Key Points
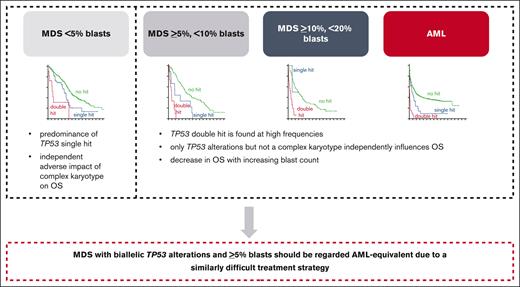
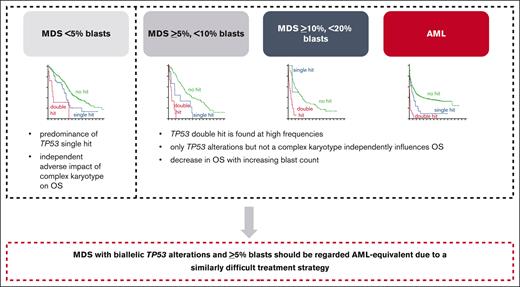
TP53dh is the strongest prognostic factor in AML and MDS, followed by blast count and CK.
We encourage the combination of biallelic TP53-altered MDS with ≥5% blasts and biallelic TP53alt AML in all classification systems.
Abstract
Several clinical and genetic factors impact overall survival (OS) in myelodysplastic neoplasms (MDS) and acute myeloid leukemia (AML), including complex karyotype (CK), TP53 allelic state, and blast count. We analyzed the interplay of these factors by performing Cox regression analysis and by determining the frequency of TP53 single-hit (sh) and double-hit (dh) events and OS in MDS (n = 747) with <5% blasts, with ≥5% but <10% blasts, and ≥10% but <20% blasts and AML (n = 772). MDS with <5% blasts showed the best outcome, followed by with ≥5% but <10% blasts, and ≥10% but <20% blasts, and AML (median OS: 75, 54, 27, and 18 months, respectively). The same hierarchy was observed when each subgroup was divided into TP53sh, TP53dh, and without TP53 alterations (alt), revealing a dismal outcome of TP53dh in all subgroups (17, 10, 8, and 1 month[s], respectively). MDS with <5% blasts differed from the other subgroups by showing predominantly TP53sh (76% of TP53alt cases), and by an independent adverse impact of CK on OS (hazard ratio, 5.2; P < .001). The remaining subgroups displayed many similarities, with TP53dh found at high frequencies (67%, 91%, and 71%, respectively) and only TP53alt but not CK independently influencing OS, and TP53dh showing the strongest influence. When the total cohort was split based on TP53 state, only the blast count and not CK had an independent adverse impact on OS in all subgroups. Thus, TP53dh is the strongest prognostic factor, further supporting its integration into risk stratification guidelines and classification as a separate entity. However, the blast count also influences OS independent of TP53 state, whereas CK plays a minor prognostic role.
Introduction
TP53 is one of the most frequently mutated and also most studied genes in human cancer. Mutations in TP53 are present in ∼10% to 20% of patients with myelodysplastic neoplasms (MDS)1-3 and acute myeloid leukemia (AML).4,5 In recent years, a lot of progress has been made in further elucidating the influence of different TP53 alterations on survival in these entities. TP53 is located on the short arm of chromosome 17, and gene mutations as well as allelic imbalances comprising deletions in TP53 and regions of copy-neutral loss of heterozygosity (CN-LOH) comprising 17p/TP53 have been described.6-8 If only 1 allele is altered, this is called monoallelic alteration or single hit, whereas a number of terms have been used in case of disruption of both alleles (caused by multiple mutations or mutation with concurrent deletion or CN-LOH of the other allele), including biallelic, double hit, or multi hit. Although biallelic alteration and double hit are biologically the most correct descriptions, the term multihit has frequently been used in the literature, possibly because of the fact that if multiple alterations are detected, it is not always possible in routine diagnostic workups to correctly determine whether both alleles are altered, in particular in cases with >1 TP53 mutation.6-12 For clarification, the terms single hit and double hit will be used throughout this manuscript. Beside these differences in terminology, data on the impact of different TP53 alterations on prognosis have also been conflicting in recent studies: it was shown in a number of publications that TP53 double hit leads to dismal outcome in both AML and MDS, whereas the influence of TP53 single hit was controversially discussed. Although several reports have shown a negative prognostic impact of TP53 single hit caused by 1 TP53 mutation, other studies observed this negative influence on overall survival (OS) only when both TP53 alleles were altered.6-12 These differences might be because of different definitions of double hit or because of consideration of various additional parameters, which makes comparison of studies challenging.
