Key Points
Erythroblasts at different stages of differentiation have distinct responses to infection by P falciparum.
P falciparum infection of erythroblasts alters expression of genes related to oxidative and proteotoxic stress and erythroid development.
Abstract
During development down the erythroid lineage, hematopoietic stem cells undergo dramatic changes to cellular morphology and function in response to a complex and tightly regulated program of gene expression. In malaria infection, Plasmodium spp parasites accumulate in the bone marrow parenchyma, and emerging evidence suggests erythroblastic islands are a protective site for parasite development into gametocytes. Although it has been observed that Plasmodium falciparum infection in late-stage erythroblasts can delay terminal erythroid differentiation and enucleation, the mechanism(s) underlying this phenomenon are unknown. Here, we apply RNA sequencing after fluorescence-activated cell sorting of infected erythroblasts to identify transcriptional responses to direct and indirect interaction with P falciparum. Four developmental stages of erythroid cells were analyzed: proerythroblast, basophilic erythroblast, polychromatic erythroblast, and orthochromatic erythroblast. We found extensive transcriptional changes in infected erythroblasts compared with that in uninfected cells in the same culture, including dysregulation of genes involved in erythroid proliferation and developmental processes. Although some indicators of cellular oxidative and proteotoxic stress were common across all stages of erythropoiesis, many responses were specific to cellular processes associated with developmental stage. Together, our results evidence multiple possible avenues by which parasite infection can induce dyserythropoiesis at specific points along the erythroid continuum, advancing our understanding of the molecular determinants of malaria anemia.
Introduction
Malaria is a mosquito-borne infectious disease caused by parasites of the genus Plasmodium. Recent data estimate 593 000 fatalities due to malaria in the year 2021, the large majority of which occurred among young children and pregnant women in sub-Saharan Africa.1 Although 5 different species of Plasmodium parasites can cause disease in humans, most malaria deaths are caused by Plasmodium falciparum.2 During infection, parasites undergo rounds of intracellular replication in erythrocytes (red blood cells) and sequester in the microvasculature and deep tissues, including sites of hematopoiesis in the bone marrow.2-4 Consequently, malaria infection is often complicated by severe anemia related to increased destruction or clearance of erythrocytes.5-8 This adverse effect can be further compounded by the failure of the bone marrow to adequately produce and release new erythrocytes into circulation.9-12
Bone marrow dyserythropoiesis remains a poorly understood aspect of the pathogenesis of malarial anemia, but it has been observed in acute, severe disease and chronic infections that are common in regions of high transmission. Bone marrow aspirates of patients with malaria with severe anemia demonstrate morphological abnormalities in developing erythroblasts, including nuclear fragmentation, multinucleate cells, and intercytoplasmic bridges.10,12 Asexual- and sexual-stage Plasmodium spp parasites have been detected in the bone marrow of patients with malaria, including adjacent to erythroblastic islands.13-15 Indeed, the bone marrow is now recognized as a protective niche for developing P falciparum gametocytes, and recent work suggests that sexual commitment is influenced by the nutrient state of the host cells.13,14,16,17 Similar findings have also been reported in rodent malaria models.18,19 Hemozoin, an iron biocrystal produced upon parasitic digestion of hemoglobin, is also present in the bone marrow during malaria infection and has been implicated in the pathogenesis of malaria anemia.10,13,14,20-22 Together, these studies raise the hypothesis that the interaction of developing erythroid precursor cells with Plasmodium parasites and metabolites in the hematopoietic niche may contribute to disordered erythropoiesis.
Advances in culture systems for ex vivo erythropoiesis enable the study of host-parasite interactions during erythroid differentiation.20 Using specialized growth media, primary human hematopoietic stem/progenitor cells (HSPCs) can be directed to differentiate down the erythroid lineage to produce enucleated reticulocytes.23-25 Along the way, erythroblasts pass through the canonical stages of terminal differentiation: proerythroblast (ProE), basophilic erythroblast (BasoE), polychromatic erythroblast (PolyE), and orthochromatic erythroblast (OrthoE). Efforts to characterize the dynamic changes in the expression of membrane surface proteins during terminal erythroid differentiation have helped identify markers for each erythroblast stage, facilitating stage-specific isolation for functional analyses.26-28
Primary human erythroblasts cultured ex vivo are susceptible to infection with P falciparum as early as the BasoE stage, but the impact of the parasitic infection on erythroid differentiation is poorly understood.17,29 Increased reactive oxygen species and reduced enucleation have been observed in late-stage erythroblasts harboring gametocytes, and hemozoin has been shown to inhibit growth of primary erythroblasts and induce cell cycle changes in K562, an erythroleukemic cell line.21,30-32 Although transcriptional profiling via microarray has shown that coculture of P falciparum with PolyE and OrthoE may perturb expression of host gene pathways related to metabolism and protein chaperones, the bulk nature of these studies limit firm conclusions.33 Hemozoin has also been shown to alter transcription of genes involved in apoptosis in late-stage erythroblasts.34 Overall, the host and parasite factors that underlie these effects are largely unknown.
To bridge the gap in knowledge, we leveraged an ex vivo erythropoiesis culture system for primary human HSPCs to develop a strategy for the transcriptomic profiling of infected and uninfected (bystander) erythroblasts at distinct stages of terminal erythroid differentiation. We used fluorescence-activated cell sorting (FACS) followed by RNA sequencing (RNA-seq) to investigate the host transcriptional response to infection or exposure to P falciparum in ProE, BasoE, PolyE, and OrthoE. Our results demonstrate shared and distinct host cell responses to P falciparum according to the differentiation state of the erythroblast and suggest that direct infection alters the expression of genes that are critical for the progression of healthy erythropoiesis.
