TO THE EDITOR:
Standard hydroxyurea treatment for sickle cell anemia (SCA) is often initiated at an oral dose of 15 to 20 mg/kg once daily. In 2014, the Expert Panel Report of the National Heart, Lung, and Blood Institute recommended starting doses of 15 to 20 mg/kg per day for adults and children, with increases of 5 mg/kg per day every 8 weeks “if dose escalation is warranted.”1 However, escalating the dose to the maximum tolerated dose (MTD) vs maintaining a fixed dose has been controversial,2 and relatively little has been published regarding hydroxyurea treatment for infants.3-6
We developed the Hydroxyurea Management in Kids: Intensive vs Stable Dosage Strategies (HUGKISS; NCT03020615), a single-blinded, multi-institutional trial, to determine the feasibility of enrolling, randomizing, and treating very young children with SCA with either a fixed or intensified dose of hydroxyurea. Secondary objectives of HUGKISS were comparisons of the laboratory effects, clinical outcomes, and toxicities from fixed dose vs intensified treatment.
HUGKISS was approved by the 4 centers’ institutional review boards. The study was conducted in accordance with the Declaration of Helsinki. Eligibility criteria were: hemoglobin SS (HbSS) or HbSβ0 thalassemia, aged 9 to 36 months, Hb ≥ 6.0 g/dL, absolute reticulocyte count (ARC) ≥ 80 × 109/L, absolute neutrophil count (ANC) ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L, creatinine and alanine transaminase < 2 × normal upper limit, and no transfusion within 2 months.
Hydroxyurea powder was distributed to clinical centers in prefilled bottles and was reconstituted locally to 100 mg/mL.7 Participants underwent clinical and laboratory assessments every 4 (±2) weeks and were monitored for excessive myelosuppression: ANC < 1.0 × 109/L, platelets < 80 × 109/L, or Hb < 6.0 g/dL (with ARC < 80×109/L). In both treatment arms, participants began hydroxyurea at 20 (±2.5) mg/kg per day, and subsequent dose adjustments were made for growth. No additional dose escalation occurred in the standard arm, but intensive arm doses were escalated by increments of 5 mg/kg per day to a maximum of 35 mg/kg per day, adjusting every 8 weeks to maintain an ANC of 1.5 × 109/L to 3.0 × 109/L. If toxicity occurred, hydroxyurea was temporarily discontinued.
After the first 8 (±2) weeks, participants were randomized to receive standard or intensive therapy (1:1 ratio, stratified based on the clinical center and age) by the data coordinating center. The medical coordinating center and local principal investigators were blinded to treatment allocation, but each local center had an experienced medical provider who had access to treatment allocation, allowing for real-time dose adjustment.
Participants received standard management for SCA,8 including a complete blood count and ARC, at 4-week visits. HbF, chemistry panel, and urinalysis were performed every 20 weeks. Adverse clinical event definitions were those used in the BABY HUG trial.6 Medication adherence was defined by medication possession ratio (MPR; days medication possessed / days medication prescribed × 100).9
Feasibility of a phase 3 trial was defined by successful enrollment (50 patients randomized within a 27-month period) and by having ≥80% of randomized subjects with a ≥80% MPR over the study’s course. Categorical variables were compared using the χ2 test or Fisher exact test, and continuous variables using the 2-sample t test or Mann-Whitney/Wilcoxon test.
