Key Points
Approximately 20% of older patients with CML had no molecular monitoring during the first year of TKI therapy.
Nearly half of the patients had suboptimal molecular monitoring, which was associated with decreased TKI adherence and overall survival.
Abstract
Tyrosine kinase inhibitor (TKI) use is critical in the care of patients with chronic myeloid leukemia (CML). Quantitative polymerase chain reaction (qPCR) testing for BCR-ABL1 every 3 months during the first year of TKI treatment is recommended to assure achievement of milestone response goals. Real-world evidence for the patterns of qPCR monitoring and TKI adherence in the older patient population is lacking. Using the Surveillance, Epidemiology, and End Results–Medicare database, we identified 1192 patients aged ≥66 years (median age, 74 years) with newly diagnosed CML who were followed up for ≥13 months from TKI initiation. In total, 965 patients (81.0%) had ≥1 test, with 425 (35.7%) and 540 (45.3%) of the patients tested during 1, 2, and ≥3 quarters (optimal monitoring) of the first year from TKI initiation, respectively. In multivariable analysis, diagnosis in later years and influenza vaccination before diagnosis, a proxy for health care access, were associated with optimal qPCR monitoring. Use of low-income subsidy and residing in census tracts with the lowest socioeconomic status were associated with less optimal monitoring. Patients with optimal monitoring were 60% more likely to be TKI adherent (odds ratio, 1.60; 95% CI, 1.11-2.31; P = .01) and had improved 5-year survival (hazard ratio, 0.66; 95% CI, 0.49-0.90; P < .01) than those without such monitoring. In this large, real-world study of CML management patterns, many older patients had suboptimal molecular monitoring, which was associated with decreased TKI adherence and worse survival.
Introduction
Chronic myeloid leukemia (CML) is a disease that occurs mainly among older adults, with a median age at diagnosis of 65 years, an incidence that increases with age, and the observation that the majority of patients who die of CML are aged ≥75 years.1-3 The survival of patients with CML has significantly improved with the introduction of the BCR-ABL1–directed tyrosine kinase inhibitors (TKIs), and, as such, TKIs are considered the standard-of-care frontline treatment for patients with CML.
Patients with CML, including those with chronic phase disease (80% of all cases), are recommended to have quantitative polymerase chain reaction (qPCR) testing for BCR-ABL1 every 3 months during the first year of therapy with a BCR-ABL1–directed TKI until major molecular remission is achieved.4 This is to assure that the disease is sufficiently sensitive to the particular frontline TKI chosen and to, consequently, guide the need to consider BCR-ABL1 mutational analysis for resistance, switching to an alternative TKI, or, in some cases, allogeneic hematopoietic stem cell transplantation. In addition to these potentially impactful clinical factors that the provider may evaluate, appropriate CML management recommendations are shared with the patient. Adherence to TKI therapies is critical to deriving their imparted benefit(s), which in addition to achieving responses may include an operational cure and eventual treatment-free remission with functional cure.5-7
Prior evaluations of qPCR testing patterns among patients with CML have demonstrated that between 30% and 40% of patients have no clear evidence of molecular monitoring during the first year of TKI therapy, and among those that do, it is not guideline congruent.8-10 Patients with suboptimal molecular monitoring (<3 qPCR tests during the first year) are found to have a higher risk of progression and shorter progression-free survival.9 Furthermore, patients having qPCR testing performed at guideline-recommended time points during the first year have been shown to have higher rates of TKI adherence, which itself is shown to predict higher rates of response and progression-free survival.5-7 However, these studies mostly included younger patients (median age, 54-62 years) who were almost exclusively treated with imatinib.5-10 Given that CML is a disease that occurs mainly among older adults, who encounter different threats to optimal care, and there is a need for a more contemporary evaluation inclusive of the currently available TKIs, we sought to study patterns of qPCR testing among older patients in the United States using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
Methods
Data source
Using the SEER-Medicare linked database, we assembled a population-based cohort of patients with CML who initiated TKIs after CML diagnosis. The SEER-Medicare database links patient-level information on incident cancer diagnoses reported to the SEER registries with a master file of Medicare enrollment and claims for inpatient, outpatient, physician services, hospice care, home health agencies, durable medical equipment, and prescription drugs.11 The SEER registries are population-based, covering ∼35% of the US population.11 The Yale Human Research Protection Program determined that this study did not directly involve human subjects.
