Key Points
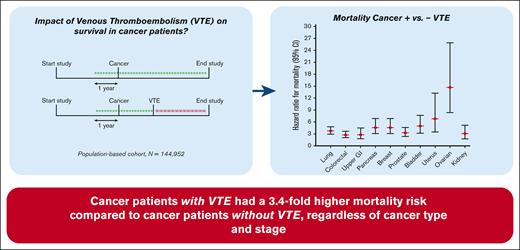
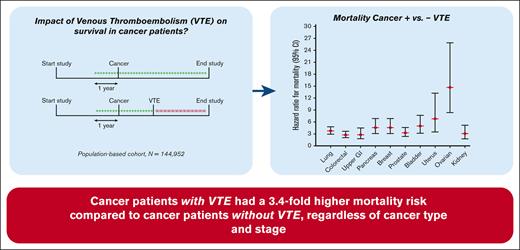
The risk of death for patients with cancer-related VTE was increased by 3.4-fold, compared with patients with cancer but without VTE.
The occurrence of VTE increased the mortality risk consistently throughout different cancer types, independent of tumor severity.
Abstract
Patients with cancer have an increased risk of developing venous thromboembolism (VTE), and this combination is reported to result in poorer survival compared with cancer alone. This study aimed to investigate the impact of VTE on the survival of patients with cancer in a general population. The Scandinavian Thrombosis and Cancer (STAC) cohort, a population-based cohort including 144 952 participants without previous VTE or cancer, was used. During follow-up, cancer and VTE incidences were registered. “Cancer-related VTE” was defined as VTE diagnosed in patients with overt or occult cancer. The survival of participants without cancer and/or VTE (“disease-free”) was compared with the survival of participants with cancer and cancer-related VTE. Cox regression models with cancer and VTE as time-varying exposures were performed to calculate hazard ratios for death. Subanalyses were performed across cancer types and stages and VTE type (deep vein thrombosis or pulmonary embolism). During follow-up (mean, 11.7 years), 14 621 participants developed cancer, and 2444 developed VTE, of which 1241 were cancer-related. The mortality rates (per 100 person years) for disease-free participants, VTE only, cancer only, and cancer-related VTE were 0.63, 5.0, 9.2, and 45.3, respectively. Compared with patients with cancer only, the risk of death for patients with cancer-related VTE was increased 3.4-fold. Within all cancer types, the occurrence of VTE increased the mortality risk 2.8- to 14.7-fold. In a general population, patients with cancer with VTE had a 3.4-fold higher mortality risk than patients with cancer without VTE, independent of cancer type.
Introduction
The risk of developing venous thrombosis, comprising deep vein thrombosis (DVT), and pulmonary embolism (PE), is reported to be ninefold higher for patients with cancer compared with the general population.1 Of all first-time venous thromboembolism (VTE) events, 20% to 30% are estimated to be cancer-associated.2 However, VTE is a multifactorial disease, and the exact size of the risk depends on many factors.2 These risk factors can be divided into individual patient- and cancer-associated risk factors.3 Individual patient risk factors include age, ethnicity, comorbidities, inherited prothrombotic conditions, or a prior history of VTE.3 As for the cancer-associated risk factors, a high VTE incidence is especially reported in the first few months after cancer diagnosis,4 in case of metastatic diseases4,5 and in association with biologically aggressive tumors like pancreas, brain, and lung tumors.6 Furthermore, factors related to the treatment of the cancer can also influence the development of VTE. Surgery, hospitalization, and immobilization have been shown to increase the risk, and patients receiving chemotherapy have a sixfold to sevenfold increased risk of VTE development.3,7
The presence of both VTE and cancer in a patient is an unfavorable combination of events. Patients with cancer with VTE experience high rates of bleeding in the presence of anticoagulant treatment as well as recurrent thrombotic disease.8 The 1-year cumulative incidence of major bleeding for patients with cancer and thrombosis has been reported to be as high as 10% to 15% when receiving anticoagulation.9
Moreover, VTE in patients with cancer is not only associated with increased morbidity but also with increased mortality. Mortality studies have shown that patients with cancer with VTE have decreased survival rates compared with matched control patients with cancer without VTE.10,11 Low survival rates have particularly been reported for patients who were diagnosed with cancer and VTE at the same time: the 1-year survival rate for these patients was 12%, compared with 36% in patients with only cancer.11 The occurrence of VTE in the first year after cancer diagnosis was a predictor of decreased survival, with hazard ratios (HRs) ranging from 1.6 to 4.2 for diverse cancer types.5 In addition, patients in whom cancer was diagnosed within 1 year after a VTE, probably representing patients with occult cancer at the time of the VTE diagnosis, also had a lower 1-year survival rate (38%) than patients without a VTE in the preceding year (47%).11 This observation is well-established but still poorly understood for different cancer types and cancer stages.
