Abstract
The International Staging Evaluation and Response Criteria Harmonization for Childhood, Adolescent, and Young Adult Hodgkin Lymphoma (SEARCH for CAYAHL) seeks to provide an appropriate, universal differentiation between E-lesions and stage IV extranodal disease in Hodgkin lymphoma (HL). A literature search was performed through the PubMed and Google Scholar databases using the terms “Hodgkin disease,” and “extranodal,” “extralymphatic,” “E lesions,” “E stage,” or “E disease.” Publications were reviewed for the number of participants; median age and age range; diagnostic modalities used for staging; and the definition, incidence, and prognostic significance of E-lesions. Thirty-six articles describing 12 640 patients met the inclusion criteria. Most articles reported staging per the Ann Arbor (72%, 26/36) or Cotswolds modification of the Ann Arbor staging criteria (25%, 9/36), and articles rarely defined E-lesions or disambiguated “extranodal disease.” The overall incidence of E-lesions for patients with stage I-III HL was 11.5% (1330/11 602 unique patients). Available stage-specific incidence analysis of 3888 patients showed a similar incidence of E-lesions in stage II (21.2%) and stage III (21.9%), with E-lesions rarely seen with stage I disease (1.1%). E-lesions likely remain predictive, but we cannot unequivocally conclude that identifying E-lesions in HL imparts prognostic value in the modern era of the more selective use of targeted radiation therapy. A harmonized E-lesion definition was reached based on the available evidence and the consensus of the SEARCH working group. We recommend that this definition of E-lesion be applied in future clinical trials with explicit reporting to confirm the prognostic value of E-lesions.
Introduction
Hodgkin lymphoma (HL) is a relatively rare cancer with excellent cure rates; but because of differences in staging criteria and risk stratification, outcomes cannot be directly compared between clinical trials.1-6 HL primarily affects the lymph nodes and the spleen but extranodal involvement does occur. The 1965 Rye Classification stated that involvement of the bone marrow, lung parenchyma, pleura, liver, bone, skin, kidneys, gastrointestinal tract, or any tissue outside of the lymphatic system was considered stage IV disease.7 Musshoff8 first noted that extralymphatic disease contiguous with a region of lymphatic involvement treated with intensive radiotherapy alone does not have the same poor prognostic implication as widespread extralymphatic disease; he divided the existing Rye Classification group IV into stage IV per continuitatem and stage IV per disseminationem.8 This led to proposals of the subclassification of “EN”8 or subscript “E”9 for extralymphatic disease within stages I, II, and III HL; “E-lesions” were initially defined as proximal or contiguous extranodal extensions that did not require modification of the nodal irradiation field or dose and did not have negative prognostic implications.10 These findings were incorporated into a new classification system9 and disease staging procedures11 defined at the Ann Arbor meeting in 1971 that were rapidly and widely adopted for adult and pediatric HL.10
Over time, this definition has continued to be modified and studied. In 1982, Yarnold showed that disease that is contiguous but too large to meet this radiotherapy definition, is associated with a poor prognosis (possibly worse than other stage IV without this feature).12 In 1984, the complexity of staging E-lesions was demonstrated when 14 internationally recognized HL centers disagreed on the classification (E-lesion vs stage IV) of 4 representative cases of nearby but not contiguous extranodal disease of the lung or bone; 2 cases were complete stalemates.13 The subsequent 1989 Cotswolds modification of the Ann Arbor staging system incorporated success with chemotherapy in early unfavorable disease, improved imaging diagnostics (computed tomography [CT] and magnetic resonance imaging [MRI]), and prognostic advancements (bulk disease and number of involved nodal sites as risk factors); the E-lesion definition was retained with different examples of local extralymphatic organ involvement provided.10
In 2011, a dramatic change for adult HL staging was made in Lugano, Switzerland in response to outcomes with the widespread use of chemotherapy and combined modality therapy (CMT).14 This update deemed “E” subclassification no longer clinically relevant for advanced-stage disease (stages IIx, III, and IV); the “E” distinction remains meaningful in cases of limited extranodal disease in the absence of nodal involvement (stage IE) or for patients with stage II disease and direct extension to a nonnodal site (stage IIE).14 Notably, these modifications have not been applied to the pediatric population.