Methods
Primary human CD34+ HSPC culture
A 3-stage protocol was used to differentiate human bone marrow CD34+ cells (Stemcell Technologies) down the erythroid lineage, as previously described.25 Briefly, CD34+ cells were cultured in Iscove modified Dulbecco medium with plasma and supplemented with interleukin-3, hydrocortisone, stem cell factor, and erythropoietin. On day 7 of differentiation, interleukin-3 and hydrocortisone were removed from the supplementation to induce terminal differentiation. From day 11 to the end of culturing, cells were switched to a medium supplemented with only erythropoietin. Differentiation was monitored using cytospins stained with May-Grünwald and Giemsa and visualized via light microscopy.
P falciparum culture
P falciparum parasites were grown in a RPMI 1640–based medium (Sigma) supplemented with 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Gibco), 50 mg/L hypoxanthine (Sigma), and 0.5% Albumax (Invitrogen) at 37°C in 1% O2 and 5% CO2 with gentle agitation. Parasites were maintained at 2% hematocrit in deidentified human erythrocytes obtained from Stanford Blood Center. Erythroblast infections used P falciparum strain D10-pfPHG (provided by Dave Richard).35 D10-pfPHG is a laboratory-adapted strain expressing cytosolic green fluorescent protein (GFP) under the PfHsp86 promoter.
Infection of primary human erythroblasts
Erythroblasts were removed from the parent culture and plated at 1 × 106/mL in Iscove modified Dulbecco media supplemented with Albumax, hypoxanthine, and cytokines, corresponding to the day of differentiation. One cycle before infection, parasites were synchronized to a 1- or 2-hour window. Schizont-stage parasites were purified using an LS magnetic column (Miltenyi) and mixed with erythroblasts at a multiplicity of infection of 5 (day 7) or 3 (day 13), unless otherwise specified. Static cocultures were incubated for 24 hours at 37°C in 1% O2 and 5% CO2.
Flow cytometry and cell sorting
Cells were analyzed and sorted using FACSAriaII (BD Biosciences). Erythroblasts were staged using CD235a-APC eFluor780 (Thermo Fisher), CD233-PE (IBGRL), and CD49d-APC (Miltenyi). Viability was determined using Calcein-Violet 450AM. Infected erythroblasts were identified based on GFP expression from the parasite. Cells were sorted directly into buffer RLT (Qiagen) for RNA extraction or into fetal bovine serum for downstream microscopy.
Library preparation and sequencing
Total RNA was extracted from sorted cells using the RNeasy Micro kit (Qiagen) and checked for quality and concentration using the Agilent Eukaryotic RNA 6000 Pico kit, run on an Agilent 2100 Bioanalyzer at the Stanford Protein and Nucleic Acid Facility. Paired-end libraries were prepared using the Tecan Trio RNA-seq kit for low-input samples with automation on the Agilent BRAVO. Custom AnyDeplete probes (Tecan) were added against the mitochondrial genome, HBB, EEFA1, MALAT1, and HSP90aa1 to improve sequencing depth of low and moderately expressed transcripts. Pooled libraries were sequenced on an Illumina NovaSeq 6000 S1 flowcell using a 200 cycle sequencing by synthesis kit.
Quantification and statistical analysis
Reads were aligned to a concatenation of the Homo sapiens and P falciparum genomes. Subsequent analysis of human and parasite gene expression was conducted separately. Differential gene expression analysis was performed using DESeq2. Gene list enrichment analysis was conducted using EnrichR36-38 and the MSigDB Hallmark 2020 gene sets.39-41 Comparison of the parasite transcriptome in erythroblast infection to the published transcriptome of the asexual stage parasites42 was conducted using Spearman correlation on gene lists ranked based on expression.
Further information on methods is available in the supplemental Appendix.
Results
P falciparum infects erythroblasts at all stages of terminal erythropoiesis
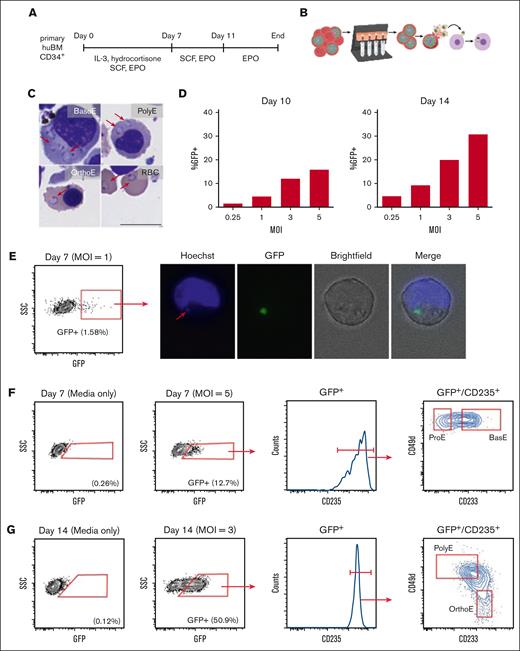
To investigate the infection of developing erythroblasts with P falciparum, we differentiated primary CD34+ cells from human bone marrow down the erythroid lineage using our previously published approach for ex vivo erythropoiesis (Figure 1A).43 We performed infections by incubating excess schizont-stage parasites with erythroblasts at 7, 10, or 14 days of ex vivo culture (Figure 1B). Ring-stage parasites were visible in the cytoplasms of BasoE, PolyE, and OrthoE on cytospins prepared after 18 or 20 hours of coculture, but typically dark staining of the cytoplasm precluded the observation of infection in ProE cells (Figure 1C). To measure parasitemia in the erythroblast cultures, we used flow cytometry after infection with a GFP-expressing parasite strain, D10-PfPHG.35 The percent of GFP+ erythroblasts was higher on day 14 infection than on day 10 of the infection, consistent with prior reports of robust infection in late-stage erythroblasts (Figure 1D). Cell sorting was used to confirm that the GFP+ population represented infected erythroblasts (Figure 1E).