Here, we used a large population-based cohort to determine the impact of VTE on the long-term survival of patients with cancer, considering time between diagnoses as well as different cancer types and stages and the type of VTE.
Methods
Study population and data collection
Three prospective population-based Scandinavian cohorts (Tromsø IV Study, Diet, Cancer and Health Study, and Hunt II Study) were merged to form the Scandinavian Thrombosis and Cancer Study (STAC cohort). Details of these cohorts are described elsewhere.12 The Tromsø IV study included 27 158 participants (77% of the eligible population) between 25 and 97 years of age living in Tromsø, the largest city in Northern Norway (about 70 000 inhabitants in total).
The HUNT2 study included 65 237 participants (69.5% of the eligible population) aged 20 years or older living in the Nord-Trondelag county in central Norway (between 12 000 and 21 000 inhabitants in total). The Diet Cancer and Health (DCH) study included 57 054 participants (35% of the eligible population) from 50 to 64 years of age living in Aarhus or Copenhagen county in Denmark (about 700 000 inhabitants in total).
Inclusion ranged from 1993 to 1997. All participants were free from VTE and cancer at baseline. In all 3 studies, data (including information about smoking, body mass index, diabetes, and use of antihypertensive medication) were collected by means of questionnaires, physical examinations, and blood samples, as described before.12 For follow-up with regard to mortality and cancer incidence, records were linked to central person registers, registers of causes of death, and the national cancer registries in Norway and Denmark. Cancer diagnoses were coded into ICD10 groups. Non-melanoma skin cancer was coded as non-cancer. Only first-time and objectively confirmed symptomatic VTE events were included in the STAC cohort. In brief, a broad search was performed using ICD codes, followed by validation of medical records or discharge letters or diagnostic images using predefined criteria.9,12 VTE events were furthermore categorized into DVT and PE.12
Follow-up and statistical analysis
During follow-up, incident cancer and VTE cases (only first diagnoses) were registered. Follow-up started at the day of inclusion and ended on the date of emigration, date of death, or date of last VTE registration (31 December 2012, 30 April 2008, and 31 December 2007 for Tromsø, DCH, and Hunt, respectively), whichever occurred first.
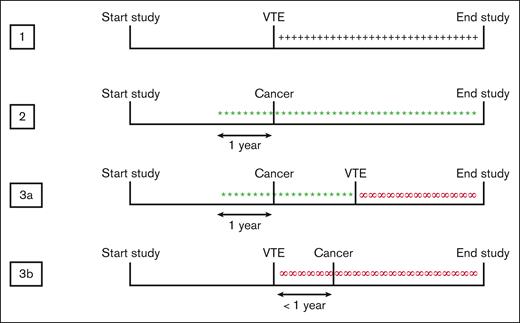
Person years (pys) from all participants in the cohort were divided into 4 possible exposure categories, namely, “disease-free,” “VTE-exposed,” “cancer-exposed,” and “cancer-related VTE–exposed” pys (Figure 1). For all the cancer-exposed participants, the date of diagnosis was brought forward by 1 year, assuming that the cancer was already present in the patient 1 year before the diagnosis and therefore to take the unknown time between the start of the tumor growth and cancer diagnosis into account. Based on this assumption, VTE was both classified as “cancer-related” if it was diagnosed in a patient with known cancer or if the cancer was diagnosed within 1 year before or after the VTE. Participants who got diagnosed with cancer more than 1 year before or after VTE were classified as “cancer” from that time on, unrelated to VTE. In the same manner, participants who got diagnosed with VTE more than 1 year before or after cancer were classified as “VTE” from that time on, unrelated to the cancer. Participants who received a diagnosis of cancer and VTE on the same day were classified as “cancer-related VTE” from that time point onward, and the year before as having cancer alone. As patients who were diagnosed with VTE at the day of death would not be counted as VTE-exposed, the date for VTE diagnosis for all VTE-exposed participants was also corrected and brought forward by 1 day. This specific time frame was chosen because it was estimated that the VTE would be present during at least 1 day before diagnosis.
