined after 5 days of stimulation (Figure 6D-F). We, however, could not address these findings for rat erythroblasts, because we were unable to generate a synchronized rat proerythroblast culture. Because we found erythroblasts in the rat BM to expand and express increased levels of β-catenin in response to anemia, inhibition of GSK3β may perturb erythroblast formation in a β-catenin–independent fashion.34
CHIR99021 hampers expansion and differentiation of cultured human EBs. (A) Examination of β-catenin protein levels in human EBs and HEK293T cells by western blot in response to 24-hour stimulation with either 10 or 100 ng/mL Wnt3a or 1 or 10 μM CHIR99021. (B) Relative β-catenin protein levels as defined by western blot quantified for EBs and (C) for HEK 293T cells. (D) Representative flow cytometry plots of erythroid differentiation, using CD71 and CD235a cell surface staining at t = 0 and 5 days after stimulation with CHIR99021 (10 μM) compared with control (DMSO)-treated cells. (E) Quantification of EB cell viability, as determined by cell counts, 5 days after stimulating cells with DMSO, or 1 μM or 10 μM CHIR99021 (n = 3; Student t test for DMSO vs 10 μM ChiR99021, ∗P = 0.049). (F) Calculated fold change in CD71−CD235a−, CD71+CD235a−, CD71+CD235a+, and CD71−CD235a+ defined cell stages of EB development, using CD71 and CD235a cell surface staining, 5 days after stimulating cells with 1 or 10 μM CHIR99021 as compared with control, treated cells with DMSO (n = 3; two-way ANOVA; ctrl vs 1 μM CHIR99021, P < .0001; and ctrl vs 10 μM CHIR99021, P < .0001).
CHIR99021 hampers expansion and differentiation of cultured human EBs. (A) Examination of β-catenin protein levels in human EBs and HEK293T cells by western blot in response to 24-hour stimulation with either 10 or 100 ng/mL Wnt3a or 1 or 10 μM CHIR99021. (B) Relative β-catenin protein levels as defined by western blot quantified for EBs and (C) for HEK 293T cells. (D) Representative flow cytometry plots of erythroid differentiation, using CD71 and CD235a cell surface staining at t = 0 and 5 days after stimulation with CHIR99021 (10 μM) compared with control (DMSO)-treated cells. (E) Quantification of EB cell viability, as determined by cell counts, 5 days after stimulating cells with DMSO, or 1 μM or 10 μM CHIR99021 (n = 3; Student t test for DMSO vs 10 μM ChiR99021, ∗P = 0.049). (F) Calculated fold change in CD71−CD235a−, CD71+CD235a−, CD71+CD235a+, and CD71−CD235a+ defined cell stages of EB development, using CD71 and CD235a cell surface staining, 5 days after stimulating cells with 1 or 10 μM CHIR99021 as compared with control, treated cells with DMSO (n = 3; two-way ANOVA; ctrl vs 1 μM CHIR99021, P < .0001; and ctrl vs 10 μM CHIR99021, P < .0001).