P falciparum infects erythroblasts at all stages of terminal differentiation. (A) Schematic diagram summarizing our primary cell culture system for ex vivo erythropoiesis. (B) Schematic diagram depicting positive selection of late-stage P falciparum using a magnetic column before infection of erythroblast cultures. (C) May-Grünwald Giemsa staining showing ring-stage parasites in the cytoplasm of erythroid cells at multiple stages of terminal differentiation. Samples were collected from 18 to 20 hours after mixing with schizonts. Scale bars represent 10 μm. Arrows indicate ring-stage parasites. (D) Percentage of GFP+ erythroblasts measured via flow cytometry from 18 to 20 hours after mixing with schizonts from a GFP-expressing parasite strain, D10-PfPHG. Erythroblasts were infected on day 10 (left) or day 14 (right) of differentiation. (E) Gating for FACS of GFP+ erythroblasts 18 or 20 hours after infection with D10-PfPHG at multiplicity of infection = 1. Erythroblasts were infected on day 7 of differentiation. Fluorescence microscopy showing localization of GFP in an infected erythroblast sorted from the GFP+ population. Arrow indicates Hoechst staining overlapping the GFP. (F) Flow cytometry analysis showing transition of infected, day 7 erythroblasts into ProE and BasoE. (G) Flow cytometry analysis showing the transition of infected, day 14 erythroblasts into PolyE and OrthoE. EPO, erythropoietin; IL-3, interleukin-3; MOI, multiplicity of infection; RBC, red blood cell; SCF, stem cell factor; SSC, side scatter.
P falciparum infects erythroblasts at all stages of terminal differentiation. (A) Schematic diagram summarizing our primary cell culture system for ex vivo erythropoiesis. (B) Schematic diagram depicting positive selection of late-stage P falciparum using a magnetic column before infection of erythroblast cultures. (C) May-Grünwald Giemsa staining showing ring-stage parasites in the cytoplasm of erythroid cells at multiple stages of terminal differentiation. Samples were collected from 18 to 20 hours after mixing with schizonts. Scale bars represent 10 μm. Arrows indicate ring-stage parasites. (D) Percentage of GFP+ erythroblasts measured via flow cytometry from 18 to 20 hours after mixing with schizonts from a GFP-expressing parasite strain, D10-PfPHG. Erythroblasts were infected on day 10 (left) or day 14 (right) of differentiation. (E) Gating for FACS of GFP+ erythroblasts 18 or 20 hours after infection with D10-PfPHG at multiplicity of infection = 1. Erythroblasts were infected on day 7 of differentiation. Fluorescence microscopy showing localization of GFP in an infected erythroblast sorted from the GFP+ population. Arrow indicates Hoechst staining overlapping the GFP. (F) Flow cytometry analysis showing transition of infected, day 7 erythroblasts into ProE and BasoE. (G) Flow cytometry analysis showing the transition of infected, day 14 erythroblasts into PolyE and OrthoE. EPO, erythropoietin; IL-3, interleukin-3; MOI, multiplicity of infection; RBC, red blood cell; SCF, stem cell factor; SSC, side scatter.
We next investigated the stage-specific susceptibility of human erythroblasts to infection with P falciparum. We hypothesized that erythroblasts at all stages of terminal erythropoiesis are infectable by P falciparum, although not all stages may support growth to the schizont stage. To identify erythroblast stages, we used a panel of antibodies targeting surface proteins that distinguishes between stages (anti-CD235a, anti-CD233, and anti-CD49d26), as confirmed by using the cytospin of sorted populations (supplemental Figure 1). When erythroblasts were infected at 7 days of differentiation, we detected GFP+ ProE and BasoEs at 18 or 20 hours after infection (time from the addition of schizonts; Figure 1F). Infection at 14 days of differentiation resulted in GFP+ PolyE and OrthoE at 18 or 20 hours after infection (Figure 1G). Thus, parasites can establish infection in erythroblasts at all stages of terminal erythropoiesis.
RNA-seq after FACS reveals host cell responses to P falciparum infection of erythroblasts
To uncover stage-specific host cell responses to P falciparum infection of erythroid precursors, we designed and implemented a FACS-based RNA-seq strategy that could disentangle the effect of erythroblast maturity on transcriptomic measurements and distinguish the response of infected cells from their uninfected neighbors (Figure 2A). Primary human CD34+ cells were induced to proliferate and differentiate down the erythroid lineage. At 2 timepoints of differentiation, days 7 and 13, erythroblasts were removed from the culture and plated with D10-PfPHG schizonts in 6 replicate wells that served as biological replicates. Erythroblasts mixed with media only were included as a reference for erythroid development in the absence of P falciparum. A condition in which erythroblasts were incubated with dead, syringe-ruptured schizonts (SRSs) was also included to identify host responses due to parasite debris and metabolites released upon rupture. Cells were harvested 24 hours after infection and stained with a panel of antibodies targeting surface proteins that distinguishes between erythroblast stages. Using FACS, we isolated ProE, BasoE, PolyE, and OrthoE erythroblasts from 4 different exposure categories: media-only (media), SRS-exposed (SRS), P falciparum-infected (GFP+ infected), and P falciparum-exposed but uninfected (GFP– uninfected). After completion of the workflow, each population had at least 4 biological replicates for analysis (supplemental Data sets 1 and 2).
