Key Points
VOE requiring medical care are significantly reduced in patients with SCD after allogeneic transplantation.
The reduction in VOE improves over time, especially beyond 1 year after allogeneic transplantation.
Abstract
Hematopoietic stem cell transplantation (HSCT) is potentially curative for patients with sickle cell disease (SCD). Patients with stable donor engraftment after allogeneic HSCT generally do not experience SCD-related complications; however, there are no published data specifically reporting the change in vaso-occlusive events (VOE) after HSCT. Data regarding the number of VOEs requiring medical attention in the 2 years before allogeneic HSCT were compared with the number of VOEs in the 2 years (0-12 months and 12-24 months) after allogeneic HSCT in patients with SCD. One-hundred sixty-three patients with SCD underwent allogeneic HSCT between 2005 and 2019. The average age at the time of HSCT was 21 years (range, 7 months – 64 years). Most patients underwent nonmyeloablative conditioning (75% [N = 123]) and had a matched sibling donor (72% [N = 118]). The mean number of VOEs was reduced from 5.6 (range, 0-52) in the 2 years before HSCT to 0.9 (range, 0-12) in the 2 years after HSCT (P < .001). Among the post-HSCT events, VOE was more frequent during the first 12 months (0.8 [range, 0-12]) than at 12 to 24 months after HSCT (0.1 [range, 0-8) (P < .001)). In patients who had graft rejection (12%, N = 20), VOEs were reduced from 6.6 (range, 0-24) before HSCT to 1.1 (range, 0-6) and 0.8 (range, 0-8) at 0 to 12 months and 12 to 24 months after HSCT, respectively (P < .001). VOEs requiring medical care were significantly reduced after allogeneic HSCT for patients with SCD. These data will inform the development of novel autologous HSCT gene therapy approaches.
Introduction
Sickle cell disease (SCD) is the most common inherited hemoglobinopathy worldwide and is associated with substantial morbidity and premature mortality.1,2 Pain is the most prominent aspect of the disease and is characterized by recurrent acute painful vaso-occlusive events (VOE) that become more frequent and severe over the lifetime of the individual. Hydroxyurea, chronic blood transfusions, and recently approved therapies may decrease VOE and other SCD-associated complications. However, these therapies do not eliminate these complications and must be continued indefinitely. Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only available curative option for patients with SCD, although autologous gene therapy may soon become an alternative curative therapy.
Patients with stable donor engraftment after allogeneic HSCT generally do not experience SCD-related complications and have stabilization or even improvement in end organ pathology.3,4 Patients are reported to have a lack of painful or other clinical events after HSCT, but data specifically reporting changes in pain that require medical attention after HSCT are lacking. The only published study that examined pain events after allogeneic HSCT reported the presence of a subgroup of patients who continued to receive opioids for chronic pain 12 months after HSCT.5 This finding suggesting that while acute pain events may be resolved, chronic pain may persist.
Preliminary data after autologous gene therapy for SCD suggest a significant reduction and/or elimination of SCD-related clinical events after gene therapy.6,7 To adequately inform and assess the results of newer autologous HSCT gene therapy approaches, published data comparing VOE before and after allogeneic HSCT are needed. Therefore, our objective was to evaluate the changes in VOE before and after allogeneic HSCT with at least 2 years of follow-up.
Methods
The selection criteria for the study population included patients with SCD who underwent allogeneic HSCT at the National Institutes of Health (NIH) or Children’s National Hospital (CNH). Eligible patients were enrolled in separate institutional and ethics review board-approved studies (NIH: NCT00061568, NCT02105766, NCT03077542, and NCT00977691; CNH: NCT02165007, NCT03587272, NCT00745420, NCT02766465, NCT03263559, NCT04018937, and NCT00029380). All participants provided informed consent before HSCT enrollment. Medical record data were gathered after obtaining approval from the institutional review board to study VOE events before and after allogeneic HSCT.