TP53 mutations are also known to be associated with a complex karyotype, in particular, patients with TP53 double hit harbor a complex karyotype in the vast majority (>90%) of cases.7,9,13,14 However, recent studies showed that the presence of TP53 alterations (in particular TP53 double hit) within cases with complex karyotype identifies an even more aggressive subgroup, both in de novo and MDS/AML postcytotoxic therapy.13,14 Moreover, the role of the blast count was controversially discussed regarding its impact on survival in MDS: on the 1 hand, TP53 double hit was more frequently detected in cases with increased blasts; on the other hand, it was shown that the prognosis of MDS (with increased blasts) with TP53 double hit does not appear to be dependent on the blast percentage, which is in contrast to other MDS subtypes.7-9,14,15 In risk stratification guidelines, TP53 alterations were clearly associated with adverse risk: for AML, mutations in TP53 and alterations involving 17p are included in the adverse risk category per European LeukemiaNet 2022; for MDS, TP53 double-hit alterations belong to 1 of the most important genetic predictors of adverse outcome in the Molecular International Prognostic Scoring System.16-19 Moreover, MDS with TP53 double hit has recently been classified as a separate entity per the World Health Organization (WHO) 2022 classification, belonging to the category of MDS with defining genetic abnormalities.20 By contrast, TP53 alterations do not constitute an individual entity in patients with AML, but 17p-/TP53 deletions, together with complex karyotype, define the entity acute myeloid leukemia, myelodysplasia-related (AML-MR), which is known to have a rather poor prognosis.20 However, in the International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemias, TP53 alterations constitute individual entities in both AML and MDS: the category myeloid neoplasms with mutated TP53 comprises separate diagnoses of MDS, MDS/AML, and AML with mutated TP53, separated only based on the blast percentage.21 Thus, in the future, these discrepancies between both classification systems should be harmonized. Nevertheless, a number of studies recently proposed that TP53-mutated AML and MDS with increased blasts (10%-19%) should be regarded as a single molecular disease entity,14,15 irrespective of the blast count currently separating AML from MDS, because they do not differ with respect to molecular, biological, and clinical features as well as survival. Furthermore, it was shown that double-hit TP53 identifies a particular aggressive disease, independent of the blast count or prior therapies (AML/MDS postcytotoxic therapy).9,10,14,15
Despite growing knowledge regarding the impact of parameters including complex karyotype, blast count, and TP53 allelic state on survival in patients with TP53-altered AML/MDS,6,7,9,14,22-24 questions remain regarding the interplay of these factors. Moreover, a number of different terms and definitions have been used to describe and analyze the impact of alterations on 1 or both TP53 alleles. Thus, the aim of this project was to disentangle these discrepancies by the analysis of the frequency of single-hit TP53 events (including single TP53 mutations, deletions, or CN-LOH covering 17p/TP53) and double-hit TP53 (comprising ≥2 TP53 mutations, or mutation in TP53 with accompanying deletion or with accompanying CN-LOH) events in MDS with <5% blasts, ≥5% but <10% blasts, and ≥10% but <20% blasts, and in AML. Furthermore, we determined OS per the TP53 allelic state in the subgroups, and performed univariate and multivariate Cox regression analyses of the impact of TP53 state, complex karyotype, and blast count on OS.