RNA-seq after FACS enables the study of host responses to P falciparum at the terminal stages of erythroid differentiation. (A) Schematic diagram of experimental design for characterizing transcriptional host responses to P falciparum in specific erythroblast populations. Erythroblasts were removed from culture on day 7 or day 14 and mixed with GFP-expressing P falciparum schizonts or an equivalent volume of media. After 24 hours, infected and uninfected erythroblast populations were collected via FACS for RNA extraction and transcriptomic analysis by RNA-seq. (B-C) Principal component analysis of RNA-seq data set. Marker shape indicates erythroblast population. Color represents infection condition. (D) Heatmap showing Spearman correlation between the parasite transcriptome in nucleated erythroblasts and the intraerythrocytic parasite transcriptome measured every 8 hours, as published by Otto et al.42 Colored annotations represent cell type, and each column represents a single replicate. Hrs, hours.
RNA-seq after FACS enables the study of host responses to P falciparum at the terminal stages of erythroid differentiation. (A) Schematic diagram of experimental design for characterizing transcriptional host responses to P falciparum in specific erythroblast populations. Erythroblasts were removed from culture on day 7 or day 14 and mixed with GFP-expressing P falciparum schizonts or an equivalent volume of media. After 24 hours, infected and uninfected erythroblast populations were collected via FACS for RNA extraction and transcriptomic analysis by RNA-seq. (B-C) Principal component analysis of RNA-seq data set. Marker shape indicates erythroblast population. Color represents infection condition. (D) Heatmap showing Spearman correlation between the parasite transcriptome in nucleated erythroblasts and the intraerythrocytic parasite transcriptome measured every 8 hours, as published by Otto et al.42 Colored annotations represent cell type, and each column represents a single replicate. Hrs, hours.
Principal component analysis based only on human gene counts of each sample highlighted the trajectory of erythroid development (Figure 2B-C). Samples from the SRS condition grouped with uninfected and media-only conditions (supplemental Figure 2). In contrast, infected populations at each erythroblast stage appeared distinctly grouped from the other stage-matched populations based on host gene expression (Figure 2B-C; supplemental Figure 2). From this preliminary analysis, we chose to focus our detailed analysis on the distinct host responses found in infected cells compared with those in uninfected cells and the media-only condition. Notably, we found that the proportion of reads mapping to the parasite genome in infected populations increased as erythroblasts matured, consistent with the dynamic reduction in transcriptional activity known to occur in the final stages of terminal erythropoiesis (supplemental Table 1). Comparison of our RNA-seq data of P falciparum with published data of P falciparum gene expression during asexual growth in erythrocytes42 showed a strong correlation with the ring-stage of development and did not appear to differ based on the erythroblast stage (Figure 2D).
Infection with P falciparum induces stage-specific changes in the host transcriptome at all stages of terminal erythropoiesis
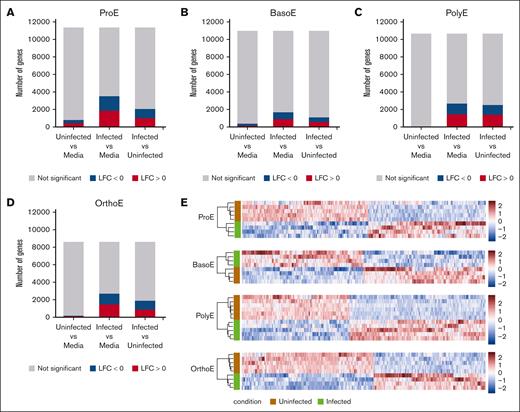
To explore the host cell response to P falciparum infection of the erythroid precursors, we applied differential gene expression analysis to pairwise comparisons of media-only, uninfected, and infected populations at each erythroblast stage (supplemental Data sets 3-6). As expected, the total number of detected human genes decreased over each consecutive stage of differentiation to reflect an increasingly specialized transcriptome (Figure 3A-D). We found that <4% of the transcriptome was differentially expressed between uninfected ProE (cells exposed to but not infected by P falciparum) and media-only conditions, and <2% of the transcriptome was differentially expressed at other erythroblast stages. These results suggest that the erythroblasts had minimal transcriptional responses when exposed to infected cells and parasite debris, at least at the 24-hour time points used in these experiments.
Differential gene expression detected in erythroblasts infected with P falciparum. (A-D) Summaries of differential gene expression analysis for the pairwise comparison of populations by infection condition within each erythroblast cell type. Color indicates genes with log fold change >0 (red), log fold change <0 (blue), or nonsignificant fold change (gray). P values were adjusted using the method of Benjamini and Hochberg and the threshold for significance set at .05. (E) Heatmaps showing z score–normalized expression of significant DEGs (infected vs uninfected) for each cell type. LFC, log fold change.
Differential gene expression detected in erythroblasts infected with P falciparum. (A-D) Summaries of differential gene expression analysis for the pairwise comparison of populations by infection condition within each erythroblast cell type. Color indicates genes with log fold change >0 (red), log fold change <0 (blue), or nonsignificant fold change (gray). P values were adjusted using the method of Benjamini and Hochberg and the threshold for significance set at .05. (E) Heatmaps showing z score–normalized expression of significant DEGs (infected vs uninfected) for each cell type. LFC, log fold change.