Study population
We identified older adults diagnosed with CML (international classification of diseases for oncology, third edition codes: 9875 and 9863) between 2007 and 2017 who fulfilled the following criteria: (1) were aged between 66 and 99 years (to avoid potential changes in the pattern of care for patients ≥100 years12,13) at diagnosis, (2) had continuous Medicare parts A and B coverage from 1 year before diagnosis to the end of follow-up (change in Medicare status, death, or 31 December 2019, whichever came first), (3) had Medicare part D coverage from 3 months before diagnosis to the end of follow-up, (4) did not receive a TKI within 3 months before diagnosis, and (5) received a TKI after diagnosis. To ensure adequate time to assess first year adherence/monitoring, we only included patients who were followed up for ≥13 months since TKI initiation.
TKI treatments and adherence
We used part D claims to identify treatment with TKIs (imatinib, bosutinib, dasatinib, nilotinib, and ponatinib) during our study period. We created a TKI admission diary within the first year after treatment initiation. As performed in our previous study,14 if a person had overlapping supply of more than one TKI, we assumed the patient started to use the second TKI and discontinued the first TKI. If a dispense coverage exceeded the end of follow-up, then the dispense was censored at the end of follow-up date. We measured TKI adherence among those with at least 2 TKI claims and calculated the proportion of days covered (PDC); adherence was defined as a PDC of ≥80%.
Molecular monitoring
All dates of molecular monitoring were identified from the initiation of first-line TKI to 1 year after the initiation of first-line TKI. We used current procedural terminology codes to identify qPCR tests. The earliest available date for specific current procedural terminology codes of qPCR tests (81206, 81207, 81208, 81479, and 0040U) was 1 January 2013. For claims before 2013, we included a group of related procedure codes to qPCR tests (83902, 83896, 83898, and 83900). To be considered as a separate molecular monitoring test, the test must be at least 30 days apart from other tests. We partitioned qPCR testing using 2 timeframes. Firstly, as suggested in the guidelines, we assessed qPCR tests at milestones at months 3, 6, 9, and 12 (all ± 30 days to allow for slightly early or late testing) after TKI initiation. Secondly, we evaluated whether a patient had qPCR tests within each quarter during the 12 months after TKI initiation. Optimal monitoring was defined as the evidence of at least 3 milestones or quarters with qPCR testing during the first year.
Statistical analysis
Descriptive statistics were used to summarize patient baseline characteristics. Categorical variables were presented using frequencies and percentages and compared between groups using Pearson χ2 test. Continuous variables were summarized using median and interquartile ranges (IQRs). Consistent with SEER-Medicare requirement to preserve confidentiality, all categories with ≤10 patients were reported as <11. Baseline patient characteristics included age at diagnosis, sex, race, year of diagnosis, marital status, SEER region (northeast, midwest, south, or west), modified Elixhauser comorbidity index (described later in the article), history of other malignancies, previous cardiovascular diseases (CVDs), previous pulmonary diseases, previous CVD risk factors (hypertension, hyperlipidemia, diabetes mellitus, or tobacco use), low-income subsidy (a marker for reduced out-of-pocket cost sharing and low socioeconomic status [SES]), census tract Yost index (a proxy for neighborhood SES),15,16 and receipt of influenza vaccination during the 12 months before CML diagnosis (an indicator for access to the health care system17). The Yost score is a composite index of SES, which is based on principal component analysis from variables measuring different SES aspects, such as education, income, and occupation of a census tract.15 High Yost scores indicate high neighborhood SES. To assess comorbidities, CVD, pulmonary diseases, and CVD risk factors, we used inpatient, outpatient, and carrier claims within 12 months before CML diagnosis that appeared on any inpatient claims or at least 2 outpatient/physician claims >30 days apart.18 As we created individual indicators of prior CVD, pulmonary diseases, and CVD risk factors, a modified Elixhauser score was developed by removing those already included in the CVD and pulmonary diseases-related indicators from the original Elixhauser score.18,19 The trend of adherence and PDC based on the number of milestones with qPCR tests was examined using Cochran-Armitage trend test for binary variables and Jonckheere-Terpstra test for ordinal variables, respectively. Multivariable logistic regression was used to assess factors associated with optimal monitoring and TKI adherence. Testing for the interaction between low-income subsidy and Yost index was only significant in the model to identify factors associated with optimal monitoring; thus, we created a combined variable of low-income subsidy and Yost index in that model. Kaplan-Meier survival analysis was used to estimate the median survival of patients after TKI initiation. Multivariable Cox proportional hazards regressions were used to assess the impact of optimal monitoring on overall survival. Because the results from using the 2 timeframes of qPCR monitoring (ie, quarters or milestone) were similar, and because we wanted to compare our findings with those of previously published studies on this topic,20 we presented our main results using quarterly qPCR testing. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC) with two-sided tests and a type I error of 5% as the threshold for statistical significance.
