TO THE EDITOR:
Life expectancy of people with myelodysplastic syndromes (MDS) is limited and ranges from 0.8 to 8.8 years depending on the risk group.1 The most common causes of death in patients with MDS are progression of underlying myeloid malignancy and cardiovascular events.2 Somatic mutations frequently observed in MDS (eg, DNMT3A, SF3B1) are associated with increased expression of inflammatory cytokines3 that drive disease progression4 and atherosclerosis.5 Currently, patients with MDS have limited treatment options that suppress inflammation and reduce mortality. HMG-Co-A reductase inhibitors (ie, statins) present an attractive class of drugs in this situation because they can suppress inflammation through mechanisms dependent and independent of HMG-CoA inhibition.6,7 Although preclinical evidence supports the ability of statins to suppress leukemia cell growth in vitro,8 there are sparse clinical data supporting their use in MDS. Available literature presents conflicting results.9-11 We, therefore, conducted a retrospective study in a national cohort of patients with MDS to explore the effect of statins on overall survival (OS) and progression to leukemia.
We used the national Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database for this study.12 We identified patients diagnosed with MDS between 2007 and 2018 using the International Classification of Diseases for Oncology version 3 histology codes (supplemental Table 1). Notable exclusions are listed in supplemental Figure 1. The study was approved by the SEER-Medicare Review Board and conducted in accordance with the Declaration of Helsinki.
Intervention of interest was “new statin use,” defined as ≥1 filled prescriptions for atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, or simvastatin within 3 months before and after MDS diagnosis and absence of a prescription in the 4 months before this window. Primary outcome was OS, defined as time between MDS diagnosis and death from any cause. Secondary outcome was the cumulative incidence of leukemia progression (supplemental Table 1). MDS risk group was approximated by the SEER-Medicare myelodysplastic syndromes risk score (SMMRS) using previously reported methods,13 with inclusion of ICD10 codes. SMMRS categories correspond to International Prognostic Scoring System risk groups for estimation of OS.13
To study the effect of statins on outcomes (OS and progression to leukemia), we performed time-to-event analyses in propensity-score matched pairs of cases and controls (1:3). We generated propensity scores for statin use by logistic regression model adjusting for cardiovascular comorbidities. Cases were new statin users at MDS diagnosis and controls were patients with MDS who did not use statins at any point during study follow-up. We used Cox regression models to adjust for potential confounders (demographics, SMMRS, and treatments for higher and lower-risk MDS) in these analyses. Variables used to generate propensity scores and SMMRS category (ie, age, history of cardiovascular comorbidities, Charlson comorbidity Index, histological category of MDS, cytopenias, transfusion dependence, and recent hospitalization) were not used as covariates in these analyses to avoid dual adjustment. We also performed a sensitivity analysis using similar methods to study the effect of statins on mortality from causes other than cardiovascular events. All analyses were performed using the SAS Enterprise Guide 8.3 (SAS Institute Inc, Cary, NC). Survival curves were created using SPSS 24 (IBM Corp, Armonk, NY).
Using SEER-Medicare dataset, 56 768 consecutive patients with MDS were identified between 2007 and 2018. After exclusions, 12 674 were eligible of which 6712 (53.0%) started statins at time of MDS diagnosis, and 86% had prescription coverage for at least 3 months The final analysis set included 657 cases and 1971 controls (supplemental Figure 1). Cases and controls were balanced with respect to cardiovascular comorbidities after propensity-score matching (Table 1).
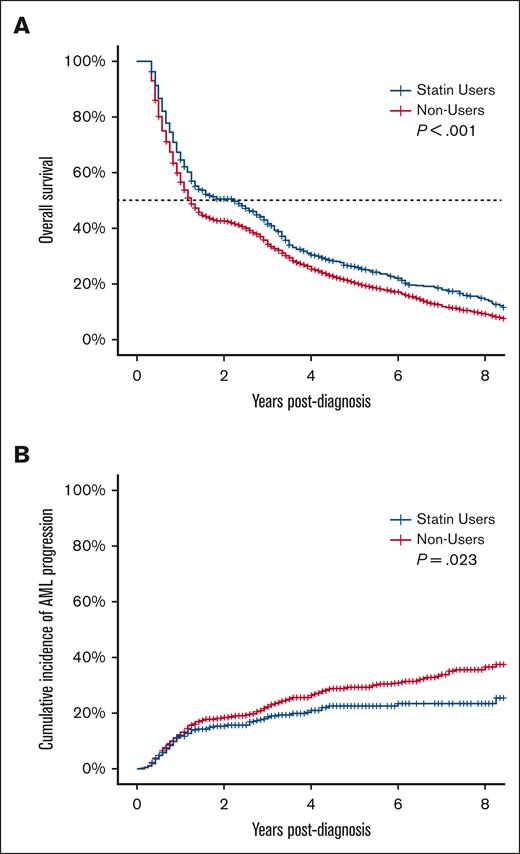
During the study period, 2161 of the 2628 patients (82.2%) died. The median OS for the entire cohort was 16 months (95% confidence interval [CI], 15-17). Among cases (ie, statin users), the median OS was 27 months (95% CI, 18-32) compared with 15 months (95% CI, 14-16) for the controls (P < .001) (Figure 1A). Death was attributed to malignancy (MDS or another malignancy) in 41.3% of the patients, and cardiovascular events in 14.0% (supplemental Table 2). In the unadjusted analysis, statin use was associated with a 36% decrease in hazard for death (hazards ratio [HR], 0.64; 95% CI, 0.46-0.89; P = .008). After adjusting for demographics, SMMRS category, and MDS treatment, statin use was associated with a 38% decrease in hazard for death (adjusted HR [aHR], 0.62; 95% CI, 0.45-0.87; P = .005). Mortality was lower among non-White races and higher among those belonging to higher-risk SMMRS categories and requiring MDS directed treatments (supplemental Table 3). In the multivariable sensitivity analysis which censored death from cardiovascular events, statin use was associated with a 39% decrease in hazard for death (aHR, 0.61; 95% CI, 0.42-0.88; P = .008).