Electronic medical records were used to determine the number of VOE events in the 2 years before HSCT and in the 2 years (0-12 months and 12-24 months) after HSCT. The outcome measure, VOE, was prespecified and clearly defined as follows: vaso-occlusive pain episode requiring a ≥24 -hour hospital or emergency room (ER) observation unit visit or at least 2 visits to a day unit or ER over 72 hours with both visits requiring intravenous (IV) treatment, acute chest syndrome (ACS) requiring oxygen or red blood cell transfusion, hepatic sequestration involving an increase in liver size with right upper quadrant pain, abnormal liver function tests, and a drop in hemoglobin (Hb) level >2 gm/dL from baseline, splenic sequestration involving an enlarged spleen and drop in Hb >2 gm/dL from baseline, or recurrent priapism requiring a visit to a medical facility. Not all eligible participants who met the selection criteria were included in the analysis. Patients were excluded from the analysis if the medical records incompletely documented the number of VOE events in the 2 years before transplant and/or documentation was inadequate to meet the VOE definition, or if patients did not have 2 years of post-HSCT follow-up. All patients underwent regular post-transplant follow-up at the study institution, which documented any nonstudy institution management of VOE since the last follow-up visit. Additional clinical data gathered included SCD-related comorbid conditions, opioid use before and after HSCT, rate of acute and chronic GVHD, and rate of rejection. Hematologic data included the percentage of donor myeloid chimerism at 2 years after HSCT and the percentage of sickle hemoglobin (HbS%) at 2 years after HSCT in patients who had graft failure. The VOE inclusion criteria for the clinical trials at NIH and CNH, as compared with one of the ongoing gene therapy studies (NCT02140554), are listed in Supplementary Table S1.
Data were analyzed using a two-tailed paired sample t test or ANOVA for multiple group comparisons. A P value of < .05 was deemed statistically significant.
Results
Two hundred and three patients with SCD underwent allogeneic HSCT at either the NIH (N = 113) or the CNH (N = 90) between 2005 and 2019. Forty patients were excluded from the analysis due to incomplete documentation (N = 22) or less than 2 years of follow-up at the time of data analysis (N = 18). One-hundred sixty-three patients with SCD were included in the analysis (Table 1). Sixty percent (N = 98) of the patients were male (P < .001). Ninety one percent (N = 148) had HbSS (P < .001). The average age at the time of HSCT was 21 years (range, 7 months-64 years). Most patients received nonmyeloablative conditioning (75%, N = 123, P < .001) from a human leukocyte antigen (HLA)-matched sibling donor (72%, N = 118, P < .001). At the time of data collection, there were 16 deaths reported in 203 patients who were treated between 2005 and 2019. Of these, 9 died within 2 years of transplantation and 1 died after 2 years but had incomplete pretransplant documentation; therefore, these patients were not included in the analysis. For patients with greater than 2 years of follow-up, the average time to death was 81 months (range, 54-137 months) due to gastrointestinal bleeding, intracranial hemorrhage, or infection/sepsis. Details regarding the 40 patients who were excluded from the analysis, including those who died with less than 2 years of follow-up data, are included in supplemental Table S2.
Pain was the most frequently reported SCD-related comorbid condition before HSCT (55%, N = 89), followed by recurrent ACS (37%, N = 60). Pain was reported less frequently in patients aged <18 years (33%, N = 25). Thirty-eight percent of patients (N = 62) reported regular use of long-acting (14%, N = 23) or as needed short-acting (24%, N = 56) opioids at the time of HSCT. At 2 years after HSCT, there was a significant reduction in long acting (3%, N = 5, P = .02) opiates and a reduced, but nonsignificant, difference in the prescription of needed short-acting opiates (15%, N = 24, ns).
Eighty-eight percent (N = 143) achieved stable donor engraftment, defined as the presence of persistent donor myeloid chimerism with a switch to donor type Hb (Table 2). The average myeloid chimerism at 2 years after HSCT was 81.1% (range, 0%-100%). Twelve percent (N = 20) had graft rejection, defined as the complete loss of donor cells or restarting sickle-specific therapy. Of these, 25% (N = 5) restarted hydroxyurea and 25% (N = 5) restarted chronic transfusions. The acute and chronic GVHD rates in the cohort were 13% (N = 22) and 8% (N = 13), respectively.