Results
We identified 1192 patients with newly diagnosed CML who received TKIs and met our selection criteria (Figure 1). The majority of patients were female (52.3%) and non-Hispanic White (81.5%), with a median age at diagnosis of 74 years (IQR, 70-80 years). The median time from diagnosis to TKI initiation was 42 days (IQR: 27-72 days), with 973 patients (81.6%) initiating TKI within 90 days of diagnosis. During the first year after TKI initiation, among 748 patients starting frontline imatinib, 17.8% switched to a second generation TKI; furthermore, among 444 patients starting a frontline second generation TKI, 137 (30.9%) received a subsequent TKI, with 89 (65.0%) switching to imatinib.
Molecular monitoring patterns
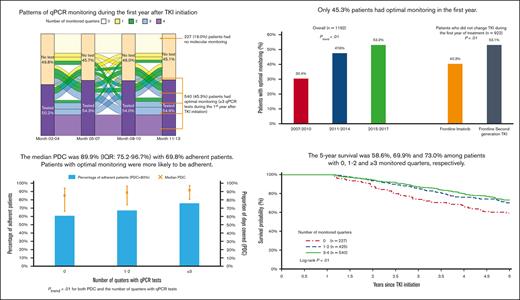
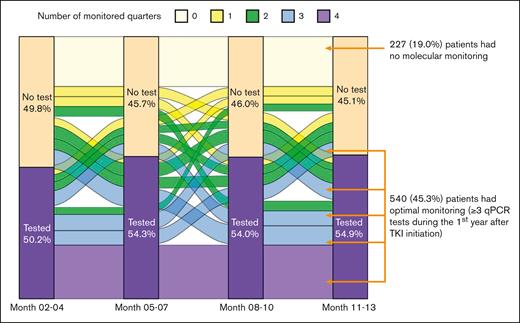
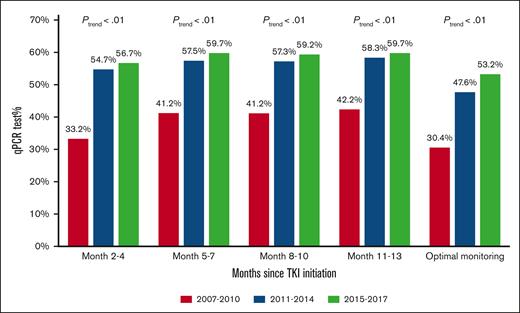
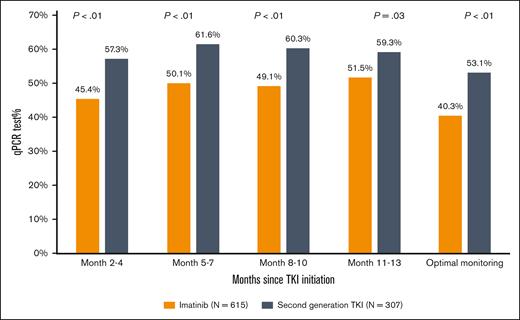
During the first year after TKI initiation, 965 patients (81.0%) had a qPCR test, with a median of 3 tests (IQR, 2-4 tests) performed. Among patients who had ≥2 tests, the median time between 2 consecutive tests was 89 days (IQR, 63-105 days). The pattern of qPCR testing within the first year after TKI initiation is shown in Figure 2. A total of 425 (35.7%) and 540 (45.3%) patients had tests during 1 or 2 and ≥3 quarters of the first year after TKI initiation, respectively. Patients diagnosed more recently during the study period were more likely to have optimal monitoring (Figure 3), but even among patients diagnosed in the latest study period of 2015 to 2017, only 210 (53.2%) had optimal monitoring. Among 922 patients who did not switch to a second generation TKI during the first year, patients receiving frontline second generation TKIs (53.1%) were more likely to have optimal monitoring when compared with patients treated with imatinib (40.3%; P < .01; Figure 4).