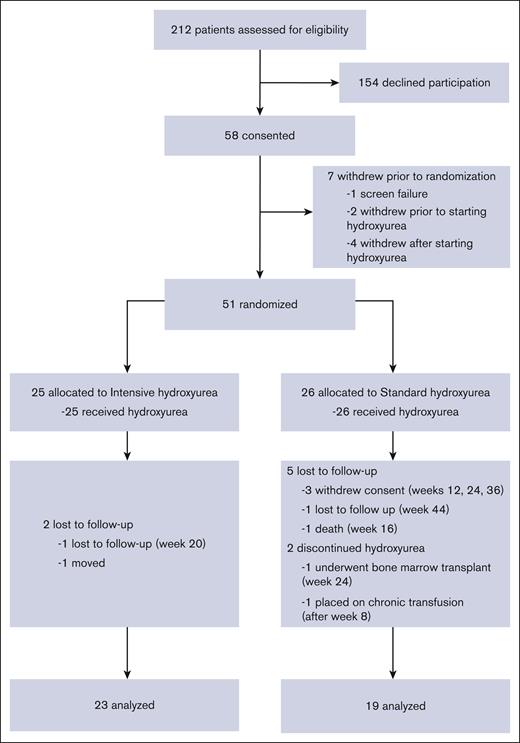
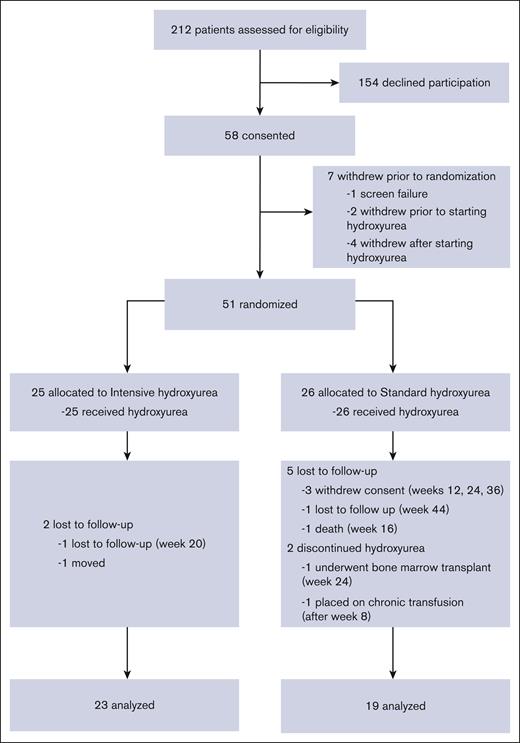
Between May 2017 and May 2019, 58 patients enrolled in the study and 55 began treatment with hydroxyurea (Figure 1). Of these, 51 patients were randomized over 24 months, thereby reaching the targeted accrual rate and number. In total, 25 patients were randomized to intensive hydroxyurea, and 26 to continued standard hydroxyurea. There were no significant differences between the intensive and standard arm subjects regarding age, genotype, sex, growth, or blood counts (supplemental Tables 1 and 2).
CONSORT diagram for HUGKISS trial. Fifty-eight subjects gave consent, 51 were randomized, and 42 completed the study.
CONSORT diagram for HUGKISS trial. Fifty-eight subjects gave consent, 51 were randomized, and 42 completed the study.
Forty-two subjects (23 in the intensive and 19 in the standard arm) completed the 1-year study. Nine subjects (18%) withdrew or were lost to follow-up after randomization (2 in the intensive and 7 in the standard arm). More than 80% of randomized subjects had ≥80% MPR. At exit (Table 1), higher median values for HbF (38.8% vs 26.1%; P = .002), mean corpuscular volume, mean corpuscular Hb, and mean corpuscular Hb concentration and lower median values for ANC and HbS were found with intensive therapy. The median increases from the entry to the exit for HbF, hemoglobin, mean corpuscular volume, and mean corpuscular Hb level were greater in the intensive arm, but the white blood cell count, ANC, ARC, bilirubin, and lactate dehydrogenase levels had greater decreases in that arm. The median hydroxyurea doses (mg/kg per day) at exit were 28.1 (intensive arm) and 19.0 (standard arm).
After randomization, 13 of 25 (52%) in the intensive arm and 6 of 26 (23%) in the standard arm experienced neutropenia toxicity (P = .033). In the intensive arm, the only severe adverse event was thrombocytopenia. In the standard arm, severe adverse events were norovirus/rotavirus, removal of a foreign object, and 1 fatality, which occurred in a 1-year-old patient with HbSS and methicillin-resistant Staphylococcus aureus sepsis (without neutropenia).
Sickle cell disease–related event rates (pain, acute chest syndrome, splenic sequestration, dactylitis, priapism, and unanticipated transfusion) were not significantly different between the 2 arms (supplemental Table 3). Pain event rates per 100 patient-years were 52 in the intensive arm and 96 in the standard arm (P = .13). Adverse event rates unrelated to sickle cell disease were most commonly infection related (667 in the intensive group and 578 in the standard group; P = .21).
After randomization, 13 of 25 patients (52%) in the intensive arm and 6 of 26 patients (23%) in the standard arm had neutropenia toxicity (P = .033), but there was no significant difference in the infection rate.
The attainment of enrollment and follow-up goals in the HUGKISS study indicated that a phase 3 randomized trial is feasible. After 1 year of treatment, the most remarkable difference in response was in HbF, which reached a median of 38.8% in the intensive arm (compared with 26.1% in the standard arm). In addition, the median Hb level in the intensive arm increased by 1.2 g/dL (vs increase of 0.4 g/dL in the standard arm).