Classification of exposure categories. Participants were divided into 3 different exposure categories: VTE-exposed, cancer-exposed, and cancer-related VTE–exposed. The date of cancer diagnosis was brought forward by 1 year, assuming that the cancer is already present 1 year before diagnosis. 1, +++ VTE-exposed; 2, ∗∗∗ cancer-exposed; 3a+b, ∞∞∞ cancer-related VTE–exposed.
Classification of exposure categories. Participants were divided into 3 different exposure categories: VTE-exposed, cancer-exposed, and cancer-related VTE–exposed. The date of cancer diagnosis was brought forward by 1 year, assuming that the cancer is already present 1 year before diagnosis. 1, +++ VTE-exposed; 2, ∗∗∗ cancer-exposed; 3a+b, ∞∞∞ cancer-related VTE–exposed.
Summary statistics of the baseline characteristics are shown as numbers (percentages) or mean ± standard deviation. Incidence rates per 100 pys were calculated for VTE, per cancer exposure category. Mortality rates were calculated for the different exposure groups. A time-dependent sex- and age-adjusted Cox regression was performed to calculate HRs for death in the different exposure groups.
Four subanalyses were performed. In the first subanalysis, VTE-exposed pys were split into PE- and DVT-exposed pys. Patients in whom the type of VTE (n = 34) was unknown were excluded from this analysis. In the second subanalysis, pys classified as exposed to cancer were further categorized into 11 major ICD10 cancer groups (lung, colorectal, upper GI [gastrointestinal tract], pancreatic, breast, prostate, bladder, uterus, ovarian, cervical, and kidney cancer). Patients with a cancer type different from 1 of these groups were excluded from this analysis. For the third subanalysis, cancer groups were split up by stage of cancer at diagnosis. The cancer stage was categorized as localized, regional spread, distant metastasis, or unknown, according to the classification system used in Denmark and Norway.13
Only categories with >10 events present were included in our analyses; therefore, only lung and colorectal cancer were studied in the third subanalysis. Finally, a sensitivity analysis was performed, consisting of patients in whom cancer was diagnosed >1 year after VTE diagnosis, who were counted as a separate exposure category.
STATA version 12.1 (Stata Corporation, College Station, TX) was used for all statistical analyses.
Results
Baseline characteristics
The STAC cohort included 144 952 participants free of cancer and VTE at baseline. Mean age at inclusion was 51.9 years, and 47.4% of participants were male. The median follow-up from start to end of the study was 11.7 years (range, 0-18.3). Baseline characteristics of the included patients are shown in Table 1.
During the follow-up period, 14 621 participants were diagnosed with cancer, and 2444 participants were diagnosed with a VTE (993 PE, 1417 DVT, and 34 unspecified) (Table 2). Of the 2444 VTEs, 1241 (50.8%) were cancer-related (in 113 participants, cancer was diagnosed within 1 year after the VTE; the other 1128 events occurred in patients with known cancer). Another 124 VTE events were diagnosed more than 1 year before cancer diagnosis, so these were classified as only VTE-exposed. The most common cancer types were prostate (2387), breast (2232), and colorectal cancer (2144). A total of 37.4% of the cancers were localized, 25.9% of patients had regional metastasis, 15.8% of patients had distant metastasis, and for 20.9% of cancers, the information regarding the stage was missing or unknown. In total, 17 194 patients died during a total of 1 699 625 pys. The mortality rate for the overall cohort was 1.0 per 100 pys.