Pattern of qPCR monitoring during the first year after TKI initiation.
Proportion of patients with qPCR monitoring during the first year after TKI initiation based on the year of diagnosis.
Proportion of patients with qPCR monitoring during the first year after TKI initiation based on the year of diagnosis.
Proportion of patients with qPCR monitoring using frontline TKI among those who did not switch therapy within the first year after treatment initiation.
Proportion of patients with qPCR monitoring using frontline TKI among those who did not switch therapy within the first year after treatment initiation.
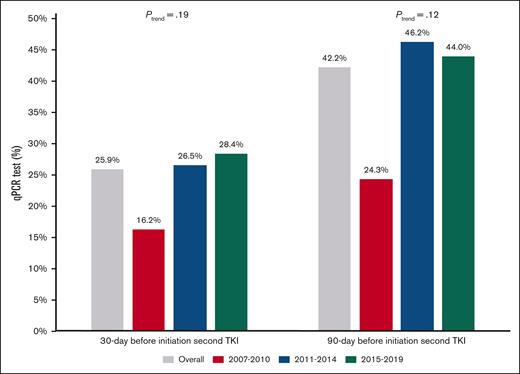
Among patients who switched during the first year, 25.9% and 42.2% patients had a qPCR testing within 30 and 90 days before the initiation of the second TKI, respectively. However, the proportions of patients who had qPCR testing within 30 and 90 days did not differ based on the year of diagnosis (Figure 5). Although a similar percentage of frontline imatinib users (27.1%) and frontline second generation TKI users (24.8%; P = .67) had a qPCR testing within 30 days before second-line TKI initiation, frontline imatinib users were more likely to have qPCR testing (48.9% vs 35.8%; P = .03) within 90 days before second-line TKI initiation.
Proportion of patients with qPCR monitoring before TKI switching based on the year of diagnosis and frontline TKI among those who switched TKIs within the first year of treatment.
Proportion of patients with qPCR monitoring before TKI switching based on the year of diagnosis and frontline TKI among those who switched TKIs within the first year of treatment.
Factors associated with optimal molecular monitoring
When compared with less frequently monitored patients, those with optimal monitoring were younger (P < .01), were diagnosed in more recent years (P < .01), and had received an influenza vaccination within 12 months preceding the diagnosis of CML (P = .02; Table 1). Patients with optimal monitoring were less likely to have low-income subsidy (P < .01) or reside in a neighborhood with low SES (P = .01). In the multivariable model, the odds of having optimal monitoring were higher among patients diagnosed in more recent years than those diagnosed between 2007 and 2010 (2011-2014 odds ratio [OR], 1.97; 95% confidence interval [CI], 1.43-2.71; P < .01; whereas, 2015-2017 OR, 2.33; 95% CI, 1.66-3.27; P < .01; Table 2). Patients who received influenza vaccination before diagnosis had 31% increased odds of being optimally monitored than those who did not receive influenza vaccination (OR, 1.31; 95% CI, 1.01-1.70; P = .04). When compared with patients who did not receive a low-income subsidy and resided in the highest SES census tracts, patients receiving a low-income subsidy and residing in a neighborhood with the lowest SES (OR, 0.44; 95% CI, 0.25-0.77; P < .01), or in the third quintile of SES (OR, 0.47; 95% CI, 0.26-0.87; P = .02) were less likely to have optimal monitoring. However, patients receiving a low-income subsidy and residing in a neighborhood with the highest SES were more likely to have optimal monitoring (OR, 2.02; 95% CI, 1.11-3.69; P = .02).
