TO THE EDITOR:
Chimeric antigen receptor (CAR) T cells and bispecific antibodies are mainly used to treat hematologic malignancies,1 including multiple myeloma (MM). MM is the second most common hematologic malignancy and remains a largely incurable disease.2,3 It is characterized by the proliferation of clonal plasma cells in the bone marrow2,3 and high expression of CD38 and B-cell maturation antigen (BCMA).4 Patients who are refractory to a proteasome inhibitor, immunomodulatory agents, and monoclonal antibodies have a poor prognosis. Therefore, new treatment strategies are necessary.5 Targeting BCMA, present on malignant and normal plasma cells, either by CAR T cells or by anti-BCMA bispecific antibodies, has become the gold standard to treat refractory MM.5 Persistence and expansion of CAR T cells is associated with superior outcomes.6 Therefore, the detection of CAR T cells in the blood is an essential parameter to make clinical decisions and to define medical intervention.
However, the diagnosis of patients treated with immunotherapeutic agents may fail to reflect the correct results. As reported previously, the treatment of patients with MM with the anti-CD38 antibody daratumumab leads to an interference with blood compatibility tests, which are necessary if patients require blood transfusions.7-9
Here, we show that teclistamab, a BCMAxCD3 bispecific antibody, can interfere with flow cytometry–based BCMA CAR T-cell detection in the blood of patients with MM, leading to false-positive results.
To this end, we performed flow cytometry and polymerase chain reaction (PCR) analyses of EDTA- or heparin-anticoagulated blood samples from patients with MM at the time of leukapheresis before the treatment with CAR T cells (n = 10) and 12 to 14 days after BCMA CAR T-cell treatment (idecabtagene vicleucel; Ide-cel, n = 5). The detailed experimental methods are explained in the supplemental File.
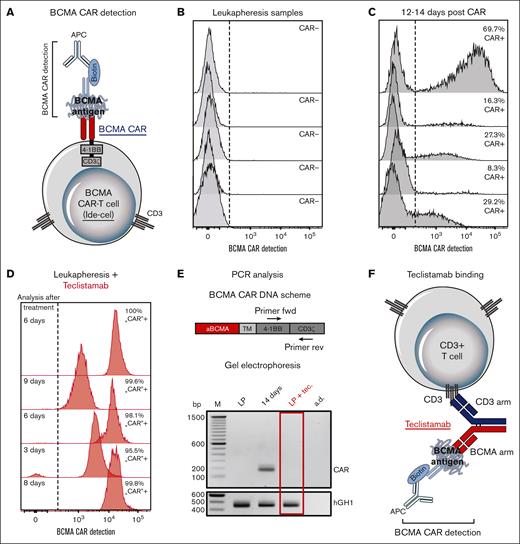
As part of a large-scale flow cytometry analysis of the fate and immunological impact of CAR T cells, we focused on the detection of BCMA CAR-T cells (Ide-cel) using a BCMA CAR detection reagent (Miltenyi Biotec). The assay recognizes the binding of a biotin-labeled BCMA antigen to the antigen-binding site of the BCMA CAR (Figure 1A). Bound BCMA was detected using a fluorescent-labeled antibiotin antibody.10 As expected, no CAR T cells were detected in the samples of patients undergoing leukapheresis (Figure 1B). These samples served as controls for samples analyzed from day 12 to 14 after CAR T-cell treatment, the time at which the BCMA-CAR T cells were present (Figure 1C). Surprisingly, some samples from patients undergoing leukapheresis appeared to be BCMA-CARpositive, although CAR-T cells were not administered (Figure 1D). However, these patients were treated with the BCMAxCD3 bispecific antibody, teclistamab, 3 to 9 days before leukapheresis. To verify the absence of CAR T cells, we performed PCR analysis, which failed to detect any BCMA CAR DNA in leukapheresis controls; whereas at day 14, after CAR T-cell treatment, the CAR DNA test was positive (Figure 1E).