Agreement regarding E-lesions vs disseminated extranodal disease is challenging and yet essential for both individual patients and the interpretation of clinical trials. Central imaging reviews for large cooperative group studies have shown that discrepancies in the identification of E-lesions affect both the stage and potential treatment for individual patients.15,16 Further emphasizing the importance of appropriate identification of E-lesions, the presence of extranodal disease is used as a poor prognostic criterion to identify unfavorable early-stage disease by the adult German Hodgkin Study Group (GHSG)17,18 and in the determination of treatment groups in the EuroNet Pediatric Hodgkin Lymphoma (PHL) C1 and C2 trials.19,20 Inappropriate patient risk assignment may lead to inappropriate conclusions drawn about the effectiveness of a given treatment regimen. The distinction between patients with local (E-lesions) or disseminated (stage IV) extranodal HL is often unclear in the literature, making it challenging to determine the prognostic value of either group.
The International Staging Evaluation and Response Criteria Harmonization for Childhood, Adolescent, and Young Adult HL (SEARCH for CAYAHL) initiative provides a platform for global collaboration to improve the cure rates of children with HL by achieving consensus between consortia.21 As part of this effort, we conducted a systematic review of the literature on the reporting of E-lesions in patients with HL. Here, we propose a universally acceptable and harmonized definition for E-lesions in pediatric patients with HL.
Methods
Search strategy
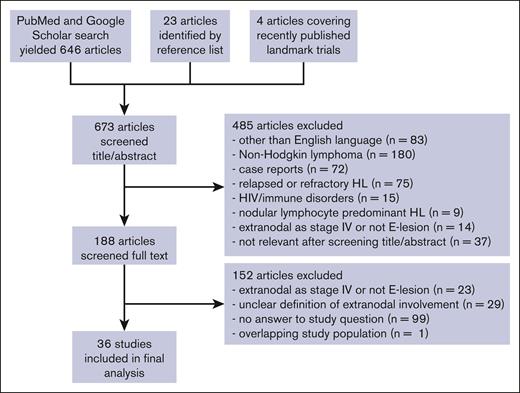
A systematic literature search was performed through the PubMed and Google Scholar databases for articles on E-lesions in patients with HL for articles published from 1 January 1960 to 1 August 2022 by using the combination of free-text keywords: “Hodgkin disease” [MeSH] AND (extranodal OR extralymphatic OR “E lesions” OR “E stage” OR “E disease”; Figure 1). Two authors (E.A.M.Z. and A.B.) independently screened each abstract per screening criteria for articles published from January 1960 to December 2016; if no exclusion criteria were met, the full manuscript was retrieved and reviewed. Reasons for exclusion were recorded. Reference lists were reviewed for additional candidate papers. A third author (J.Z.) repeated this process using the same search terms to screen articles published from 1 January 2017 to 1 August 2022. Although they did not meet the search criteria, several additional contemporary landmark trials (EuroNet PHL-C1, Hodgkin Lymphoma High Risk protocol 13, and GHSG Hodgkin's disease [HD]17) were also reviewed for inclusion. The date of the last database search was 6 September 2022. The manuscript was amended to add 1 significant recent publication that was published while this manuscript was under review. A fourth author (J.E.F.) reviewed articles as needed to determine appropriateness of inclusion or clarify interpretation.
Flowchart of literature search. Diagram representing information flow in the review of literature describing E-lesions in studies of patients with Hodgkin lymphoma.
Flowchart of literature search. Diagram representing information flow in the review of literature describing E-lesions in studies of patients with Hodgkin lymphoma.
Inclusion and exclusion criteria
Articles met criteria for inclusion if they offered information on the incidence or prognostic significance of E-lesions in HL and gave an explicit definition of E-lesions or reference staging per the Ann Arbor classification, the Cotswold modification, or Lugano criteria, or treatment protocols with E-lesion definitions. Acceptable examples of staging classifications and the latest international treatment protocols are listed in Tables 1-2.19,20 We included articles without explicit definitions of extranodal involvement if they were limited to stages I-III, as the term “extranodal” could not be referring to stage IV disease in these patients. All patient ages were included, because many articles combined both pediatric and adult patients.