Methods
Patients and samples
A total number of 1519 samples were used for analysis. These were sent to the MLL Munich Leukemia Laboratory between September 2005 and January 2020 and comprised 772 AML and 747 MDS cases. Diagnoses (from the peripheral blood and/or bone marrow) were established based on cytomorphology, immunophenotype, cytogenetics, and molecular genetics, as previously published per WHO 2022 classification.20,25-27 The AML cohort comprised 349 (45%) female and 423 (55%) male cases, and the median age was 68 years. Median age of the MDS cohort was 73 years, and the cohort included 316 (42%) female and 431 (58%) male patients. MDS cases were subdivided into cases with <5% blasts (n = 419), ≥5% but <10% blasts (n = 175), and ≥10% but <20% blasts (n = 153). All patients had given written informed consent to the use of genetic and clinical data in accordance with the Declaration of Helsinki. The study was approved by our internal institutional review board. Clinical data were available for 1454 patients (AML: n = 717; MDS: n = 737). The cohort is part of a larger data set containing 5000 whole genomes, all investigated by the Munich Leukemia Laboratory, and cases were used for analysis of TP53 alterations dependent on AML and MDS subgroups in a previous study.8
Whole-genome sequencing (WGS) and variant filtering
WGS analysis was performed for all 1519 patients (median coverage, 100×). For this, total genomic DNA was extracted from lysed cell pellets of diagnostic bone marrow or peripheral blood using the MagNA Pure 96 with DNA and Viral Nucleic Acid Large Volume Kit and Cellular RNA Large Volume Kit (Roche, Basel, Switzerland). WGS libraries were prepared from 1 μg of DNA with the TruSeq PCR-Free library preparation kit following the manufacturer’s recommendations (Illumina, San Diego, CA) and 2× 150–base pair paired-end sequences were generated on a NovaSeq 6000 or HiSeqX instrument with 100× coverage (Illumina). Further alignment and variant filtering were performed as previously described.8 Final analysis was performed on protein-altering and splice-site variants only.
Statistical analysis
SPSS (version 19.0.0) software (IBM Corporation, Armonk, NY) was used for statistical analysis including univariate and multivariate Cox regression analysis. OS curves were calculated using the Kaplan-Meier method and compared using the 2-sided log-rank test. All reported P values are 2-sided and were considered significant at P ≤ .05. OS was measured from the date of diagnosis until last follow-up or death.
Results
Frequency of TP53 alterations dependent on blast count
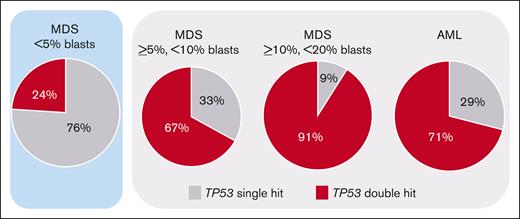
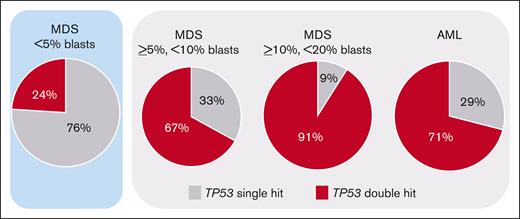
TP53 alterations (comprising mutations, deletions, CN-LOH, and the respective combinations) were detected in 11% of AML (83 of 772) and in 13% of MDS (95 of 747) cases, as previously published.8 Regarding single-hit events, in both AML and MDS, TP53 mutations were detected most frequently (AML: 13%, 11 of 84; MDS: 40%, 38 of 95) followed by TP53 deletions (AML: 14%, 12 of 83; MDS: 7%, 7 of 95). CN-LOH was only rarely detected as single-hit event and was not included into the OS analysis because of lack of clinical importance (AML: 1%, 1 of 83; MDS: 0%, 0 of 95). The most abundant double-hit event constituted a TP53 mutation with accompanying deletion (AML: 39%, 32 of 83; MDS: 20%, 19 of 95), followed by ≥2 TP53 mutations (AML: 16%, 13 of 83; MDS: 17%, 16 of 95) and TP53 mutation with accompanying CN-LOH (AML: 17%, 14 of 83; MDS: 16%, 15 of 95) showing comparable frequencies. When the MDS cohort was split based on blast count into cases with <5% blasts, ≥5% but <10% blasts, and ≥10% but <20% blasts, the frequency of TP53 alterations was also comparable between these subgroups (MDS with <5% blasts: 11% [45 of 419] of cases with TP53 alteration; MDS with ≥5% but <10% blasts: 15% [27 of 175] of cases with TP53 alteration; and MDS with ≥10% but <20% blasts: 15% [23 of 153] of cases with TP53 alteration). However, MDS cases with <5% blasts clearly differed from the other subgroups by showing predominantly TP53 single-hit events (76% [34 of 45] of cases with TP53 alteration), whereas in the other subgroups and AML a TP53 double hit was detected at high frequencies (in 67% [18 of 27], 91% [21 of 23], and 71% [59 of 83] of cases with TP53 alteration, respectively) (Figure 1; Table 1). A complex karyotype was found to be significantly associated with the presence of a TP53 double hit, because it was detected in 84% (92 of 109) of TP53 double-hit cases, compared with 17% (12 of 69) of TP53 single-hit cases (P < .001; Table 1). Consequently, in all subgroups a complex karyotype was found with higher frequencies in the TP53 double-hit cases compared with TP53 single-hit cases (MDS with <5% blasts: 82% vs 6%; MDS with ≥5% but <10% blasts: 94% vs 22%; MDS with ≥10% but <20% blasts: 95% vs 50%; and AML: 78% vs 29%; Table 1). Median variant allele frequency (VAF) of the total cohort with TP53 mutation was 0.5; 15% (24 of 158) showed a VAF of <20%, and the remaining 85% (134 of 158) had a VAF of ≥20%.