Interference of BCMA CAR-T cell detection with teclistamab. (A) Schematic overview of BCMA CAR-T cell detection. (B-D) BCMA CAR-T cell detection by flow cytometry of 5 samples of patients with MM (B) at the time of leukapheresis and (C) 12 to 14 days after BCMA CAR-T cell treatment (Ide-cel). Furthermore, samples from 5 patients pretreated with teclistamab were analyzed (D) at the time of leukapheresis. Dashed lines indicate cutoff values for defining CAR-positive T cells. (E) (Top) schematic overview of BCMA CAR DNA, including the location of forward (fwd) and reverse (rev) primers. (E) (Bottom) gel electrophoresis of quantitative PCR amplified BCMA CAR DNA products from samples of patients with MM, taken at the time of leukapheresis (LP); 14 days after CAR-T cell administration (Ide-cel) (14 days) and at leukapheresis with teclistamab pretreatment (LP + tec.). Human Growth Hormone 1 (hGH1) served as the reference gene and aqua dest. (a.d.) as the negative control. Shown is 1 experiment out of 5. The gel image was adjusted for contrast and brightness, with no nonlinear adjustments. (F) Schematic overview of the recognition of teclistamab binding to CD3 T cells using BCMA CAR detection reagent. TM, transmembrane domain.
Interference of BCMA CAR-T cell detection with teclistamab. (A) Schematic overview of BCMA CAR-T cell detection. (B-D) BCMA CAR-T cell detection by flow cytometry of 5 samples of patients with MM (B) at the time of leukapheresis and (C) 12 to 14 days after BCMA CAR-T cell treatment (Ide-cel). Furthermore, samples from 5 patients pretreated with teclistamab were analyzed (D) at the time of leukapheresis. Dashed lines indicate cutoff values for defining CAR-positive T cells. (E) (Top) schematic overview of BCMA CAR DNA, including the location of forward (fwd) and reverse (rev) primers. (E) (Bottom) gel electrophoresis of quantitative PCR amplified BCMA CAR DNA products from samples of patients with MM, taken at the time of leukapheresis (LP); 14 days after CAR-T cell administration (Ide-cel) (14 days) and at leukapheresis with teclistamab pretreatment (LP + tec.). Human Growth Hormone 1 (hGH1) served as the reference gene and aqua dest. (a.d.) as the negative control. Shown is 1 experiment out of 5. The gel image was adjusted for contrast and brightness, with no nonlinear adjustments. (F) Schematic overview of the recognition of teclistamab binding to CD3 T cells using BCMA CAR detection reagent. TM, transmembrane domain.
Because weeks can pass between leukapheresis and CAR T-cell application, patients often receive bridging therapy to stop the progression of the disease.11 Some of our patients with MM received the BCMAxCD3 bispecific antibody teclistamab as bridging therapy before starting BCMA CAR T-cell treatment (Ide-cel). Teclistamab was approved by the US Food and Drug Administration in October 2022 for the treatment of adult patients with relapsed and refractory MM who had received at least 4 previous therapies, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody.12 The European Medical Agency recommended conditional marketing authorization of the antibody in July 2022.13
The antimyeloma effect of teclistamab is based on its binding to BCMA on target cells with 1 arm and to CD3 on T cells with the other arm, thus inducing T cell activity.14 In case teclistamab binds to CD3 T cells but not to target cells, the BCMA recognizing arm is not occupied. In the presence of the BCMA CAR detection reagent, which was used for the analysis of CAR expression, the recombinant BCMA antigen could bind and induce false-positive signals, suggesting the presence of BCMA CAR-T cells (Figure 1F). This false-positive signal was detectable at least 9 days after teclistamab treatment (Figure 1D). The presence of the antibody which was administered weekly at a dose of 1.5 mg/kg subcutaneously agrees well with published data showing a half-life of 3.8 (±1.7) days (individual values ranging up to 8.8 days) when applied intravenously.15