Articles were excluded if they were case reports or written in languages other than English, or if the study population did not include frontline classical HL (ie, nodular lymphocyte predominant HL, only relapsed or refractory HL, or patients with HIV or other immune disorders). We also excluded articles with unclear or incomplete definitions of extranodal or extralymphatic involvement. This encompassed the exclusion of articles that did not distinguish between E-lesions and stage IV extranodal involvement in their descriptive tables or analyses.
Outcomes
Two authors (E.A.M.Z. and A.B.) independently extracted data from identified articles published from January 1960 through December 2016; a third author (J.Z.) extracted data from articles published from 1 January 2017 through 1 August 2022. The following data were extracted from each article and recorded in a spreadsheet: the number of participants, median age and age range of participants at diagnosis, type of diagnostic tool(s) used for staging, the incidence of E-lesions, treatment, and the prognostic value of E-lesions. One author (J.Z.) manually verified all extracted data on a second occasion. Overall and stage-specific incidences of E-lesions were calculated. Pairs of articles with overlapping patient populations were individually considered for inclusion in incidence calculations; attempts were made to maximize sample size and minimize double counting. Incidence calculations were repeated without articles that used risk-based inclusion criteria (with exception of disease stage) to evaluate for selection bias.
Results
Study selection
Our literature search produced 646 unique articles; 23 additional articles were identified within the reference lists of these publications, and 4 articles reporting on contemporary landmark trials were also reviewed. After screening the abstracts, 485 were excluded per our screening criteria. We reviewed 188 full-text articles and excluded an additional 152 publications (Figure 1). In total, 36 articles were included in our final incidence analysis (Table 3). Several articles contained overlapping patient populations. If the articles provided meaningfully distinct incidence and/or prognostic information, they were both included and collated under the same study number.
The first 2 articles with overlapping populations were by Levi et al22 and Wiernik and Slawson.23 Wiernik and Slawson reported a follow-up of the patient population described by Levi et al and also added unique patients to the group. The only stage-specific E-lesion incidence data available was from the Levi et al study. To avoid double counting patients, only the larger population reported by Wiernik was used for the overall incidence calculation. Both articles were used in the prognosis analysis because they provided unique outcomes analyses. Another article by Levi24 was excluded because it did not include unique E-lesion incidence or prognostic data.
A second instance of overlapping populations was encountered when Hoppe25 and Crnkovich26 described Stanford HL outcomes during a similar time period. The Hoppe article reported on patients with stage I-II HL. The Crnkovich article appeared to include a longer-term follow-up of a subset (stage IIB) of the same patients. The only stage-specific E-lesion incidence data available was from the Crnkovich article. To avoid double counting patients, only the larger population reported by Hoppe was used for the overall incidence calculation. Both articles were used in the prognosis analysis because they provided unique outcomes analyses.
A third set of overlapping populations studied by Loeffler et al27,28 reported unique prognostic data from patients treated on the German HD1 study. The only stage-specific data available were from the 1989 article. To avoid double counting of patients, we used the 1997 article (with the larger population) in the overall incidence calculation.
The final set of overlapping populations were patients treated on the German HD5 treatment protocol described by Franklin18 and Sieber.29 Both articles were included because they each described discrete prognostic data. To avoid double counting patients, only the larger population reported by Sieber was used for the overall incidence calculation.
Table 3 denotes which of the overlapping studies were used for incidence calculations. Because the presence of E-lesions was used to determine EuroNet PHL-C1 treatment groups, the stage-specific incidence data from the separate articles30,31 were interpreted as a single study population. All articles included in our final analysis were published between 1977 and 2022.
Participant characteristics
In the 36 articles analyzed, 12 640 patients (aged 2-88 years) with stage I-III HL were included. Five articles included solely pediatric patients (aged 0-18 years),15,30-33 19 articles included children and adults,18,22,27-29,34-47 7 included only adults,48-54 and 5 articles23,25,26,55,56 provided limited information about patient age.