In contrast, direct infection with P falciparum produced host responses distinct from those found in uninfected erythroblasts or those exposed only to media (Figure 3A-E). Infection of ProEs resulted in the differential expression of 18% of detected genes compared with that in the uninfected population (Figure 3A). Only 10% of genes detected in BasoEs were differentially expressed, whereas we found differential expression of 24% in the PolyE transcriptome and 22% in the OrthoE transcriptome (Figure 3B-D). These findings suggest that the sensitivity of erythroblasts to parasite-induced transcriptional perturbation may vary based on the developmental stage. The proportion of upregulated and downregulated genes of the total differentially expressed genes (DEGs) was roughly equal regardless of the erythroblast stage (Figure 3E).
To dissect patterns of differential gene expression across cell types, we used set analysis to visualize the intersection of DEGs found by comparing the infected and uninfected transcriptomes of each erythroblast stage (Figure 4A-B). The intersection of DEGs was considered separately for upregulated (Figure 4A) and downregulated genes (Figure 4B). At least half the DEGs detected in each erythroblast stage, regardless of whether the DEGs had high or lower expression in infected erythroblasts, were unique to a single erythroblast stage. In contrast, only 27 DEGs were common to all 4 erythroblast stages. This result likely reflects the global changes in the composition of the erythroblast transcriptome over differentiation and suggests that the response to P falciparum correlates with stage-specificity rather than a general, cell type–independent response to an insult. The largest intersection in DEGs was observed between PolyE and OrthoE. A total of 587 DEGs were unique to these 2 late erythroblast populations, whereas only 171 DEGs were common to ProE and BasoE. This could be due to fewer DEGs detected at the BasoE stage or a consequence of stage-specific differences related to the host response in early erythroblasts that are less pronounced at later stages.
Erythroblasts have shared and distinct host responses to P falciparum at different points along the erythroid trajectory. (A) Set intersections based on the cell type for DEGs that are more highly expressed in infected than in uninfected erythroblasts (log fold change >0). Color indicates the set intersection for day 7 ProE and BasoE (magenta), day 13 PolyE and OrthoE (orange), and all erythroblast populations (purple). (B) Set intersections for genes that have lower expression in infected than uninfected erythroblasts (log fold change <0).
Erythroblasts have shared and distinct host responses to P falciparum at different points along the erythroid trajectory. (A) Set intersections based on the cell type for DEGs that are more highly expressed in infected than in uninfected erythroblasts (log fold change >0). Color indicates the set intersection for day 7 ProE and BasoE (magenta), day 13 PolyE and OrthoE (orange), and all erythroblast populations (purple). (B) Set intersections for genes that have lower expression in infected than uninfected erythroblasts (log fold change <0).
Host responses in ProEs and BasoEs
Next, we used gene set analysis to characterize the host responses to P falciparum infection in ProE and BasoE, which differ from that in PolyE and OrthoE in the composition and complexity of the transcriptome as well as hemoglobin content, a key factor in parasite nutrition.44-47 We applied enrichment analysis to identify cellular processes associated with differential gene expression in infected compared with uninfected erythroblasts (Figure 5A; supplemental Data set 7). EnrichR was used to determine whether the genes associated with processes in the MSigDB Hallmark 2020 gene set library were overrepresented among DEGs. Our analysis revealed that upregulated genes in the host response to the infection of ProEs and BasoEs are significantly enriched for genes associated with E2F targets, G2M checkpoint, heme metabolism, and the mitotic spindle (Figure 5A). None of the gene sets enriched among downregulated genes were common to ProEs and BasoEs.
Host responses to infection with P falciparum in early erythroblasts involve genes implicated in cell cycle regulation and dyserythropoiesis. (A) Enrichment analysis results using MSigDB Hallmark gene sets with DEGs (infected vs uninfected) detected in ProE and BasoE populations. Analysis of DEGs with log fold change > 0 (UP), and analysis of DEGs with log fold change < 0 (DOWN) are shown. Size indicates significance. Color indicates the odds ratio based on a Fisher exact test. (B) Expression of GDF15 and (C) NUF2 in ProE and BasoE populations. (D) Expression of cell cycle–related genes in ProEs and (E) BasoEs. (F-G) Expression of genes encoding ribosomal proteins in ProEs and BasoEs. Data are shown as median with 95% confidence intervals. cpm, counts per minute; TNF, tumor necrosis factor.
Host responses to infection with P falciparum in early erythroblasts involve genes implicated in cell cycle regulation and dyserythropoiesis. (A) Enrichment analysis results using MSigDB Hallmark gene sets with DEGs (infected vs uninfected) detected in ProE and BasoE populations. Analysis of DEGs with log fold change > 0 (UP), and analysis of DEGs with log fold change < 0 (DOWN) are shown. Size indicates significance. Color indicates the odds ratio based on a Fisher exact test. (B) Expression of GDF15 and (C) NUF2 in ProE and BasoE populations. (D) Expression of cell cycle–related genes in ProEs and (E) BasoEs. (F-G) Expression of genes encoding ribosomal proteins in ProEs and BasoEs. Data are shown as median with 95% confidence intervals. cpm, counts per minute; TNF, tumor necrosis factor.