The false-positive signal described here also occurs when teclistamab is administered during treatment with CAR T cells. One strategy to distinguish between BCMA CAR-T cells and teclistamab-bound CD3+ T cells might be to disrupt teclistamab binding using the reducing agent dithiothreitol (supplemental Figure 1), as described for the CD38 antibody daratumumab, which interferes with the blood compatibility tests.7,8 However, care must be taken not to destroy the CAR and other surface molecules important for immunophenotyping by flow cytometry. Alternatively, PCR analysis of CAR DNA can be performed to avoid manipulation of cells. However, PCR analysis, in contrast to flow cytometry, does not allow for the identification and characterization of CAR T-cell subsets at the single cell level nor does it provide information on the function of CAR T cells.16
In view of the increased number of bispecific antibodies and CAR T-cell products being developed,5 the phenomenon described here should be considered when performing immunophenotyping by flow cytometry of samples from patients treated with both bispecific antibodies and CAR T cells targeting the same antigen. This could also happen in case CD19 CAR T cells and CD19xCD3 bispecific antibodies are applied and detected by a CD19 antigen-based CAR detection reagent. However, CD19 CAR antibodies are available that circumvent this phenomenon (supplemental Figure 2). In contrast to antigen-based CAR detection, CD19 CAR antibodies recognize the variable single chain fragment (scFv) based on the FMC63 antibody used by all currently approved CD19 CAR T-cell products. Alternatively, CAR T-cell determination can always be performed using PCR analysis.
Acknowledgments: The authors acknowledge the support from Leipzig University for Open Access Publishing.
The project on which this publication is based was partially funded by the German Federal Ministry of Education and Research under the funding code 03ZU1111KA as part of the Clusters4Future cluster SaxoCell and by the imSAVAR project, which received funding from the Innovative Medicine Initiative 2 Joint Undertaking (JU) under grant agreement number 853988. The JU receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA and JDRF International. The responsibility for the content of this publication lies with the authors.
Contributions: B.G., K.W., and R.W. planned and performed the experiments; R.W., S.H., A.G., M.F., A.B., and L.F. performed the data analysis; R.W., S.H., A.G., and M.F. wrote the manuscript; M.M. and V.V. were responsible for sample collection and treatment of patients; K.R., M.A., and M.H. provided crucial research components; P.F., S.F., U.K., U.S., and U.P. provided administrative, logistic, and budget support; and all authors were involved in data interpretation and read and approved the manuscript.
Conflict-of-interest disclosure: M.M., V.V., and U.P. receive honoraria from Janssen and BMS. M.M. is also supported by Janssen Research. S.F. was a consultant for Janssen-Cilag. S.F., P.F., R.W., and A.G. are involved, in collaborations with Novartis and Miltenyi Biotec, in the production of CAR-T cells. M.H. receive speaker honoraria from Novartis, Janssen, Celgene/BMS, and has participated in scientific advisory boards for Janssen and Celgene/BMS. M.H. is listed as an inventor of patent applications and granted patents related to CAR technologies and CAR T-cell therapy that have been filed by the Fred Hutchinson Cancer Research Center and the University of Wuerzburg, and that have been, in part, licensed to industry. M.H. is the cofounder and equity owner of T-CURX GmbH, Wuerzburg, Germany. U.K. is a consultant in immune-oncology for AstraZeneca, Affimed, Glycostem, GammaDelta, and Zelluna and has collaborations with Novartis and Miltenyi Biotec regarding the production of CAR-T cells. The remaining authors declare no competing financial interests.
Correspondence: Ronald Weiss, Institute of Clinical Immunology, Leipzig University, Johannisallee 30, 04103 Leipzig, Germany; e-mail: ronald.weiss@uni-leipzig.de.
References
Author notes
∗B.G. and K.W. contributed equally to this study.
Data are available on request from the corresponding author, Ronald Weiss (ronald.weiss@uni-leipzig.de).
The full-text version of this article contains a data supplement.