Definition of E-Lesions
We observed that 26 articles used the Ann Arbor criteria for staging classification,12,15,18,22,23,25-29,32,34-41,45-47,51,53-56 9 articles used the Cotswolds revision of the Ann Arbor staging classification,30,33,42-44,48-50 and 1 study used the Lugano criteria.52 Fourteen studies provided some description of E-lesion location.12,22,23,25,26,28,32-35,39,45,46,53,56
Diagnostic tools
A wide variety of imaging modalities were used in these studies because of the evolution of imaging technology over time. Studies used a combination of X-ray imaging, CT (or focal plane tomography), nuclear medicine (liver spleen scan, positron emission tomography [PET], and bone scan), ultrasound, and MRI, in addition to surgical sampling (biopsies, laparotomies, and splenectomies) for staging. Several studies in our analysis concluded that CT scanning was more sensitive than X-ray imaging for the detection of E-lesions of the lung parenchyma, pleura, pericardium, and chest wall.57-61 Gaudio et al noted more extranodal localizations were found with the use of PET/CT than with contrast-enhanced CT for staging.45 One pediatric article reported their findings with full-body MRI with diffusion-weighted imaging for staging in 50 pediatric patients with HL (aged 5-19 years) enrolled on Euronet PHL-C1 or PHL-LP1 trials and concluded that the technology was not acceptably equivalent to PET-CT for staging purposes (Ann Arbor staging concordant in 78%, 39/50),62 including at extranodal sites (28% discordance rate; 95% confidence interval exact, 17.8-40.3).62
Incidence of E-lesions by stage
In the 36 articles analyzed, 1330 of the 11 602 unique patients (12.4%) had an E-lesion (results summarized in Table 4). Sixteen articles15,22,26,28,30,31,33,38,43,44,46,47,52,53,55,56 encompassing 3888 patients provided stage-specific E-lesion incidence data. E-lesions were rarely present in stage I disease, affecting 1.1% (4/365) of patients (range, 0%-6%). Available data did not show a difference in incidence between IA and IB subgroups. E-lesions were similarly prevalent in stage II and III disease, affecting 21.2% (560/2646) of patients (range, 0%-53%) and 21.9% (192/877) of patients (range, 4%-33%), respectively. Overall, there were notably more E-lesions in patients with stage IIB disease (32.4% [284/877]; range, 0%-53%) than in those with stage IIA disease (15.6% [252/1618]; range, 3%-28%). A similar relationship was seen with more E-lesions in patients with stage IIIB disease (26.0% [90/346]; range, 21%-27%) than in those with stage IIIA disease (18.8% [79/420]; range, 4%-33%).
Nine studies18,27,36,40,44,47,49,53,54 used risk-based inclusion criteria, including a combination of stage, bulk disease (mediastinal or other sites), extranodal disease, B-symptoms, massive spleen involvement, or GHSG unfavorable early-stage disease. When these studies were excluded, the overall and stage-specific incidence of E-lesions were relatively unchanged (stage I-III: 12.5% [982/7848]; stage I: 1.3%, stage II: 21.7%, stage III: 22.5%).