Notably, we also uncovered the differential expression of genes implicated in the regulation of erythropoiesis in health and disease. GDF15, a member of the transforming growth factor–β superfamily and marker of ineffective erythropoiesis,48-50 was upregulated in infected ProEs (2.1-fold; Padj = 8.6 × 10−7) and BasoEs (1.8-fold; Padj = 2.9 × 10−6) compared with that in uninfected neighboring cells (Figure 5B). Among downregulated genes in infected ProEs and BasoEs, we found several genes encoding ribosomal proteins. Decreased expression of transcripts for ribosomal proteins included RPS19 and RPL28, genes implicated in the pathogenesis of hereditary Diamond-Blackfan anemia (DBA)51,52 (Figure 5C-D).
Perturbation of gene expression associated with the cell cycle and cell division was a common signature of infection. Upregulation of NUF2, a component of the kinetochore that is crucial for nuclear division,53-55 was common to both ProEs and BasoEs (twofold; Padj= 6.5 × 10−10; Figure 5E). Although the full set of genes associated with cell cycle and division function differed between ProEs and BasoEs, the host response of both cell types involved increased expression of regulators of cell division. In ProE, these included the mitotic regulator STIL56 (1.4-fold; padj = 7.4 × 10−11), centriole elongator regulator C2CD357 (1.6-fold; Padj = 1.2 × 10−7), and spindle-assembly regulator BUB1B58 (1.4-fold; Padj= 1.2 × 10−5; Figure 5F). In BasoEs, we found the upregulation of cyclin B1 CCNB1 (1.7-fold; Padj = 3.9 × 10−5), cyclin-dependent kinase inhibitor CDKN3 (twofold; Padj= 4.0 × 10−5), and mitotic regulator NEK2 (2.4-fold; Padj = 1.6 × 10−7; Figure 5G). Together, the observed host responses indicate that P falciparum infection at the start of terminal differentiation induces host responses that are common to the pathogenesis of dyserythropoiesis in anemia of inflammation as well as hereditary disorders of hematopoiesis.
Host responses in PolyEs and OrthoEs
We hypothesized that PolyEs and OrthoEs would respond differently to infection than early erythroblasts, reasoning that terminal differentiation would affect host defenses and capacity to support parasite development. Enrichment analysis uncovered gene sets that were overrepresented in the host response of PolyEs and OrthoEs to direct P falciparum infection, compared with uninfected neighboring cells (Figure 6A; supplemental Data set 8). The upregulated DEGs in infected PolyEs were enriched for genes belonging to the apoptosis and protein secretion gene sets, whereas downregulated DEGs were enriched for genes related to E2F targets, G2-M checkpoint, and the mitotic spindle. In contrast, members of the E2F targets, G2-M checkpoint, and Myc targets V1 and V2 were overrepresented in DEGs upregulated in infected OrthoEs. Both populations of late erythroblasts upregulated genes associated with mammalian target of rapamycin complex 1 signaling, the p53 pathway, tumor necrosis factor–α signaling via the NF-κB pathway, the unfolded protein response, and the UV response. In addition, we found the downregulation of genes involved in heme metabolism and management of reactive oxygen species. Both processes are crucial for red blood cell development.
Host responses to infection with P falciparum in late erythroblasts. (A) Enrichment analysis results using MSigDB Hallmark gene sets with DEGs (infected vs uninfected) detected in PolyE and OrthoE populations. Analysis of DEGs with log fold change > 0 (UP), and analysis of DEGs with log fold change < 0 (DOWN) are shown. Size indicates significance. Color indicates the odds ratio based on a Fisher exact test. (B-C) Expression of stress-related genes in PolyE and OrthoE populations. (D) Expression of MXI1, an enucleation factor, in PolyEs and OrthoEs. (E) Expression of FECH, encoding protoporphyrin ferrochetalase, in PolyEs and OrthoEs. Data are shown as median with 95% confidence intervals.
Host responses to infection with P falciparum in late erythroblasts. (A) Enrichment analysis results using MSigDB Hallmark gene sets with DEGs (infected vs uninfected) detected in PolyE and OrthoE populations. Analysis of DEGs with log fold change > 0 (UP), and analysis of DEGs with log fold change < 0 (DOWN) are shown. Size indicates significance. Color indicates the odds ratio based on a Fisher exact test. (B-C) Expression of stress-related genes in PolyE and OrthoE populations. (D) Expression of MXI1, an enucleation factor, in PolyEs and OrthoEs. (E) Expression of FECH, encoding protoporphyrin ferrochetalase, in PolyEs and OrthoEs. Data are shown as median with 95% confidence intervals.
Among the shared responses between PolyEs and OrthoEs were genes involved in proteotoxic stress and several genes essential for the final stages of erythroid differentiation. We found increased expression of DNAJA1, a member of the Hsp40 family of protein chaperones, in infected PolyEs (2.8-fold; Padj = 4.5 × 10−18) and OrthoEs (1.6-fold; Padj = 6.9 × 10−4; Figure 6B). Expression of the HSP90B1 gene, which encodes a chaperone whose client proteins include certain integrins and toll-like receptors,59 was also increased in infected PolyEs (2.7-fold; Padj = 8.7 × 10−21) and OrthoEs (3.6-fold; Padj = 9.8 × 10−8; Figure 6C). Perturbation of genes involved in healthy erythroid development was also evident through our analysis. MXI1, a critical factor for enucleation, was downregulated in PolyEs (1.7-fold; Padj = 3.6 × 10−11) and OrthoEs (4.9-fold; Padj = 4.2 2 × 10−50; Figure 6D). Knockdown of MXI1 expression in murine erythroblasts reduces enucleation and results in decreased expression of other genes involved in erythroid development, such as FECH1, the murine homolog of the human FECH gene.60 Expression of FECH, which encodes a key enzyme in heme biosynthesis, was, indeed, decreased 1.6-fold in infected PolyE (1.6-fold; Padj = 1.6 × 10−10) and greater than threefold in infected OrthoE (3.7-fold; Padj = 8.6 × 10−28; Figure 6E). Deficient activity of the ferrochetalase, encoded by FECH, is implicated in hereditary erythropoietic protoporphyria,61 highlighting the detrimental effect of perturbed heme metabolism on the host.