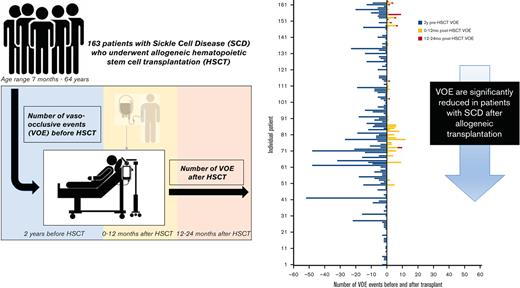
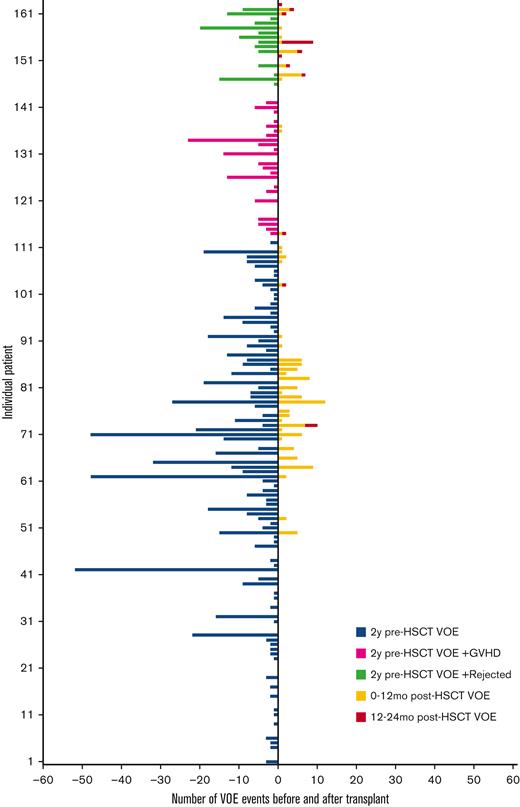
The average number of VOEs in the 2 years before HSCT for all patients was 5.6 events (range, 0-52) or 1.7 events per 100 patient-years vs 0.9 events (range, 0-12) or 0.3 events per 100 patient-years in the 2 years after HSCT (P < .001) (Table 2, Figures 1 and 2). Of the post-HSCT events, VOE was more frequent during the 0 to 12 months after HSCT (0.8 events [range, 0-12] or 0.5 events per 100 patient-years) compared with 12 to 24 months after HSCT (0.1 events [range, 0-8] or 0.06 events per 100 patient-years) (P < .001). There was no difference in pre- and post-VOE based on conditioning (myeloablative vs nonmyeloablative) when controlling for age, given that the majority of nonmyeloablative transplants were performed in an older population from the NIH cohort. Compared with the pediatric cohort, in which ACS and splenic sequestration were more likely (47% of VOE were related to ACS in the pediatric cohort vs 28% in the adult cohort), the majority of VOEs in the 2 years before HSCT in the adult cohorts were pain related, with recurrent hepatic sequestration (N = 1) and recurrent priapism (N = 1) noted in the adult cohort, based on the prespecified VOE definition. Most post-HSCT VOEs were pain related and required IV pain medication. After HSCT, ACS occurred in only 2 patients who experienced graft rejection.
Number of VOEs before and after HSCT. HSCT, hematopoietic stem cell transplantation; GVHD, graft-vs-host disease; VOE, vaso-occlusive event.
Number of VOEs before and after HSCT. HSCT, hematopoietic stem cell transplantation; GVHD, graft-vs-host disease; VOE, vaso-occlusive event.
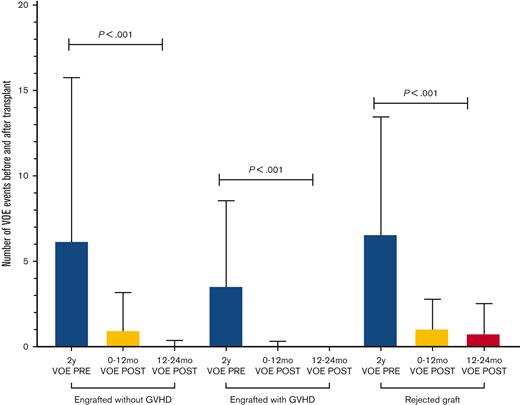
Number of VOEs before and after HSCT in engrafted patients with and without GVHD and in patients with graft rejection. GVHD, graft-vs-host disease; PRE, pretransplantation; POST, post-transplantation; VOE, vaso-occlusive event.
Number of VOEs before and after HSCT in engrafted patients with and without GVHD and in patients with graft rejection. GVHD, graft-vs-host disease; PRE, pretransplantation; POST, post-transplantation; VOE, vaso-occlusive event.