Association of statin use with OS and progression to AML in patients with MDS. (A) OS and (B) cumulative incidence of AML progression of patients with MDS stratified by statin use.
Association of statin use with OS and progression to AML in patients with MDS. (A) OS and (B) cumulative incidence of AML progression of patients with MDS stratified by statin use.
During the study period, 465 of the 2628 patients with MDS (17.7%) progressed to acute myeloid leukemia (AML). In the unadjusted analysis, statin use was associated with a 22% (HR, 0.78; 95% CI, 0.63-0.97; P = .023) decrease in the risk of AML progression (Figure 1B). After adjusting for demographics, SMMRS category, and MDS treatment, statin use was associated with a 20% decrease in the risk of AML progression (aHR, 0.80; 95% CI, 0.64-1.00; P = .045). Higher-risk categories of MDS and requirement for MDS-redirected treatments predicted progression to AML (supplemental Table 3).
In summary, in this SEER-Medicare cohort of 2628 patients with MDS, initiation of statin therapy at MDS diagnosis was associated with a 38% improvement in OS, higher than that observed for general population.14 Interestingly, statins also reduced progression to leukemia and mortality from noncardiovascular causes, alluding to their anticancer potential.
MDS directed treatments predicted higher mortality and risk of progression to AML in this study. This is likely because requirement for MDS-directed treatments is a marker for aggressive disease in this analysis. We used SMMRS for risk-stratification of patients with MDS using both ICD9 and ICD10 codes as compared with ICD9 codes only in the original publication.13 SMMRS categories correlated with risk of death as well as progression to AML in this study, thereby providing a useful tool for contemporary investigators conducting analyses in claims-based datasets.
There are no randomized controlled trials assessing the efficacy of statins in patients with MDS. A pilot study of lovastatin showed acceptable toxicity and signal of activity.9 Previous observational studies have shown mixed results.10,11 An observational study of US veterans showed prolongation of OS in patients with MDS who were using statins at the time of MDS diagnosis.11 A recent observational study from Canada10 showed no effect of statins on mortality of patients with MDS. The Canadian study10 had a smaller small sample size (n = 533) and cumulative exposure to statins was assessed from 1-year before MDS diagnosis until death. In contrast, we assessed the efficacy of statins when started at time of MDS diagnosis. It is plausible that anti-inflammatory properties of statins are more beneficial in arresting early progression of myeloid malignancies.
This study has limitations inherent to retrospective database analysis, eg, selection bias for intervention of interest, lack of dose-response assessment, lack of data on cytogenetics, molecular aberrations, blast percentage and degree of cytopenias, and potential for unmeasured confounding. Despite these limitations, this is the largest retrospective study of statins in patients with MDS showing an association with reduced mortality and progression to leukemia. These results should be confirmed in future randomized trials of patients with MDS.
Acknowledgments: This work was supported by grants from the Siteman Cancer Center, the Foundation for Barnes-Jewish Hospital Cancer Frontier Fund (5109), the Lottie Caroline Hardy Trust, the Edward P. Evans Center for Myelodysplastic Syndromes at Washington University, and the Edward P. Evans Foundation (to M.J.W.).
Contribution: A.A., M.A.F., and M.J.W. conceived the work; A.A. and M.A.F. designed and conducted the study; M.A.F. performed statistical analysis and provided methods, results, and data visualization; A.A. and M.A.F. wrote the manuscript; M.A.J. reviewed and edited the manuscript; and M.J.W. provided supervision, revised the manuscript, and obtained funding for research.
Conflicts-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amber Afzal, Division of Hematology, Department of Medicine, Washington University in St. Louis, 660 S. Euclid Ave, St. Louis, MO 63110; e-mail: afzalamber@wustl.edu.
References
Author notes
Data are available on request from the corresponding author, Amber Afzal (afzalamber@wustl.edu).
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
The full-text version of this article contains a data supplement.