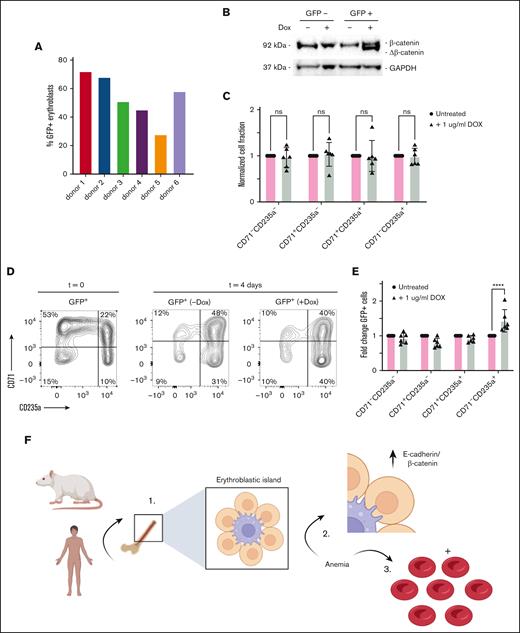
β-catenin signaling stimulates erythroblast differentiation
Next, we investigated the role of β-catenin during expansion and differentiation of human erythroblasts more directly, by having them express a lentivirus containing green fluorescent protein (GFP) and a doxycycline-inducible, stable Δ45β-catenin mutant.35 Lentiviral transduction of CD34+ HSPCs generated mixed (pro)erythroblast cell cultures comprising ∼20% to 80% GFP-marked transduced and nontransduced cells (Figure 7A). Expression of Δ45β-catenin was confirmed after doxycycline stimulation for 24 hours, as shown by a second slightly smaller protein band that appeared upon western blot analysis for β-catenin (Figure 7B). Synchronized (un)transduced lentiviral CD71+CD235a− proerythroblast cultures were examined upon doxycycline treatment for 5 days. Doxycycline treatment did not affect erythroblast expansion or differentiation of untransduced control cells (Figure 7C). In contrast, expression of stable β-catenin significantly enhanced erythroblast differentiation, as indicated by increased GFP+CD71−CD235a+ erythroblast formation with ∼1.5-fold, as compared with untransduced cells obtained from the doxycycline-stimulated mixed cell cultures (Figure 7D-E). Thus, because expression of stable β-catenin enhanced erythroid differentiation, its upregulation in rat erythroblasts by E-cadherin may support stress erythropoiesis to stimulate erythroblast differentiation (Figure 7F).
β-catenin promotes human EB differentiation. (A) Percentage of GFP+ EBs after Δ45β-catenin transduction in each of the 6 donors shown in Figure 6. (B) Western blot analysis of Δ45β-catenin expression in human EBs 24 hours after doxycycline (Dox) treatment. (C) Quantification of erythroid differentiation in response to Dox treatment compared with PBS-treated control cells, as determined by flow cytometry, revealing treatment with Dox (gray bars) not to alter the differentiation of EBs (n = 6; two-way ANOVA). (D) Representative flow cytometry plots of erythroid differentiation, using CD71 and CD235a cell surface staining, gated for GFP+ marked lentiviral transduced cells, in response to Dox-inducible Δ45β-catenin expression, as compared with untreated control conditions. (E) Quantification of flow cytometry data, representing fold change of GFP+ cells in CD71−CD235a−-, CD71+CD235a−-, CD71+CD235a+-, and CD71−CD235a+-defined cell stages of EB development, in untreated conditions (pink bars) or stimulated with Dox (gray bars) (n = 6; two-way ANOVA, ∗∗∗∗P < .0001). ns, not significant. (F) Graphical model displaying conserved expression of E-cadherin and β-catenin in human and rat erythroblatst, which upregulation in response to anemia may stimulate erythropoiesis.
β-catenin promotes human EB differentiation. (A) Percentage of GFP+ EBs after Δ45β-catenin transduction in each of the 6 donors shown in Figure 6. (B) Western blot analysis of Δ45β-catenin expression in human EBs 24 hours after doxycycline (Dox) treatment. (C) Quantification of erythroid differentiation in response to Dox treatment compared with PBS-treated control cells, as determined by flow cytometry, revealing treatment with Dox (gray bars) not to alter the differentiation of EBs (n = 6; two-way ANOVA). (D) Representative flow cytometry plots of erythroid differentiation, using CD71 and CD235a cell surface staining, gated for GFP+ marked lentiviral transduced cells, in response to Dox-inducible Δ45β-catenin expression, as compared with untreated control conditions. (E) Quantification of flow cytometry data, representing fold change of GFP+ cells in CD71−CD235a−-, CD71+CD235a−-, CD71+CD235a+-, and CD71−CD235a+-defined cell stages of EB development, in untreated conditions (pink bars) or stimulated with Dox (gray bars) (n = 6; two-way ANOVA, ∗∗∗∗P < .0001). ns, not significant. (F) Graphical model displaying conserved expression of E-cadherin and β-catenin in human and rat erythroblatst, which upregulation in response to anemia may stimulate erythropoiesis.