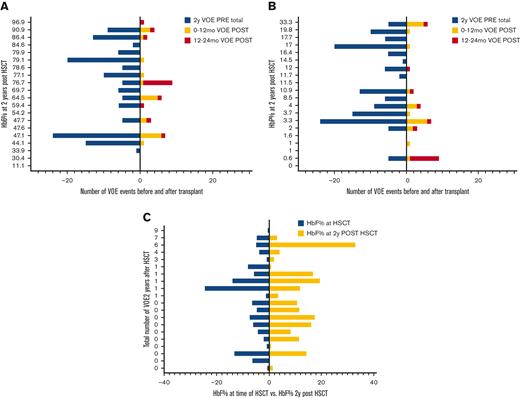
In engrafted patients, the risk of VOE was reduced by 75% in the first year after HSCT (RRR = 0.75) and 99% in the second year after HSCT (RRR = 0.99) (Table 2). Thirty-five percent (N = 51) of patients remained on immune suppression at 2 years after HSCT and were noted to report higher total 2 year rates of VOE in the post-HSCT period (1.5 events, range, 0-12, or 0.5 events per 100 patient-years) than those who were off immune suppression (0.4 events, range, 0-10, or 0.1 events per 100 patient-years) (P < .01); however, this difference was not significant when controlling for age (ns). In patients who had graft rejection, VOEs were reduced from 6.6 events (range, 0-24) or 2 events per 100 patient-years before HSCT to 1.1 events (range, 0-6) or 0.7 events per 100 patient-years and 0.8 events (range, 0-8) or 0.5 events per 100 patient-years in the 0 to 12 months and 12 to 24 months after HSCT, respectively (P < .001). In patients who had graft rejection, there was no correlation between the HbS% or percent fetal hemoglobin (HbF) at 2 years after HSCT and the total number of VOEs at 2 years after HSCT (ns) (Figure 3A,B), nor was there an association between HbF% at the time of HSCT and HbF% 2 years after HSCT (ns) (Figure 3C). Absolute reticulocyte counts (ARC) were higher 2 years after HSCT compared with ARCs around the time of HSCT (P < .01) in patients who had graft rejection; however, there was no difference in other markers of hemolysis, such as total Hb, aspartate transaminase, total bilirubin, or lactate dehydrogenase after rejection (supplemental Figure S3). The changes in VOE before and after HSCT for all participants, engrafted participants, those who were rejected, and those with GVHD are listed in Table 2.
Number of VOEs before and after HSCT in patients with graft rejection. Number of VOEs before and after transplant in 20 patients with graft rejection displayed by (a) sickle hemoglobin and (b) fetal hemoglobin percentages at 2 years post transplantation. (C) Fetal hemoglobin percentages at the time of transplant vs 2 years post transplantation. HbF, fetal hemoglobin; HbS, sickle hemoglobin; HSCT, hematopoietic stem cell transplantation; VOE, vaso-occlusive event.
Number of VOEs before and after HSCT in patients with graft rejection. Number of VOEs before and after transplant in 20 patients with graft rejection displayed by (a) sickle hemoglobin and (b) fetal hemoglobin percentages at 2 years post transplantation. (C) Fetal hemoglobin percentages at the time of transplant vs 2 years post transplantation. HbF, fetal hemoglobin; HbS, sickle hemoglobin; HSCT, hematopoietic stem cell transplantation; VOE, vaso-occlusive event.
Younger patients generally had less VOEs both at 2 years before (P = .002) and 2 years after HSCT (P = .005) (supplemental Figure S1). The difference in VOEs after HSCT based on age was eliminated at 12 to 24 months after HSCT (ns). In a regression model including age, gender, and VOE rates in the 2 years before HSCT, age (P < .01) and number of VOE before HSCT (P < .01) but not gender (ns) were predictive of VOE rates in the first 0 to 12 months after HSCT. Neither age nor pretransplant VOE rates were predictive of VOE rates 12 to 24 months after HSCT (ns). Patients with a higher percentage of donor myeloid chimerism at 2 years after HSCT had less VOEs at 0 to 12 months after HSCT (P = .01), with no difference at 12 to 24 months after HSCT (ns) (supplemental Figure S2). There was no difference in the 2 year myeloid chimerism or VOEs based on donor status (HbAS N = 110, HbAA N = 50, HbAC N = 2, HbAbetathal = 1). The average HbS% at 2 years after HSCT in engrafted patients with an HbAS donor was 35.7% (range, 5.1%-43.7%) vs 0.1% (range, 0%-5.1%) in engrafted patients with an HbAA donor. Patients on either long- or short-acting opioids at the time of HSCT had significantly more VOEs pre-HSCT (P < .001) and in the 0 to 12 months (P < .001), but not 12 to 24 months after HSCT (ns).