Erythroblasts at all stages upregulate HMOX1 in response to infection with P falciparum
Although many changes in the host transcriptome were dependent on the erythroblast stage, we found that direct infection induced changes to genes involved in mitigating oxidative stress in a stage-independent manner. HMOX1, which encodes heme oxygenase 1, was upregulated in each population of infected erythroblasts (Figure 7). We found a 3.6-fold increase in expression in infected ProEs (Padj = 3.9 × 10−9) and a 5.9-fold increase in infected BasoEs (Padj = 4.0 × 10−4). Expression of HMOX1 was increased 19-fold in infected PolyEs (Padj = 2.3 × 10−29) and 6.5-fold in infected OrthoEs (Padj = 1.6 × 10−27). These data show that HMOX1 expression is involved in the erythroblast response to P falciparum infection at all stages of terminal differentiation, adding to previous evidence that HMOX1 is a critical host factor in malaria infection.62-64
Upregulation of HMOX1 in erythroblasts directly infected with P falciparum. Expression of HMOX1, encoding heme oxygenase-1, in each erythroblast population. Data are shown as median with 95% confidence intervals.
Upregulation of HMOX1 in erythroblasts directly infected with P falciparum. Expression of HMOX1, encoding heme oxygenase-1, in each erythroblast population. Data are shown as median with 95% confidence intervals.
Genes encoding parasite effectors that are exported into the host cytoplasm are transcribed in erythroblast infection
P falciparum extensively modifies the host red blood cell and hepatocytes by exporting effectors into the host cytoplasm,65-67 and related parasites are known to modulate host gene expression through effectors that traffic to the host cell nucleus.68,69 We hypothesized that the same might be true in P falciparum during infection of nucleated erythroblasts, so we queried our RNA-seq data set for the transcription of genes encoding the predicted intraerythrocytic exportome of P falciparum.42,70 We found that of 245 transcripts of exported genes expressed at 8 or 16 hours after invasion in red blood cells, 185 were expressed in parasites in at least 1 of the 4 erythroblast stages (supplemental Table 2). Among those detected were genes encoding well-studied examples of exported effectors, including ring-infected erythrocyte surface antigen (PF3D7_0102200), knob-associated histidine-rich protein (PF3D7_0202000), and members of the FIKK kinase family. These data provide a foundation for further study of effector export during infection of erythroblasts by P falciparum.
Discussion
Although dyserythropoiesis has long been recognized as a contributing cause in severe malarial anemia, little is understood about the host-parasite interactions that lead to perturbed erythroid development. In this study, we used ex vivo erythropoiesis of primary human bone marrow CD34+ HSPCs to study transcriptional host responses to infection by P falciparum asexual parasites at specific stages of erythroid development. Using a small panel of erythroid surface markers enabled us to sort and sequence the transcriptome of infected and uninfected erythroblast populations at 4 distinct stages of terminal differentiation.
We found few DEGs from the comparison of uninfected cells to cells cultured in media without stimulus or SRS. This result suggests that ∼20 or 22 hours after invasion of erythroblasts, transcriptional changes due to signaling from infected cells to neighboring uninfected cells or from exposure to releasate from ruptured schizonts were negligible. This type of paracrine signaling may be more relevant at earlier time points, and it is possible that activity by the parasite silences signaling from infected cells to uninfected neighbors, as has been found in a related member of the apicomplexan parasite family, Toxoplasma gondii.69 Although Lamikanra et al observed differential gene expression between hemozoin-treated erythroblasts and controls, the concentration of hemozoin used was much greater than that generated by the multiplicity of infection used in this study.34 Further work is warranted to better understand the implications for erythroid development from the presence of hemozoin and other inflammatory byproducts of schizont rupture in the bone marrow.
Many of the DEGs we found in infected erythroblasts compared with those in uninfected cells are implicated in hereditary anemias whose pathogenesis involves disordered erythropoiesis. Expression of GDF15, a regulator of stress erythropoiesis, could have systemic effects on regulation of iron metabolism, similar to what is found in patients with β-thalassemia.71 We also found altered expression of transcripts encoding ribosomal proteins, a hallmark of DBA. DBA is an inherited condition caused by mutations in genes that encode ribosomal proteins and characterized by macrocytic anemia, physical abnormalities, and a lack of erythroid precursors in bone marrow with otherwise normal cellularity.52 Decreased expression of RPS19, a common etiology of DBA, has been shown to reduce in vitro proliferation of erythroid progenitor cells through activation of the p53 pathway and cell cycle arrest.51,72 Clinical phenotypes in DBA can be heterogeneous, including mild anemia associated with disordered differentiation of erythroid progenitors rather than complete erythroid arrest.73 Recent work regarding the regulation of hematopoiesis has underscored that not only does expression of individual ribosomal components affect cell development, but the abundance of ribosomes can determine cell fate in stem cell precursors.74 Although the pathological effects of RPS mutations occur at earlier stages than we studied, a similar mechanism in which impaired ribosomal function or reduction in abundance at a time of high demand for the translation of specific proteins may affect critical developmental processes in malaria-infected bone marrow. Disruption of the sensitive balance between proliferation of precursor cells and erythroid commitment and differentiation, further evidenced by differential expression of genes associated with the cell division, could have a similar detrimental effect on the production of healthy erythrocytes in this context.