Discussion
Here, we revealed that E-cadherin in the rat BM is mostly expressed by erythroblasts, as also seen in humans. In the rat BM, E-cadherin marks erythroblasts in clusters that are reminiscent of erythroblastic islands. Expansion of erythroid progenitors is dependent on SCF that is provided as membrane bound factor on stromal cells of the BM.36 During their differentiation, erythroblasts migrate from the stromal cell to the central macrophage to form erythroblastic islands.26 Hence, E-cadherin predominantly marks erythroblastic islands in the rat BM similar to what is observed in the human BM.27 This is in accordance with the strongest staining on polychromatic erythroblasts (Figure 1C).
When adhered to VCAM-1 coating, cultured human erythroblasts were found to cluster and revealed E-cadherin and β-catenin to mostly reside on the cell membrane between cells. However, most adhered erythroblasts clustered sporadically (supplemental Figure 6A). It, therefore, remains to be defined whether E-cadherin requires additional niche factors, which were lacking in vitro, to stimulate cell-to-cell adhesion between erythroblasts, as seen for epithelial cells. Loss of E-cadherin did not seem to hamper proliferation or differentiation of cultured human erythroblasts. In mice, erythroblasts lack E-cadherin as well. Together, this may indicate that E-cadherin expression is not intrinsically required to drive erythropoiesis.23 We identified E-cadherin to be conserved in human and rat erythropoiesis, both of which, in contrast to mice, mostly execute stress erythropoiesis in the BM.23 E-cadherin may, therefore, instead, have an important role in the control of red blood cell formation during stress erythropoiesis in response to anemia. In line with this notion, we identified erythroblasts in the rat BM to expand and upregulate E-cadherin expression in response to anemia. However, erythroblasts that were generated by extramedullary hematopoiesis in the rat spleen also express E-cadherin. E-cadherin expression is, therefore, not restricted to the BM environment but rather seems conserved between rats and humans throughout erythropoiesis.
The role of E-cadherin in stress erythropoiesis remains to be defined, but because E-cadherin stabilizes β-catenin in cultured human erythroblasts, the regulation of E-cadherin may underly the upregulation of β-catenin in erythroblasts in rat BM in response to anemia. Enhanced E-cadherin/β-catenin signaling, as indicated by the increased cytoplasmic/nuclear localization of β-catenin in anemic rat BM, accordingly, may support stress erythropoiesis. Mechanical cues, such as cell tension, which may develop during tissue remodeling of the BM, have been shown to trigger E-cadherin–dependent nuclear translocation of β-catenin and subsequent signaling in epithelia.7,8 Accordingly, active β-catenin signaling may stimulate stress erythropoiesis by promoting erythroblast differentiation, as demonstrated in vitro in human erythroblasts upon expression of a stable β-catenin variant. Interestingly, mimicking Wnt/β-catenin signaling, by inhibiting the GSK3β activity with CHIR99012, reduced erythroblast differentiation and survival. CHIR99021 is considered to be a highly selective GSK3β inhibitor (50% inhibitory concentrations of 6.7 and 10 nM for GSK3β and GSK3α, respectively), exhibiting up to 10 000-fold selectivity for GSK3 over closely related protein kinases.37 We, therefore, anticipate 10 μM CHIR99021 to mainly inhibit GSK3β in human erythroblasts. This is also in line with the required concentration to activate Wnt signaling in primary fibroblasts.38 Canonical Wnt signaling may therefore rather negatively regulate erythropoiesis, which however would not directly affect human erythropoiesis because cultured human erythroblasts poorly respond to Wnt stimulation. Reduced formation and survival of erythroblasts upon inhibition of GSK3β activity may, however also occur independent of β-catenin signaling.34 We therefore suggest that β-catenin stability and signaling is mostly controlled by E-cadherin in human and rat erythroblasts.