Frequency of TP53 single-hit and double-hit events. The frequency of the respective TP53 states in the blast-dependent subgroups is depicted. Although <5% of MDS cases predominantly showed single-hit events (highlighted in blue), the other subgroups are dominated by TP53 double-hit events (marked in light gray).
Frequency of TP53 single-hit and double-hit events. The frequency of the respective TP53 states in the blast-dependent subgroups is depicted. Although <5% of MDS cases predominantly showed single-hit events (highlighted in blue), the other subgroups are dominated by TP53 double-hit events (marked in light gray).
OS and Cox regression analysis in blast-dependent MDS subgroups and AML
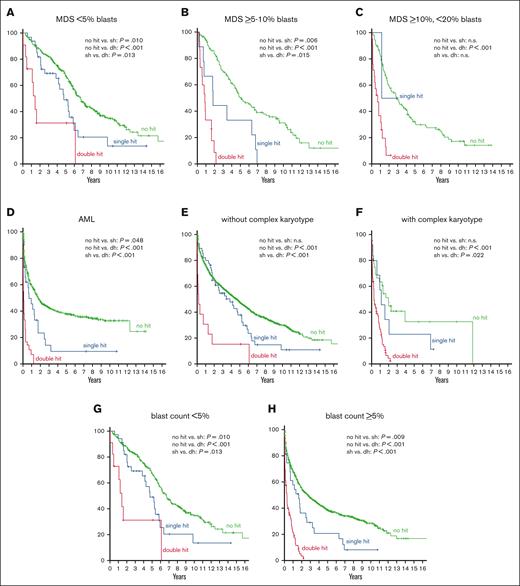
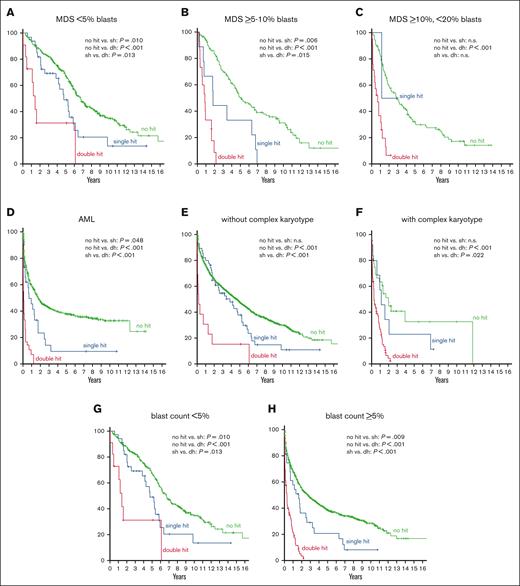
Regarding OS in the MDS subgroups with different blast counts, MDS with <5% blasts showed the best outcome (median OS, 75 months), followed by MDS with ≥5% but <10% blasts (54 months), MDS with ≥10%,<20% blasts (27 months), and AML (18 months). The same hierarchy was observed when each subgroup was divided into cases with and without TP53 alteration, cases with TP53 alteration showing a worse prognosis (56, 11, 10, and 3 months, respectively) than cases without TP53 alteration in each subgroup (80, 60, 34, and 21 months, respectively). Further subdivision of cases with TP53 alteration into TP53 single hit and double hit revealed a dismal outcome of TP53 double hit in all subgroups, again following the hierarchy described earlier (TP53 double hit: 17, 10, 8, and 1 month(s), respectively; TP53 single hit: 57, 22, 14, and 8 months, respectively; Table 1; Figure 2A-D). Of note, all comparisons regarding OS (without TP53 alteration vs TP53 single hit; without TP53 alteration vs TP53 double hit; and single hit vs double hit) was found to be statistically significant in all subgroups with the exception of MDS with ≥10% but <20% blasts in which only the difference in OS between cases without TP53 alteration vs those with TP53 double hit was statistically significant, because of the low number of TP53 single-hit cases in this subgroup (Figure 2; Table 1).