However, we acknowledge that increased HbF is not equivalent to clinical efficacy. In the randomized, multicenter, double-blind trial in children (mean age, 4.7 years) with SCA in Uganda, hydroxyurea at a fixed dose (20 mg/kg per day) was compared with dose escalation to reach an Hb level ≥ 9.0 g/dL or HbF ≥ 20%.2 End points were reached in the dose-escalation group in 86% (vs 37% for fixed dose), and this group had fewer pain events, acute chest syndrome episodes, transfusions, and hospitalizations. In other longitudinal cohort studies, greater HbF resulted in decreased hospitalization, pain events, and mortality, and increased intelligence quotient.10-13
Regarding hydroxyurea toxicity, a meta-analysis found that neutropenia was more frequent with MTD dosing.14 We noted increased neutropenia without an increase in significant infection in the intensive dose arm.
In HUGKISS, hydroxyurea treatment began at a median age of 9 months. This early age of initiation is common clinical practice in North America15 but is substantially lower than the age of children in the African MTD trial.2,5 HUGKISS, therefore, may provide a template for future clinical trials in very young children with SCA who are treated with hydroxyurea as frontline therapy.
Our trial had limitations. It was a feasibility study that enrolled a relatively small number of subjects, probably too few to demonstrate differences in clinical events. The 1-year length of treatment may not have allowed adequate time for evaluation of organ function, quality of life, or toxicities. Furthermore, HbF is a surrogate marker, not a clinical effectiveness end point.
We conclude that HUGKISS demonstrates the feasibility of enrolling and treating very young children with SCA with intensive dose hydroxyurea. The potential benefit of greater HbF from early intensive hydroxyurea dosing should be confirmed in larger phase 3 trials that include serial evaluation of organ function, quality of life, and newer antisickling agents.
Acknowledgments: The HUGKISS Investigators thank the following who provided significant effort throughout the conduct of the study, especially Teresa Carr, director of Clinical Research Quality Management and Education; Jason Hodges, director of Clinical Trials Management; Michelle Brignac, manager of Clinical Research Operations; Kristen Ryan; Gail Fortner; Ashely Cooper; Ana Discenza, data entry/management; Pei-Lin Chen, data analyst; Lisa Johnson, nurse case manager; Niccole Pankey, nurse case manager; Nicole Dockery, nurse practitioner; Nathan Gray, physician's assistant, Roynetta Lloyd, nurse practitioner; and Terri Davis, grant coordinator and manuscript facilitator. Finally, the authors especially recognize the enthusiastic participation and the generous time commitment made by the children and families who enrolled in HUGKISS.
This research was supported, in part by the National Institutes of Health grant R34HL127162 (J.H.E. and G.K.), the American Society of Hematology (J.H.E.), and the American Lebanese Syrian Associated Charities at St. Jude Children’s Research Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Society of Hematology, or the American Lebanese Syrian Associated Charities at St. Jude Children’s Research Hospital.
Contribution: J.H.E., W.C.W., G.K., M.B., and J.R. developed the concept and designed the analysis, and analyzed the results; W.C.W., J.H.E., and G.K. drafted the manuscript; J.G. and G.K. performed the statistical analyses; J.H.E., G.K., J.S.H., W.C.W., M.R.D., and J.S.P. interpreted the results; J.H.E., R.C.B., M.A.M., and Z.R.R. enrolled participants, collected and managed data, and helped interpret results; W.C.W., J.H.E., J.S.H., and M.R.D. provided critical reviews; and all authors had access to clinical trial data, agreed to be accountable for all aspects of this analysis, reviewed the final manuscript, and approved the final version for publication.
Conflict-of-interest disclosure: During the conduct of the HUGKISS trial, J.H.E. received research support from Pfizer, Eli Lilly & Co, Forma Therapeutics, Global Blood Therapeutics, the National Institutes of Health, and the American Society of Hematology, and served as a consultant for Daiichi Sankyo, Agios Pharmaceuticals, Emmaus Life Sciences, and Global Blood Therapeutics. W.C.W. served as a consultant for Novartis. Z.R.R. served as a consultant for Terumo Corporation, Celgene, and Novartis. J.S.H. served as a consultant for Global Blood Therapeutics, Forma Therapeutics, and CVS Health. J.H.E. is employed by Agios Pharmaceuticals. R.C.B. is employed by Global Blood Therapeutics. Agios Pharmaceuticals and Global Blood Therapeutics had no role in the design of the HUGKISS study, analysis, or interpretation of the results, or drafting of the manuscript.
The current affiliation for M.R.D. is McMaster University, Hamilton, ON, Canada.
The current affiliation for J.H.E. is Agios Pharmaceuticals, Cambridge, MA.
The current affiliation for R.C.B. is Pfizer Pharmaceuticals, Manhattan, NY.
Correspondence: Winfred C. Wang, Department of Hematology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: winfred.wang@stjude.org.
References
Author notes
∗W.C.W. and J.H.E. contributed equally to the development of this study.
Data are available on request from the corresponding author, Winfred C. Wang (winfred.wang@stjude.org).
The full-text version of this article contains a data supplement.