Overall, the conserved expression of E-cadherin/β-catenin signaling in human and rat erythropoiesis extends previous findings by providing additional molecular evidence, indicating that rats, as compared with mice, reflect a superior model to study human (stress) erythropoiesis.24 Our findings underscore the need to develop rat models, which are currently lacking, to manipulate stress erythropoiesis in vivo (eg, by interfering with E-cadherin/β-catenin signaling) to increase our understanding of the regulation of stress erythropoiesis in the BM as occurs in humans.
Acknowledgments
The authors acknowledge Eric Sahai for providing the HECD-1 antibody.
This research has been funded by the Landsteiner foundation for blood transfusion research (LSBR) fellowship grant, LSBR 2007F.
Authorship
Contribution: R.A.K., S.A.v.d.M., and M.N. conceived experiments and analyzed data; R.A.K., S.A.v.d.M., H.V., M.D., G.F., M.H., and M.N. conducted experiments; M.v.L., E.v.d.A., and M.N. provided expertise and feedback; R.A.K., S.A.v.d.M., and M.N. wrote the manuscript; and all authors have agreed to publish this manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Micha Nethe, Landsteiner Laboratory, Sanquin Research/Amsterdam University Medical Centers, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: m.nethe@sanquin.nl.
References
Author notes
∗R.A.K. and S.A.v.d.M. contributed equally to this study.
Data are available on request from the corresponding author, Micha Nethe (m.nethe@sanquin.nl).
The full-text version of this article contains a data supplement.


![E-cadherin also stabilizes β-protein expression in rat EBs. (A) Representative flow cytometry plot showing CD90 expression in whole rat BM and rat BM–derived CD90+ cells enriched using microbeads. (B-F) Flow cytometry plots showing His49 (B) CD71 (C), CD11b (D), CD3 (E), and CD45R (F) in rat BM–derived CD90 microbead enriched cells (blue histogram); compared rat whole BM cells positive for these markers used as a positive control (red histogram). (G) Representative microscopy picture of cultured rat CRISPR-edited EBs on day 7 after isolation from the rat BM. Cells were edited either using CRISPR with Cas9 only (left) or with Cas9 and RNPs targeting Cdh1 (right). (H) Flow cytometry plot showing erythroid differentiation, based on His49 and CD71 surface staining, of rat EBs edited using CRISPR with either only Cas9 or Cas9 + Cdh1 guides on day 7 of culturing. (I) Western blot showing E-cadherin and β-catenin protein expression of control (ctrl) EBs or EBs edited using CRISPR for Cdh1 in 3 different rats. Actin was used as a loading control. (J) Quantification of E-cadherin and β-catenin protein expression defined by western blot, as normalized to actin used as input, in rat EBs edited using CRISPR with Cas9 only (ctrl) or Cas9 + Cdh1 guides (n = 3; two-way analysis of variance [ANOVA], E-cadherin ctrl vs Cas9 Cdh1: ∗∗∗∗P < .0001; β-catenin ctrl vs Cas9 Cdh1: ∗P = .0116).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/23/10.1182_bloodadvances.2023010875/2/m_blooda_adv-2023-010875-gr5.jpeg?Expires=1764969943&Signature=Bel-pap3pHpUOyTZ7KSgU0dZ84U3aybYY823GZ~b1a76z2H~UeDJSEFkvoL-wMPWLwLjFwbmcXYg0q9Yic2BF3POIIwb7pZI73qZPIutI7Wz03NoSThQ-lID7a~brEEgMAWp5vYQ6T8szYFjNOmFdQysms09HNaKln4S6bxVkKYKBZo84s8uKIU20J1Cp-YokyHM6L53N8rW1DM~zzs~29pspLNvUwiEd2vypOcUqsOKN8dgdAshHDNhodiF3emTiQrgP0otZ132pyCbUHSUQlVyyL7G1D1Uhm2XMzYmEh6tECk55laP2RIbR71OdKMNZrv5tGzuAi7rr6SxY1V-xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)