Molecular monitoring and TKI adherence
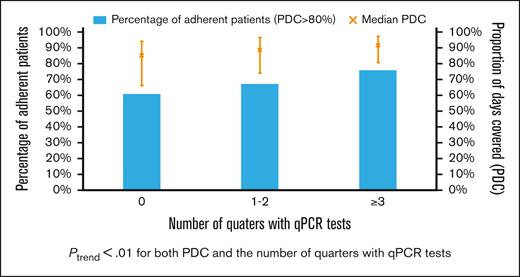
Among 1170 patients who had at least 2 claims for a TKI, the median PDC was 89.9% (IQR, 75.2%-96.7%), with 817 (69.8%) adherent patients. The median PDC among patients with qPCR tests during 0, 1 or 2, and ≥3 quarters was 84.9%, 88.8%, and 91.2%, respectively (Figure 6). Adherence was observed among 60.6%, 67.1%, and 75.8% of patients with 0, 1 or 2, and ≥3 quarters of testing, respectively. Both PDC and percentage of adherent patients increased with the number of quarters with qPCR tests (both Ptrend < .01). When compared with patients without any monitoring test during the first year, patients with optimal qPCR monitoring were found to be more adherent (75.8% vs 64.9%; P < .01). After adjusting for demographic factors, SES, and comorbidities, patients with optimal monitoring were 60% more likely to be adherent (OR, 1.60; 95% CI; 1.11-2.31; P = .01) than those who never had qPCR testing during the first year of treatment. In addition, patients diagnosed during more recent years (compared with 2007-2010 and 2011-2014; OR, 1.60; 95% CI, 1.15-2.22; P = .01; whereas 2015-2017; OR, 1.54; 95% CI, 1.09-2.19; P = .02) and received influenza vaccination within 12 months before diagnosis (OR, 1.45; 95% CI, 1.10-1.91; P = .01) were more likely to be adherent. However, patients who were aged between 85 and 99 years (OR, 0.58; 95% CI, 0.36-0.94; P = .02) were less likely to be adherent than those aged between 66 and 69 years (Table 3).
Proportion of patients who were TKI adherent and the median PDC by TKI (error bar for interquartile range) based on the number of quarters of the first treatment year using qPCR test.
Proportion of patients who were TKI adherent and the median PDC by TKI (error bar for interquartile range) based on the number of quarters of the first treatment year using qPCR test.
Molecular monitoring and 5-year survival
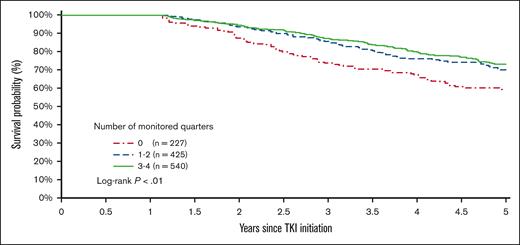
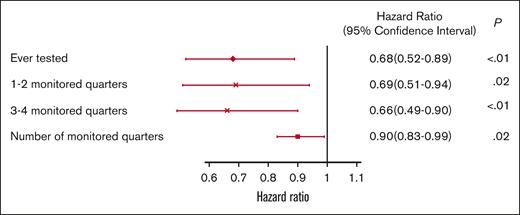
The 5-year survival after TKI initiation was 58.6%, 69.9%, and 73.0% among patients with 0, 1 or 2, and ≥3 monitored quarters, respectively (log-rank, P < .01; Figure 7). After adjusting for demographic factors, SES, and comorbidities, patients with any evidence of qPCR monitoring during the first year after TKI initiation had a 32% decreased risk of death within 5 years after initiation (hazard ratio [HR], 0.68; 95% CI, 0.52-0.89; P < .01; Figure 8). Patients with 1 or 2 (HR, 0.69; 95% CI, 0.51-0.94; P = .02) and ≥3 monitored quarters (HR, 0.66; 95% CI, 0.49-0.90; P < .01) had a lower risk of death within 5 years after TKI initiation when compared with those without any evidence of testing. The five-year survival improved with the number of monitored quarters (HR, 0.90; 95% CI, 0.83-0.99; P = .02).
Kaplan-Meier survival curves based on the number of monitored quarters during the first year of TKI therapy.
Kaplan-Meier survival curves based on the number of monitored quarters during the first year of TKI therapy.