OS analysis in respective AML and MDS subgroups. (A-D) OS in patients without TP53 alteration (green curve), TP53 single hit (blue), and TP53 double hit (red) in blast-dependent subgroups (MDS with <5% blasts, MDS with ≥5% but <10% blasts, MDS with ≥10% but <20% blasts, and AML). (E-F) OS in respective subgroups dependent on the presence of a complex karyotype. (G-H) OS in subgroups dependent on the blast count. P values are depicted and were considered significant at P ≤ .05.
OS analysis in respective AML and MDS subgroups. (A-D) OS in patients without TP53 alteration (green curve), TP53 single hit (blue), and TP53 double hit (red) in blast-dependent subgroups (MDS with <5% blasts, MDS with ≥5% but <10% blasts, MDS with ≥10% but <20% blasts, and AML). (E-F) OS in respective subgroups dependent on the presence of a complex karyotype. (G-H) OS in subgroups dependent on the blast count. P values are depicted and were considered significant at P ≤ .05.
In univariate Cox regression analysis, TP53 single hit (hazard ratio [HR], 1.4; P = .043), TP53 double hit (HR, 4.9; P < .001), blast count (<5% vs ≥5%; HR, 1.9; P < .001), and complex karyotype (HR, 3.2; P < .001) had an adverse impact on OS in the total cohort. Multivariate Cox analysis that included these parameters revealed an independent adverse impact on OS for all factors (TP53 single hit: HR, 1.6; P = .003; blast count: HR, 1.8; P < .001; complex karyotype: HR, 1.3; P = .048), with TP53 double hit being the strongest prognostic factor (HR, 3.9; P < .001). Furthermore, univariate Cox regression analysis were also performed for the respective subgroups (MDS with <5% blasts; MDS with ≥5% but <10% blasts; MDS with ≥10% but <20% blasts; and AML) using TP53 single hit, TP53 double hit, and complex karyotype as parameters. In these analyses, all factors showed a negative influence on OS in all subgroups (except TP53 single hit in MDS with ≥10% but <20% blasts because of the low number of cases; Table 2). However, in multivariate analysis, an independent adverse impact on OS regarding complex karyotype was found only in cases with MDS with <5% blasts (HR, 5.2; P < .001) but not in the other subgroups. By contrast, TP53 single hit and double hit were independent factors in all subgroups (with the exception of TP53 single hit in MDS with ≥10% but <20% blasts, see earlier discussion), TP53 double hit again showing the strongest influence (MDS with <5% blasts: TP53 single hit: HR, 1.7; P = .021 and double hit: HR, 2.2; P = .041; MDS with ≥5% but <10% blasts: TP53 single hit: HR, 2.3; P = .026 and double hit: HR, 9.2; P < .001; MDS with ≥10% but <20% blasts: double hit: HR, 4.6; P = .004; and AML: TP53 single hit: HR, 1.6; P = .044 and double hit: HR, 4.2; P < .001; Table 2). Regarding VAF, there was a trend toward a worse OS in cases with ≥20% VAF compared with those with <20% VAF, however this was not found to be statistically significant (19 vs 10 months).