Discussion
Curative strategies for SCD aim to replace the ineffective erythropoiesis of SCD with predominant donor derived erythropoiesis, thereby eliminating the clinical complications resulting from sickle derived vascular occlusion, such as pain, ACS, hepatic and splenic sequestration, and priapism. This study used a strict definition of VOE in alignment with current gene therapy clinical trials to more adequately inform and assess the results of these newer approaches. After allogeneic HSCT in this large cohort of patients from infancy to late adulthood, with a mix of donor and conditioning regimens, the average number of VOEs requiring medical attention for patients with SCD in the 2 years before HSCT was significantly reduced in the 0 to 12 months and more significantly in the 12 to 24 months post-HSCT period in all patients, including those with GVHD and those who rejected their graft.
Early preliminary data after autologous gene therapy for SCD suggest a similar significant reduction and/or elimination of SCD-related clinical events after gene therapy6,7; however, a direct comparison with the patients included in this study is cautioned. The nonmyeloablative protocols at the NIH8-10 were specifically designed to broaden the scope of patients with SCD who are eligible for curative therapy and included older, sicker patients with increased comorbid conditions who would otherwise be unable to tolerate myeloablative therapy. In the allogeneic setting, frequent pain despite hydroxyurea treatment and other SCD supportive care is an acceptable indication for HSCT in patients with an HLA-matched sibling donor11; however, the pain was often not the sole indication for HSCT in this study, and the rates of pre-HSCT pain in patients referred to the NIH may not be reflective of the general adult sickle cell population. Patients treated at the NIH often had multiple comorbid conditions in addition to frequent pain, whereas patients treated at the CNH were younger and had less pain, presumably because of less accumulation of organ injury resulting from repeated VOEs. This is in contrast to autologous gene therapy trials, which are currently limited to patients who meet strict inclusion criteria, are primarily characterized by severe recurrent VOE as an easily measurable efficacy end point, and often exclude sicker patients who may otherwise meet the eligibility criteria for allogeneic curative strategies. For example, patients who were eligible and underwent HSCT at NIH often had multiple comorbid conditions that would exclude them from gene therapy, such as cerebrovascular disease, liver cirrhosis, red cell alloimmunization, cardiovascular disease, pulmonary hypertension, and/or chronic kidney disease. Furthermore, this study included only 2 clinical sites. Although 4 clinical trials from the NIH gathered pain data as a secondary end point, 7 studies from the CNH did not prospectively collect pain data.
Pain is a hallmark of SCD and a frequent comorbid condition in patients undergoing curative therapy. Acute pain events are the most common cause of SCD-related hospitalization and treatment in the ER and are associated with increased morbidity and mortality.12,13 The inflammatory response that occurs with vaso-occlusion worsens with recurrent episodes, resulting in organ damage over time, contributing to neuropathic and chronic pain syndromes. Recent evidence further suggests that mechanisms in addition to acute vaso-occlusion contribute to pain in patients with SCD.14 Pain in SCD is therefore broad in its definition, incidence, frequency, and severity, and exhibits wide heterogeneity in its acute and chronic manifestations. The complexity and overlap of pain syndromes in SCD make SCD pain unique, and mixed mechanisms may operate simultaneously. Hospitalizations for acute painful episodes are largely eliminated after successful HSCT,10 resolution of pain in the first year after curative therapy is not guaranteed5 and was more common in the 0 to 12 vs 12 to 24 months after HSCT in this study. Untangling chronic pain, neuropathic pain, and non–SCD-related pain from the lifetime of SCD-related pain remains a challenge. Many patients may have pain phenotypes where acute and chronic pain are intertwined and may not be distinguished clinically.15 Given this overlap, and given the retrospective nature of this study, there is likely an overestimation of pain both before and after HSCT. For example, post-HSCT VOEs were included if the patient received IV narcotics for any reason during hospitalization for post-transplant complications such as neutropenic fever or viral reactivation. Such pain may not have been SCD-related and is often multifactorial, requiring frequent reassessment of the pain etiology. Management of pain after HSCT for patients with SCD involves acknowledging the individual’s pain history, pain memory, confounding, and exacerbating psychological stressors such as depression,16 chronic pain disorders not altered by HSCT, such as avascular necrosis or leg ulcers, opioid hyperalgesia, or opioid withdrawal and rebound pain that can occur with rapid tapering of narcotics.