Incidence of VTE
Supplemental Table 1 shows the incidence rates (IR) per 100 pys for VTE in all cancer types. The median follow-up time between cancer diagnosis and VTE diagnosis was 1.2 years (range, –12.8 to 17.3). Overall, the IR for VTE in cancer patients was 4.8 per 100 pys (95% confidence interval [95% CI], 4.7-4.9). The highest rates were found for upper GI cancer (IR, 8.1 [95% CI, 7.3-8.9]), pancreatic cancer (IR, 8.0 [95% CI, 7.1-8.7]), and lung cancer (IR, 7.4 [95% CI, 6.9-7.8]). The lowest incidences were seen in breast cancer (IR, 1.2 [95% CI, 1.1-1.3]), uterine cancer (IR, 1.7 [95% CI, 1.4-2.1]), and prostate cancer (IR, 2.0 [1.9-2.1]).
Mortality rates
The median follow-up between the time of VTE diagnosis and date of death was 0.7 years (range, 0-16.6). The mortality rate in disease-free participants was 0.64 per 100 pys (95% CI, 0.62-0.65) (Table 3). Patients with VTE had a mortality rate of 5.0 per 100 pys (95% CI, 4.6-5.5), whereas patients with cancer had a mortality rate of 9.2 per 100 pys (95% CI, 9.0-9.5). Patients with cancer-related VTE had the highest mortality rate of 45.3 per 100 pys (95% CI, 41.1-50.0). In comparison with disease-free participants, the age- and sex-adjusted HRs for mortality for patients with VTE alone, with cancer alone, and with cancer-related VTE were 3.2 (95% CI, 2.9-3.5), 7.2 (95% CI, 7.0-7.4), and 24.9 (95% CI, 22.2-27.2), respectively (Table 3). Compared with patients with cancer, the HR for patients with cancer-related VTE was 3.4 (95% CI, 3.1-3.8) Adjustment for smoking, body mass index, diabetes, and use of antihypertensive medication led to HRs of 3.2 (95% CI, 2.9-3.6), 7.2 (6.9-7.4), and 25.4 (22.9-28.1) for VTE only, cancer only, and cancer-related VTE, respectively, compared with disease-free participants (Table 3). The same adjustment did not attenuate the HR for patients with cancer-related VTE vs cancer alone either (3.5 [95% CI, 3.2-3.9]).
Incidence of mortality stratified per VTE type
To determine if the effect of a VTE was different per VTE type, a distinction was made between patients with a PE diagnosis and patients with a DVT diagnosis. Patients who experienced PE without cancer were found to have a higher relative risk of death than patients who experienced DVT (HR, 3.8 [95% CI, 3.3-4.4] and 2.8 [95% CI, 2.5-3.2], respectively), compared with disease-free participants) (supplemental Figure 1; supplemental Table 2). In contrast, the mortality risk for patients with cancer-related PE was found to be lower than for patients with cancer-related DVT (HR, 20.5 [95% CI, 17.3-24.1] and 26.4 [95% CI, 23.3-30.0], respectively, compared with disease-free participants) (supplemental Figure 1; supplemental Table 2). In addition, when the mortality rates of cancer-related PE and cancer-related DVT were compared with the rates of patients with cancer alone, the same pattern was observed with HRs of 2.8 (95% CI, 2.4-3.4) and 3.7 (95% CI, 3.2-4.2), respectively (supplemental Table 2).