OS and Cox regression analysis in cases with and without TP53 alterations
Next, we analyzed the interplay of these factors from another perspective and split the total cohort of 1519 cases into cases without TP53 alteration (n = 1341), with TP53 single hit (n = 69), and with TP53 double hit (n = 109). As expected, cases without any TP53 alteration showed the best outcome (54 months), followed by cases with TP53 single hit (39 months) and TP53 double hit (4 months). The same hierarchy was detected when cases were further separated per complex karyotype, although cases without complex karyotype showed a better outcome than the respective subgroups in which a complex karyotype was detected (without complex karyotype: no TP53 alteration, 55 months; TP53 single hit, 46 months; TP53 double hit: 2 months; and with complex karyotype: 24, 14, and 4 months, respectively) (Figure 2E-F; Table 1). In total, 59 patients showed a TP53 single hit without a complex karyotype (33% [59 of 178] of all cases with TP53 alteration), which mainly belonged to the subgroup of MDS with <5% blasts (n = 32), although it was also found in patients with AML (n = 19). However, TP53 single hit without a complex karyotype was only rarely detected in MDS with ≥5% but <10% blasts (n = 6) and MDS with ≥10% but <20% blasts (n = 2). When cases were split into <5% blasts and ≥5% blasts, OS followed the same aforementioned hierarchy with cases with TP53 double hit showing the worst outcome in both subgroups (<5% blasts: 80, 57, and 17 months; ≥5% blasts: 33, 20, and 4 months, respectively) (Figure 2G-H; Table 1). Regarding univariate Cox regression analysis, the blast count (<5%, ≥5%) had an adverse impact on OS in all subgroups (no TP53 alteration: HR, 1.8; P < .001; TP53 single hit: HR, 2.2; P = .007; and TP53 double hit: HR, 2.9; P = .008), whereas a complex karyotype did not show an impact on OS in cases with TP53 double hit (no TP53 alteration: HR, 1.6; P = .038; and TP53 single hit: HR, 1.5; P = .035). Moreover, in multivariate Cox regression analysis, again only the blast count had an independent adverse impact on OS in all subgroups (HR, 1.8; P < .001; HR, 2.2; P = .013; and HR, 2.9; P = .008, respectively), whereas the presence of a complex karyotype did not show a negative impact on OS in any subgroup (Table 2).
Discussion
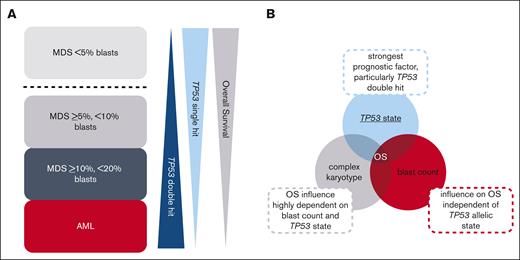
In this study, we analyzed the prognostic influence of TP53 allelic state, blast count, and complex karyotype in a large cohort of patients with AML and MDS. All patients were diagnosed per WHO and ICC standards and all also received WGS. We show that MDS cases with <5% blasts clearly separated from MDS cases with ≥5% blasts and from AML cases by the predominance of TP53 single hit and by having an independent adverse impact of complex karyotype on OS, which was not observed in the other subgroups. These remaining subgroups (MDS with 5%-19% blasts, and AML) are connected by several similarities: they are dominated by the presence of a TP53 double hit, which is found in ∼70% to 90% of cases with TP53 alteration. In addition, only TP53 alterations but not a complex karyotype independently influenced OS in these (Figure 3) subgroups. This supports recent studies proposing that mutant TP53 AML and MDS with increased blasts should be considered as a single genetically defined disease entity, because these cases show particularly poor outcomes, which separates them even from other adverse-risk AML/MDS cases, including those with complex karyotype, with a very poor OS even when intensively treated.9,14,15 Moreover, unification of these cases is also supported by a very similar biology, because >50% of patients with TP53-altered MDS with increased blasts or with AML do only rarely harbor other common myeloid mutations and, thus, also show a very low frequency of driver mutations such as NPM1 or FLT3.9,14,28,29 By contrast, these cases very often show complex karyotypes frequently including aberrations of chromosomes 5, 7, and 17.9,22,30 These similarities in biology of TP53-altered MDS with increased blasts and AML is corroborated by our results.
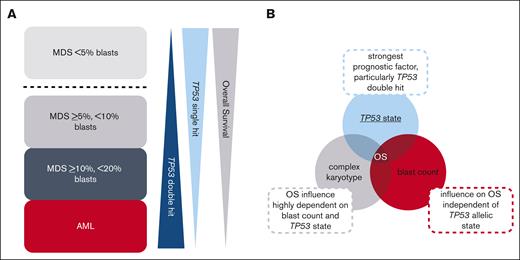
Graphical summary of interplay of TP53 state, blast count, and complex karyotype in MDS and AML. (A) Summary of the general trends of the frequency of TP53 allelic state (single hit vs double hit) and outcome of patients in the analyzed subgroups. MDS cases with <5% blasts clearly separate from MDS cases with ≥5% blasts and from AML cases by the predominance of TP53 single hit and by being the only subgroup in which a complex karyotype showed an independent adverse impact on OS. The remaining subgroups (MDS with ≥5% but <10% blasts; MDS with ≥10% but <20% blasts; and AML) display a number of similarities, with TP53 double hit found at high frequencies and only TP53 alterations but not a complex karyotype independently influencing OS. Please note that the graphic indicates a general trend but that MDS with ≥10% but <20% blasts actually showed a higher percentage of TP53 double hit compared with AML. Furthermore, a decrease in survival with increasing blast count was observed. (B) Interplay of TP53 state, blast count, and complex karyotype on OS. In the total cohort, TP53 state, in particular the double hit, was the strongest prognostic factor; however, also the blast count influenced OS independent of TP53 allelic state. By contrast, the influence of the presence of a complex karyotype on OS seems to be highly dependent on blast count and TP53 allelic state.