Treatment and prognosis
Twenty-two articles, encompassing 5836 patients, examined the prognostic implication of E-lesions (Table 5). Eight articles18,29,33,34,42,44,48,49 (3622 patients) found the presence of E-lesions to be predictive of poorer outcomes, including relapse and survival metrics. All patients in this subset received CMT, except patients in 2 studies33,42 in which response-adapted radiotherapy was also used. The interim report of the prospective, nonrandomized German Society of Pediatric Oncology and Hematology Hodgkin Lymphoma Trial 95 examining response-adapted involved field radiotherapy in pediatric early-stage HL found E-lesions to be an independent risk factor for both progressive disease (P < .002) and relapse (P < .002) at a median follow-up of 38 months.33 Two articles18,29 similarly commented on the association of E-lesions and poor relapse outcomes in the HD5 trial, which evaluated different CMT regimens in patients with stage I-II HL with GHSG risk factors, or with stage III HL. E-lesion also trended toward providing additional prognostic value beyond the International Prognostic Score for disease-free survival, reaching statistical significance for stage IIB-IIIA HL; the authors speculate this may be related to misclassification between the sometimes subtle distinction between E-lesion and stage IV disease.18 Another study comparing cooperative group risk criteria used pooled outcomes from H8 and H9 randomized trials found E-lesions to be associated with significantly worse overall survival at 42 months in patients with stage I-II disease largely treated with CMT (multivariate, stage–adjusted prognostic index, relative risk [RR], 1.2; P = .008), but the authors hypothesized that bulky mediastinal disease may be the driver of the poor outcomes in these patients.42
Nine articles,25-28,35-38,50 encompassing 1345 patients, reported that the presence of E-lesions did not influence relapse or survival outcomes. Patients in this subgroup received either radiation monotherapy or CMT. Leslie et al35 noted the frequent cooccurrence of B-symptoms (11/25) and bulk disease (13/25) in those with E-lesions; when patients were analyzed by mediastinal size, E-lesions did not appear to influence freedom from relapse or 10-year survival. Leopold et al36 retrospectively evaluated 92 patients with stage IA-IIB disease with large mediastinal adenopathy that was treated with radiation monotherapy or CMT; the substantial subset of patients with E-lesions (29%) did not have significantly different 12-year relapse rate or overall survival.
Five articles,22,23,39,41,56 encompassing 869 patients, described nuanced prognostic relationships between study groups. Hodgson et al39 noted that in patients with stage I-II HL treated with CMT, the site of extranodal extension determined the impact on disease outcomes; patients with chest-wall E-lesions but not lung E-lesions had poorer cause-specific and overall survival compared with those with only nodal disease. Three articles22,23,56 reported that the unfavorable association between E-lesions and remission duration, disease-free survival, and OS were negated when CMT was administered instead of radiation alone; authors note that many of the patients with E-lesions also had large mediastinal masses that may have been the driver of their poor outcomes. Vassilakopoulos et al41 found that the presence of E-lesions was an independent predictor of poorer 10-year failure-free survival in patients with stage IA-IIA HL treated with adriamycin(epirubicin)/bleomycin/vinblastine/dacarbazine and radiation, but the statistical significance did not hold once patients treated with mustine, oncovin, prednison, procarbazin (MOPP) were also included; E-lesions were not associated with 10-year overall survival in either group.
Pediatric studies
Five articles included only pediatric patients, with a maximum patient age of 18 years.15,30-33 All studies included patients with stage I-III HL. The incidence of E-lesions was 20.5% [604/2947]; range, 5% to 30%. Two of these articles15,32 used the Ann Arbor classification for staging, whereas 3 articles31,33 used the Cotswold modification; all 5 provided a specific definition of E-lesions in their methods or protocols. Four articles15,31,33 used CT as a diagnostic tool for all patients, and 1 study32 used CT for some patients. One study33 included information about the impact of E-lesions on prognosis, which concluded that E-lesions were a negative prognostic risk factor for progressive disease and relapse after a median follow-up period of 38 months.
Discussion
Our review of the literature has shown that the term “extranodal involvement” is frequently ambiguously used to refer to either locally contiguous disease or disseminated involvement. For example, Dieckmann et al15 clarifies that “extralymphatic involvement” included multiple scenarios: contiguous spread, noncontiguous spread, involvement of multiple sites, and diffuse involvement. Care should be taken in future manuscripts to carefully define terminology used to allow for interpretation of the significance of extension to an extranodal site. Although the appropriate use of “extranodal involvement” or “extralymphatic involvement” can be inferred when working within the boundaries of stage I-III disease, the inclusion of stage IV disease creates ambiguity. The term “E-lesion” or “extranodal extension” should be used within manuscripts (including tables) when referring to localized (contiguous) disease.
E-lesions were similarly prevalent in stage II and III, and more often seen with B symptoms; E-lesions were rarely seen in stage I disease. Although E-lesions can occur in stage IV disease, nonspecific language prevented further investigation. The inconsistent prognostic implications of E-lesions were not clearly explained by treatment era, therapies received, or risk-based inclusion criteria, although a combination of these factors could obscure their effects. The largest studies (and overwhelming cumulative population), with presumably the greatest power to detect an effect, showed that E-lesions were a negative prognostic factor.18,29,33,42 The apparent trend of greater incidence of E-lesions in patients with B symptoms and the previously described associations with E-lesions and mediastinal and/or bulk disease22,23,35,42,56 further support the need for careful description and control of other known risk factors (eg, peripheral vs mediastinal bulk) to elucidate the prognostic influence of E-lesions in the current treatment era.