Incidence of mortality stratified per cancer type and stage
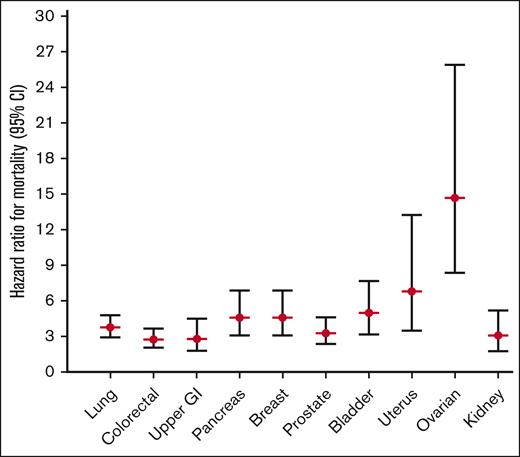
To study whether certain cancer types in combination with a VTE were differently related with the effect on mortality, we categorized all patients into 11 major cancer types, as specified in the methods section. A total of 3641 patients (24.9%) diagnosed with a cancer type different from the 11 major types were subsequently excluded from subanalyses 2, 3, and 4. Cancer types associated with the highest mortality in the absence of VTE were lung cancer (mortality rate 34.0 per 100 pys [95% CI, 32.2-36.0]; HR, 26.6 [95% CI, 25.1-28.2] compared with disease-free participants) and pancreatic cancer (mortality rate 50.6 per 100 pys [95% CI, 45.7-56.1]; HR, 33.5 [95% CI, 30.2-37.2] compared with disease-free participants) (Figure 2; supplemental Table 3). If a cancer-related VTE occurred for 1 of these 2 cancer types, mortality rates increased up to 112.0 (95% CI, 89.5-140.0) and 197.0 (95% CI, 134.1-289.3) per 100 pys, and HRs compared with disease-free participants increased to 101.5 (95% CI, 81.1-127.1) and 155.1 (95% CI, 105.4-228.2) for lung and pancreatic cancer, respectively (Figure 2; supplemental Table 3). Breast cancer was the cancer type associated with the lowest mortality (mortality rate 2.4 per 100 pys [95% CI, 2.2-2.7]; HR compared with disease-free participants, 3.1 [95% CI, 2.7-3.4]). Still, even breast cancer, with its low absolute mortality rates, showed a high increase in mortality rate in the presence of a related VTE, that is, up to 26.0 per 100 pys (95% CI, 18.0-37.7) and the HR compared with disease-free participants increased to 14.1 (95% CI, 9.7-20.4). The same increased mortality risk when a cancer-related VTE occurred was seen across all cancer types studied when compared with patients with cancer alone, with HRs ranging from 2.8 in upper GI cancer (95% CI, 1.8-4.5) and colorectal cancer (95% CI, 2.1-3.7) to 14.7 (95% CI, 8.4-25.9) in ovarian cancer (Figure 2; supplemental Table 3).
Subanalysis 2: age- and sex-adjusted HRs (95% CI) for mortality for different types of cancer with VTE, compared with the same cancer type without VTE. Date of cancer corrected by 1 year.
Subanalysis 2: age- and sex-adjusted HRs (95% CI) for mortality for different types of cancer with VTE, compared with the same cancer type without VTE. Date of cancer corrected by 1 year.
Finally, when the cancers were further split up according to stage, the same increase in mortality risk was observed when cancer-related VTE occurred (supplemental Figure 2; supplemental Table 4). Only cancer types with >10 events could be analyzed; therefore, only the HRs for lung cancer and colorectal cancer are shown here. We do however see that within all 3 cancer stages studied, there is the same increase in mortality risk.
Incidence of mortality after 1-year follow-up
To determine if the 1-year time between cancer and the VTE event was chosen correctly, a sensitivity analysis was performed with patients in whom cancer was diagnosed >1 year after the VTE. Mortality rates of 13.8 per 100 pys (95% CI, 10.7-17.9) and a HR of 8.0 (95% CI, 6.1-10.3) were found compared with disease-free participants, and 1.1 (95% CI, 0.8-1.5) compared with patients with cancer alone (supplemental Table 5). Mortality rates and HRs for the other exposure groups did not change if these patients were counted as a separate exposure group.