Graphical summary of interplay of TP53 state, blast count, and complex karyotype in MDS and AML. (A) Summary of the general trends of the frequency of TP53 allelic state (single hit vs double hit) and outcome of patients in the analyzed subgroups. MDS cases with <5% blasts clearly separate from MDS cases with ≥5% blasts and from AML cases by the predominance of TP53 single hit and by being the only subgroup in which a complex karyotype showed an independent adverse impact on OS. The remaining subgroups (MDS with ≥5% but <10% blasts; MDS with ≥10% but <20% blasts; and AML) display a number of similarities, with TP53 double hit found at high frequencies and only TP53 alterations but not a complex karyotype independently influencing OS. Please note that the graphic indicates a general trend but that MDS with ≥10% but <20% blasts actually showed a higher percentage of TP53 double hit compared with AML. Furthermore, a decrease in survival with increasing blast count was observed. (B) Interplay of TP53 state, blast count, and complex karyotype on OS. In the total cohort, TP53 state, in particular the double hit, was the strongest prognostic factor; however, also the blast count influenced OS independent of TP53 allelic state. By contrast, the influence of the presence of a complex karyotype on OS seems to be highly dependent on blast count and TP53 allelic state.
Our data clearly show that TP53 double hit is the strongest prognostic factor; however, also TP53 single-hit events showed an independently negative impact on OS in most subgroups (except in MDS with ≥10% but <20% blasts, because of the low number of single-hit cases in this subgroup) irrespective of the blast count or entity assignment. The prognostic impact of TP53 single hit particularly in MDS was discussed controversially in recent years,7-10 although it has to be noted that direct comparisons between the respective studies might be difficult because of differences in the selected cohorts used for analysis (eg, inclusion only of patients at high risk) as well as differences in underlying treatment patterns (inclusion of patients receiving transplantation) and also by differences in assessment and definition of TP53 allelic state. Recently, analysis of a cohort with previously untreated AML and higher risk MDS who were uniformly treated with a hypomethylating agent–based regimen revealed no differences in outcome between patients with TP53-mutated AML and those with MDS, further supporting the suggestion of combining these patient groups into 1 entity.11 Moreover, in the same study an adverse prognosis was observed for patients with either single-hit or double-hit TP53 alterations compared with patients with wild-type TP53, with no differences regarding TP53 allelic state,11 supporting our data that TP53 single-hit events also have an independent negative impact on OS. However, our data showed that the blast count also influences OS independent of TP53 allelic state, because a decrease in survival with increasing blast count was observed in all subgroups (hierarchy regarding OS: best outcome in MDS with <5% blasts, followed by MDS with ≥5% but <10% blasts, and MDS with ≥10% but <20% blasts, with worst outcome in AML). This is in some contrast to previous data, in which an independent influence of blast count on OS was not observed9; however, this might be explained by the larger number of patients with MDS used in this study, making a more detailed analysis of the impact of blast count possible.