It is important to note the limitations of including studies across a wide range of diagnostic and therapeutic technological eras. As imaging technologies evolved, the sensitivity of detecting small areas of extranodal extension increased. CT scans were more sensitive than X-ray imaging for detecting E-lesions.57-61 The limited imaging obtained in many of the early studies would be considered insufficient to adequately stage patients today. Current CT, MRI, and PET modalities are all able to detect E-lesions, and a combination should be used to image the neck, chest, abdomen, and pelvis for staging. Using studies with risk-based HL populations could introduce selection bias, but their absence did not appreciably affect incidence results, and there were no overt trends among prognostic outcomes.
A global consensus has not yet been reached regarding the prognostic influence of E-lesions. Historically, German consortia have been the only groups that use E-lesions in risk stratification,17 but efforts toward harmonization have led to the recent use of E-lesions in treatment group/level stratification in the EuroNet PHL-C1 and C2 trials, with slight modifications in their E-lesion definitions between trials.19,20 The presence of bulk disease and/or mediastinal masses are likely covariates that may obscure or inappropriately give prognostic influence of E-lesions. The results of this systematic review demonstrate that E-lesions likely remain predictive, but data from large consortium trials and improved granularity regarding location of E-lesions are needed to confirm this in the modern era of response-adapted therapy. Therefore, the SEARCH for CAYAHL working group proposes an update to the Cotswold-modified Ann Arbor staging criteria to reflect current practices for pediatric HL and to allow for prospective study of harmonized criteria. In conclusion, we propose a definition that an “E-lesion” is a contiguous infiltration of a lymph node mass into extralymphatic structures or organs (eg, lung or bone).30 Pleural and pericardial involvement should be considered E-lesions, but a pleural or pericardial effusion alone is not considered an “E-lesion.” Disease that extends beyond the lymphatic system without adjacent lymphatic involvement is considered stage IV; liver or bone marrow involvement is always considered stage IV disease. Unlike the adult Lugano criteria,14 E-lesions remain relevant in pediatric patients with stage I, II, and III disease. We recommend that this description of E-lesions should be consistently applied for pediatric patients with HL, with explicit reporting of the presence and location of E-lesions to confirm the prognostic value of E-lesions in the current treatment era.
Acknowledgments
J.E.F. receives support from the American Lebanese Syrian Associated Charities (ALSAC) and the Lymphoma Research Foundation.
Authorship
Contribution: E.A.M.Z. and J.Z. prepared the manuscript with contributions from A.B. and J.E.F., and all authors were involved in the preparation and editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the SEARCH working group members appears in “Appendix.”
Correspondence: Auke Beishuizen, Princess Máxima Center for Pediatric Oncology, Heidelberglaan 25, 3584 CS Utrecht, The Netherlands; e-mail: a.beishuizen-2@prinsesmaximacentrum.nl.
Appendix
The SEARCH working group members are Andishe Attarbaschi, Brad Hoppe, Reena Pabari, Jenny Belsky, Scott Howard, Monica Palese, Auke Beishuizen, Tatum Johnson, Neeta Pandit-Taskar, Nickhill Bhakta, Kara Kelly, Angela Punnett, Steve Cho, Regine Kluge, Jennifer Seelisch, Pedro de Alarcon, Lars Kurch, Dietrich Stoevesandt, Ute (Karin) Dieckmann, Hollie Lai, Paul Thacker, Richard Drachtman, Michael Link, Stephen Voss, Matt Ehrhardt, Lianna Marks, Jamie Zeal, Jamie Flerlage, Christine Mauz-Koerholz, Thomas Georgi, and Kathleen McCarten.
References
Author notes
∗E.A.M.Z. and J.Z. are joint first authors.
†J.E.F. and A.B. are joint senior authors.