Discussion
We studied mortality after the occurrence of cancer-related VTE in a large, unselected cohort of 144 952 participants, who were followed for a median period of 11.7 years. Several cancer types linked with VTE were studied, where we found the highest risks in upper GI tract cancer, pancreas cancer, and lung cancer. During this observation period, patients with cancer-related VTE had an almost 25-fold increased risk of death compared with disease-free participants and a 3.4-fold increased risk of death compared with patients with cancer alone. Here, the biologically most aggressive types of tumors (eg, pancreas and lung cancer) had the highest absolute mortality rates. These 2 cancer types and other aggressive tumors, such as brain or stomach cancer, are already known to be highly associated with the risk of developing VTE.14 However, we saw that the unfavorable prognosis for patients with cancer with a related VTE was not only present among these biologically aggressive cancer types, but instead that a cancer-related VTE consistently increased the risk by threefold to sixfold as compared with patients with cancer without VTE, within all cancer types except ovarian cancer, where this was even higher (14-fold). For example, breast carcinoma, although associated with a low absolute mortality rate, had a 4.6-fold increased risk of death when associated with VTE.
Other than distinguishing between cancer types, we also distinguished between a primary tumor, regional spread, and distant metastases of the tumor, but here only lung and colorectal cancer could be studied because of limited numbers. For lung cancer, the HRs for the primary tumor stage increased from a threefold increased risk up to an almost sixfold increased risk of mortality for patients with distant metastases, compared with patients with lung cancer having the same stage but no VTE. The HR for patients with regional spread was lower than that for primary tumors, which might be a chance finding. For colorectal cancer, the same trend was observed, with a smaller but still linear increase from a 2.5-fold increased mortality risk for local cancer to a 3.4-fold increased risk for patients with distant metastases. However, because the numbers of events in lung and colorectal cancer were small for the separate stages, all HRs were accompanied by large confidence intervals. We could not study the other 9 cancer types stratified by stage because of a limited number of events, but nevertheless, the devastating additional effect of VTE on mortality seemed consistent, and we have no reason to assume that this situation does not hold for the other types.
Both cancer-related PE and DVT were associated with an increased risk of death. Consistent with previous literature, we observed that the occurrence of PE was associated with a higher risk of death than DVT in patients without cancer, most likely because of the higher short-term mortality related to acute and severe PE. However, the opposite was observed among cancer patients with PE or DVT, where patients with cancer with DVT had a slightly higher subsequent mortality than those with PE. The distribution of DVT and PE among the aggressive and less aggressive cancer types did not differ and could therefore not explain this observation. Possibly, some PE events in cancer might have been incidental findings diagnosed in staging computerized tomography scans performed for cancer assessment and not for clinical diagnosis of possible PE. These relatively mild events would not lead to an increased mortality risk. Furthermore, it should be kept in mind that we studied the long-term association (the mean follow-up between VTE and mortality was 2 years), whereas the increased mortality risk of PE is generally limited to the first month(s).
Finally, a sensitivity analysis demonstrated that the time relation between the occurrence of VTE and cancer is of importance. Patients diagnosed with cancer more than 1 year after a preceding VTE had no increased risk of death compared with patients with cancer without any related VTE (HR, 1.1 [95% CI, 0.8-1.5] compared with patients with cancer alone). This endorsed our decision not to analyze these patients as a separate exposure group for the other analyses. This minimally elevated risk for patients with cancer >1 year after VTE was also reported before by Sorensen et al.11
Overall, patients with cancer with related VTE had an increased risk of mortality compared with patients with cancer alone. Interestingly, this threefold to sixfold increase was independent of and similar across all cancer types. The observations from our and previous studies raise the intriguing question of why VTE has such a detrimental additional effect in patients with active (or occult) cancer. For the pathophysiological mechanisms underlying the relation between cancer and VTE, there is some knowledge about factors that contribute, although none of these have been fully established yet. Direct activation of coagulation can occur via several factors upregulated and/or released by tumor cells, such as overexpression of prothrombotic factors (tissue factor or podoplanin) or neutrophil infiltration into the tumor microenvironment.3,15,16 Somatic tumor mutations (eg, STK11 or KRAS) have also been linked as risk factors for cancer-associated thrombosis (CAT).3,15,16 Furthermore, additional clinical risk factors for VTE in cancer have been extensively studied, including stasis from the tumor in the veins, vascular damage from surgery and chemotherapy, use of central venous catheters, and immobility, all of which result in the development of CAT.2 Regarding the relation between CAT and mortality, it is still unknown whether the high mortality rate in patients with CAT is causally linked to the VTE or if it is a consequence of tumor characteristics altogether. Primary prevention and anticoagulant treatment in patients with cancer and established CAT were shown to reduce the risk of (recurrent) VTE over placebo but did not sufficiently reduce mortality.17-20 Several other clinical factors might also influence the mortality risk in patients with CAT, which could explain why primary or secondary VTE prevention strategies do not seem to reduce the mortality risk. For example, a VTE diagnosis in cancer usually delays cancer treatment in a patient (eg, surgery, chemotherapy), as it is necessary to allow for a few weeks of uninterrupted anticoagulation. Furthermore, the increased mortality could be a result of certain tumor characteristics that, independent of stage and type, lead both to the occurrence of VTE and reduced survival. Therefore, more awareness is needed about this mortality burden in patients with established CAT, and at the same time, future studies should be focused on comprehending the mechanisms behind the high mortality rates after CAT as well as updated strategies to reduce it.