By contrast, the prognostic role of a complex karyotype seems to be particularly important for MDS cases with <5% blasts, for which an impact of OS was found in this study. Hence, it does not necessarily have to be determined in MDS with ≥5% blasts or in AML with TP53 alterations, corroborating previous studies.14
Therapeutic options of patients with AML and MDS with TP53 alterations remain limited, because these patients show a high rate of resistance to conventional (chemo)therapies.31 In recent years, several novel therapies have been approved or are under consideration in clinical trials, including (1) CPX-351 for therapy of AML with myelodysplasia-related changes and therapy-related AML (comprising a high frequency of TP53 alterations); (2) eprenetapopt (APR-246), a small molecule that restores wild-type p53 functions in TP53-mutant AML and MDS cells; (3) magrolimab, an antibody targeting CD47 in TP53-mutated AML and patients with high-risk MDS; as well as (4) bispecific antibodies and (5) chimeric antigen receptor T-cells therapies.32-37 However, patients with TP53-altered AML or MDS still show a very poor OS that has so far neither improved markedly in the era of novel therapies nor by application of intensive chemotherapy approaches, and only patients able to proceed to allogeneic hematopoietic stem cell transplantation after responding to induction therapy have been found to have improved OS.22,38 Thus, therapy of patients with TP53-altered AML and MDS remains challenging and further therapeutic strategies are required. Another parameter that was controversially considered to provide prognostic information is VAF of TP53 mutations, with a VAF of >40% found to be independently associated with higher rates of relapse and worse OS in patients with AML or MDS, and was suggested to provide important prognostic information that may be considered when selecting frontline therapy.23,24 However, the influence of the VAF on OS seems to be less clear when specifically TP53-altered AML/MDS with complex karyotype was investigated.9,13,14,22-24,29 This may be explained by the fact that a higher TP53 VAF and the presence of a complex karyotype are associated with TP53 double hit, known to cause a dismal outcome irrespective of other parameters.7-9 Hence, it can be hypothesized that it might be more accurate to precisely detect the TP53 allelic state (by determination of mutations, deletions, and CN-LOH) for determination of prognosis than sole analysis of the VAF.7,8 However, as mentioned earlier, comparison between respective studies is often difficult because of varying selection criteria of cohorts and treatment regimens applied.
Although our data, and also other previous analysis,6-11 clearly show that the TP53 double hit is the strongest predictor of outcome, generally the majority of patients with AML and MDS with TP53 alterations have very poor outcomes compared with cases with wild-type TP53. Our data support the hypothesis that the only subgroup of patients with AML and MDS with TP53 alterations with a more favorable prognosis are patients with are TP53 single hit without a complex karyotype. However, these cases account for only 33% of patients with TP53 alterations.
Taken together, we suggest that MDS with biallelic TP53 alterations and ≥5% blasts should be regarded AML-equivalent because of a similarly difficult treatment strategy regardless of the diagnosis of MDS or AML and because of data from other recent publications,9 although our data show that the blast count does have a minor impact on prognosis. This supports that cases with TP53 altered AML and MDS with increased blasts should be considered a homogeneous disease entity, particularly in terms of treatment options, in order to improve future outcomes. Combining these cases into 1 genetically defined disorder would also ensure access of patients with MDS to novel drugs in terms of clinical trials otherwise potentially only available for patients with AML.29 Both the recent ICC and the current WHO classification added TP53 alterations into their classification algorithm although with different definitions and restrictions, and in both classification systems the blast count still separates TP53-altered MDS from AML.20,21 Based on our results, and other studies, we would encourage the combination of biallelic TP53-altered MDS with ≥5% blasts and biallelic TP53-altered AML in all classification systems.
Acknowledgments
The authors thank all coworkers at the Munich Leukemia Laboratory for their dedicated work. The authors also thank all physicians for providing samples and caring for patients as well as collecting data.
Authorship
Contribution: A.S. designed the study, interpreted the data, and wrote the manuscript; A.S. and C.H. were responsible for cytogenetic analyses; M.M., W.W., C.B., N.N., and S.H. were responsible for molecular and bioinformatic analyses; W.K. was responsible for immunophenotyping; T.H. was responsible for cytomorphologic analyses; and all authors read and contributed to the final version of the manuscript.
Conflict-of-interest disclosure: C.H., W.K., and T.H. declare part ownership of MLL Münchner Leukämielabor. A.S., W.W., M.M., C.B., N.N., and S.H. are employed by the MLL Münchner Leukämielabor.
Correspondence: Anna Stengel, MLL Munich Leukemia Laboratory, Max-Lebsche-Platz 31, 81377 München, Germany; e-mail: anna.stengel@mll.com.
References
Author notes
The data used in this manuscript are part of a larger data set containing 5000 whole genomes all investigated by the MLL Munich Leukemia Laboratory. Data security is guaranteed by the MLL Munich Leukemia Laboratory with respect to storage and scientific analyses and strictly follows the General Protection Regulation in Europe (https://gdpr-info.eu/). Any request for scientific use of the data should be addressed to the corresponding author, Anna Stengel (anna.stengel@mll.com) of the manuscript.