We previously reported that 40% of adult patients (n = 35) after HSCT had persistent pain requiring opioid medications at 1 year after HSCT,5 but, in this expanded cohort, the majority of patients were off narcotics by 2 years after HSCT. Discontinuation of narcotics is therefore possible and likely requires slow tapering, particularly for the subgroup of patients who reported a higher pain burden, more symptoms of anxiety, and the use of long-acting opioids before HSCT. Despite opioid use at 1 year, this subgroup reported significant improvement in pain intensity, pain impact, satisfaction with social role, and physical function following HSCT, and given a longer follow-up, is likely able to discontinue long-acting and reduce short-acting narcotic use by 2 years after HSCT. Chronic pain in SCD and its complications seem to develop during adolescence and young adulthood17; therefore, transplantation before the development of chronic pain may further reduce the need for post-HSCT narcotics. None of the pediatric patients transplanted at CNH required long-acting narcotics before or after HSCT, nor were there any post-HSCT VOEs, except in 1 patient who had graft rejection, further providing supportive evidence of improved outcomes in younger patients.
Transitioning from the lifetime of SCD to a life free of SCD-related symptoms occurs gradually. For patients with successful HSCT, there is an improved quality of life with a return to normal school and work life after successful HSCT.18-20 This study reports 2 novel findings: a higher percent myeloid chimerism in the first year after HSCT is protective against pain events, and there is improvement in VOEs even in patients who rejected their graft. The mechanism of this latter phenomenon is not understood but may include significant alterations to the bone marrow niche and inflammatory microenvironment as a result of chemotherapy and/or radiation. Despite the return of elevated HbS levels, there was no correlation with VOE frequency in the first 2 years after HSCT. After graft failure, the interval between the rise in HbS levels and the return of symptoms can vary depending on the severity of the SCD and the trait status of the donor, where HbS levels could increase faster in the event of graft failure.21 Understanding the mechanism by which pain symptoms improve after HSCT despite graft failure and subsequent long-term worsening of symptoms over time is important, given the increased use of alternative sources of hematopoietic donors, which carry a higher risk of graft rejection. Longer follow-up in patients with graft failure and prospective characterization of VOEs, for example, subjective pain vs objective ACS, are important, as they may inform decisions regarding further therapy, including second transplant planning. The prospective monitoring of pain events, clearly defining an SCD-related pain event and capturing patients’ pain management after transplantation are important for future studies.
Pain is the most prominent symptom of SCD. It becomes more frequent and severe over time and significantly interferes with the function and quality of life of patients with SCD. Allogeneic HSCT significantly reduces the rate of painful episodes and other VOEs in the first 2 years after HSCT; thus, the benefit of acute pain cessation in patients with SCD who undergo HSCT may balance the harm for some. Severe recurrent VOEs indicate that patients should consider gene therapy in clinical trials. Therefore, these data demonstrating a reduction in VOE after allogeneic HSCT should inform the results of newer autologous HSCT gene therapy approaches that aim to reduce VOEs and provide a potential curative option for more patients with SCD.
Acknowledgments
A.A. received funding for the protocols discussed in this manuscript from the Doris Duke charitable foundation, Amos Medical Faculty Development Award, American Society of Hematology & Robert Wood Johnson Foundation. Trial registration numbers: NIH: NCT00061568, NCT02105766, NCT03077542, NCT00977691; CNHS: NCT02165007, NCT03587272, NCT00745420, NCT02766465, NCT03263559, NCT04018937, NCT00029380.
Authorship
Contribution: A.L. designed the research, analyzed the results, made the figures, and wrote the manuscript; D.F. designed the research, collected data, and wrote the manuscript; A.A., D.S.D., R.N., E.L., C.F., and M.H. conducted the clinical trials and wrote the manuscript; and J.T. conducted clinical trials, designed the research, and wrote the manuscript.
Conflict-of-interest disclosure: A.A. served on the safety monitoring committee for Sangamo Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: John F. Tisdale, Cellular and Molecular Therapeutics Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD; e-mail: johntis@mail.nih.gov.
References
Author notes
Individual participant data that underlie the results reported in this article, after de-identification, can be made available upon request to the corresponding author, John F. Tisdale (johntis@mail.nih.gov.).
The full-text version of this article contains a data supplement.