Our study also indicates that parasite infection induces changes in expression of genes related to protein-folding pathways and factors that mitigate proteotoxic stress. In infected PolyEs and OrthoEs, we found increased transcription of genes encoding protein chaperones such as DNAJA1 and HSP90B1. Protein chaperones function at several critical steps in erythropoiesis from shuttling cyclins into the nucleus and cooperating with cyclin-dependent kinases to regulate cell division to facilitating globin folding and assembly and preventing premature apoptosis in response to the unusual demands of remodeling the proteome in erythrocytic maturation.75 As such, dysregulation of members of the HSP70 family and other molecular chaperones is often implicated in disordered erythropoiesis. In DBA, mutations in RPL5 or RPL11 result in decreased levels of HSP70, leading to excessive degradation of GATA1 and defects in proliferation, differentiation, and regulation of apoptosis.76 Overexpression of HSP70 rescues DBA progenitors by increasing GATA1 levels and reducing free heme by restoring normal hemoglobin synthesis.77 HSP90 is also linked to the protection from cellular stress as a chaperone of FANCA, which participates in the DNA damage response. Mutation of FANC genes is the underlying etiology in Fanconi anemia, a rare disorder characterized by genomic instability, bone marrow hypocellularity, and subsequent pancytopenia of the 3 blood cell lineages.78 The increased transcription of genes encoding heat shock proteins suggest that Plasmodium infection of late erythroblasts may produce genotoxic or proteotoxic effects that result in dyserythropoiesis. Although further experiments are needed to evaluate the extent of damage to the cell during infection, increased transcription suggests a cell intrinsic host response aimes at protecting developing erythroblasts from detrimental effects of infection.
In the peripheral blood, P falciparum extensively modifies infected red blood cells by exporting proteins from the parasitophorous vacuole where the parasite resides after invasion and throughout its development.67,Plasmodium has also been shown to export proteins into the hepatocyte cytoplasm during the liver stage of infection, some of which reach the host cell nucleus, although the full exportome and activity of effectors remain largely unknown.65,66 It is possible that Plasmodium effectors are similarly involved in mediating host cell responses to infection and transforming the erythroblast into a hospitable environment for gametocytogenesis. Given that we observed differential expression of genes that regulate enucleation and heme metabolism, it is tempting to speculate that parasite activity is involved in physical and metabolic modification of the host cell.
In this study, we demonstrated that erythroblasts have specific transcriptional responses to infection with P falciparum at distinct stages of erythropoiesis. Moreover, direct infection with P falciparum induces differential expression of genes related to cell cycle regulation and key erythroid developmental processes in addition to a wide range of stress responses. The results presented here provide a foundation for further investigation of the impact of malaria infection on erythropoiesis and the pathogenesis of malaria anemia. A more complex model of the hematopoietic niche that better mimics the microenvironment of erythroblastic islands and a single cell approach to transcriptomics could reveal the broader impact of parasite infection on the development of blood cell lineages in the hematopoietic niche.20 Genetic manipulation of ex vivo differentiated erythroblasts could be used to interrogate host factors identified in this study or the effect of naturally occurring genotypes in regions of malaria endemicity, like sickle hemoglobin, on erythroblast responses to P falciparum. Further elaboration of the interactions between host and parasite in the bone marrow may yield strategies for mitigating harm to host cell development and limiting the bone marrow as a reservoir for growth and development of the parasite.
Acknowledgments
The authors thank David Schneider, Anupama Narla, John Boothroyd, and members of the Egan, Boothroyd, and Yeh labs for helpful discussions. The authors thank Stanford University and the Stanford Research Computing Center for providing computational resources and support that contributed to these research results. Some of the computing for this project was performed on the Sherlock and Stanford SCG Informatics clusters. Parts of Figures 1B and 2A were created with BioRender.com.
This work was supported, in part, by National Institutes of Health grants T32GM007276 (T.P.F.) and DP2HL13718601 (E.S.E.), and the Stanford Maternal and Child Health Research Institute. E.S.E. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund and is a Chan Zuckerberg Biohub (San Francisco, CA) investigator and a Tashia and John Morgridge–endowed faculty scholar of the Stanford Maternal and Child Health Research Institute. This work used the Genome Sequencing Service Center by Stanford Center for Genomics and Personalized Medicine Sequencing Center, supported by National Institutes of Health grant S10OD020141.
Authorship
Contribution: T.P.F. and E.S.E. conceptualized the study and wrote the final draft of the manuscript; T.P.F. analyzed and visualized the data and wrote the initial draft of the manuscript; E.S.E. provided the resources; E.S.E. supervised the study and acquired funds; and all authors performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth S. Egan, Department of Pediatrics, Stanford University School of Medicine, 240 Pasteur Dr, BMI 2400, Stanford, CA 94305; e-mail: eegan@stanford.edu.
References
Author notes
Raw sequencing data can be found in the National Center for Biotechnology Information Gene Expression Omnibus database (accession number GSE233739).
All data generated or analyzed during this study will be found in the data supplements available with the online version of this article.
Custom scripts used for processing from raw data to tabulated gene counts can be found in the repository at GitHub: https://github.com/t-feldman/transcriptomics-malaria-infected-erythroblast
The full-text version of this article contains a data supplement.