There are a few limitations to this study. First, only the first thrombotic events and the first cancer diagnoses were registered. VTE events associated with the occurrence of a second cancer were not classified as such, nor were recurrent VTEs associated with a first or subsequent cancer events. In addition, only the cancer stage at the time of the cancer diagnosis was registered. Therefore, we do not know if VTEs occurring several months or years after the cancer diagnosis were associated with a still active or maybe even a deteriorating first cancer or were not cancer-associated at all because the cancer was already cured. However, this shortcoming will actually have led to attenuation of the results. As this study population consisted only of Scandinavian people, ethnic variety may have been limited. In our cohort, no data on race or ethnicity were available, so these results need to be cautiously interpreted when translated to a country with a different distribution of race and ethnicity. The causes of death were not specified, and cancer-associated and VTE-associated mortalities could therefore not be discerned from other causes of death. Finally, as the STAC cohort consisted of relatively old data, we could not consider the more recent developments in the clinical care of patients with cancer. The prognosis of patients with cancer has improved over the years because of better treatment therapies (eg, immunotherapy), but these have recently also been linked to an increased VTE risk. Therefore, future studies with more recent and updated data are necessary to determine the updated prognosis of patients with cancer in current clinical care.
Notwithstanding these limitations, we think that this study is unique in its size and population-based design. We also think that the quality of our data can be considered high, as cancer registration is mandatory by law in Norway and Denmark, and the completeness of cancer registration in Norway and Denmark has been estimated to be 95% to 98% for invasive cancers.21
In conclusion, we have shown that in a general Scandinavian population, patients with cancer with related VTE have a 3.4-fold increased risk of death compared with patients with cancer alone, and that within each cancer type studied, this increase in mortality risk remained constant.
Acknowledgments
The work was supported by independent research grants from The Obel Family Foundation (26145) donated to S.R.K. and Stiftelsen Kristian Gerhard Jebsen (SKGJ-MED-012) to the K.G. Jebsen Thrombosis Research and Expertise Center.
The study has used data from the Cancer Registry of Norway and the Danish Cancer Registry. The interpretation and reporting of this data were the sole responsibilities of the authors, and no endorsement by these registries is intended nor should be inferred.
Authorship
Contribution: J.-B.H., S.K.B., S.R.K., M.T.S., I.A.N., and S.C.C. contributed to the conception and design of the study; J.-B.H., S.K.B., S.R.K., B.P., M.T.S., K.O., A.T., I.A.N., H.S., J.H., and S.C.C. were responsible for data collection; M.J.T.C., R.J.S.A., S. K.B., M.T.S., I.A.N., H.S., J.H., B.P., K.O., and A.T. were responsible for statistical analysis; M.J.T.C. and R.J.S.A. drafted the manuscript; and all authors critically revised the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne C. Cannegieter, Department of Clinical Epidemiology, Leiden University Medical Center, PO Box 9600, RC Leiden 2300, The Netherlands; e-mail: s.c.cannegieter@lumc.nl.
References
Author notes
Data are available on request from the corresponding author, Suzanne C. Cannegieter (s.c.cannegieter@lumc.nl).
The full-text version of this article contains a data supplement.