Multivariable adjusted HRs and 95% CIs for association between qPCR testing and 5-year overall survival.
Multivariable adjusted HRs and 95% CIs for association between qPCR testing and 5-year overall survival.
Discussion
Using a large, contemporary, population-based cohort, we studied the real-world practice patterns of molecular monitoring and TKI adherence during the first year of treatment among older patients with CML in the United States. Nearly 20% patients did not have any qPCR testing within the study period, with only 53.2% of the patients being properly monitored, that is, having testing during ≥3 quarters. Among patients who switched TKIs during the first year, 25.9% and 42.2% patients had a qPCR testing within 30 days and 90 days before the initiation of the second TKI, respectively. Patients diagnosed in more recent years were more likely to have optimal monitoring, and patients residing in the lowest SES census tracts were less likely to have optimal monitoring. Furthermore, patients with optimal qPCR monitoring were more TKI adherent and had lower risk of death within 5 years when compared with less monitored patients.
Evidence of substandard CML management, however, is not restricted to older patients. The prospective, observational SIMPLICTY study evaluated practice patterns in >1200 patients with CML diagnosed between 2010 and 2015 (median age, 56.6 years [IQR, 46.0-67.7 years]) and found that 13% of the patients in the United States had no molecular monitoring during the first year after TKI initiation, irrespective of insurance status; only 47% of the patients had ≥3 molecular tests during the first year.21 Similarly, a retrospective cohort study demonstrated that nearly one-quarter of patients (median age, 59 years) receiving frontline treatment in the community between 2006 and 2010 and being followed up for ≥18 months had no evidence of molecular monitoring8; a subsequent analysis 1 year later showed that 13.2% patients did not receive any qPCR test within a median follow-up of 35.9 months.9 Two prior claims-based studies found that ∼36% to 41% of the patients (median age, 54 years) had no qPCR testing during the 1-year post-TKI initiation study period, and only approximately half of patients had optimal monitoring.10,22 A more recent analysis using a large database of >1000 younger patients (mean age, 56.4-59.9 years) treated with a frontline TKI demonstrated that 11% patients in 2016 to 2017 and 18% patients in 2007 to 2015 did not have molecular monitoring during the first year after TKI initiation, and only 52.7% of the patients during the latter study period had optimal monitoring.20 The median age of patients in these studies was <60 years and less than that in our study, which found that increasing age was negatively associated with receiving optimal monitoring. Furthermore, we found that less than half of the patients, especially those with second generation TKI as frontline therapy, had qPCR testing within 90 days before the initiation of the second TKI. Similar to the analyses by Henk et al, we did not observe any change in testing patterns before TKI switching over time, by year of diagnosis.20
We found that patients diagnosed during more recent years had more guideline-recommended optimal management, which aligns with the incorporation of qPCR monitoring for milestone response assessment by both the European LeukemiaNet and National Comprehensive Cancer Network in 2013.4,23 Optimal qPCR monitoring and TKI adherence were also associated with the receipt of an influenza vaccination in the 12 months before CML diagnosis, which is used as a surrogate for access to the health care system.17 The same could be said for patients with a prior diagnosis of a non-CML malignancy, who were found to have better adherence, because these patients were likely already accustomed to the requirements for chronic and, in this case, malignant disease management. We also observed that patients who did not have a low-income subsidy were more likely than their subsidized counterparts to have optimal molecular monitoring, a finding corroborated previously by Shen et al in their SEER-Medicare analysis of patients diagnosed between 2007 and 2012; during that time, nonadherent patients were burdened with a 45% higher mean out-of-pocket monthly cost when compared with adherent individuals.24 Lower financial burden associated with TKI prescription costs has been previously shown to predict stable, adherent behavior.25 Furthermore, lower social support has been associated with lesser adherence, which likely contributes to the inadequacies associated with increasing age among patients in our cohort.25,26 Other factors may contribute to these shortfalls in management, including TKI-attributed side effects, emotional distress, illiteracy, or poor understanding of the implications of suboptimal management.6,27,28
Patient-specific barriers may not be the only challenges encountered that eventually influence suboptimal molecular monitoring and TKI adherence. The practice setting and behaviors and experience of providers may also be important.6,27,28 Patients treated at low-volume centers were previously shown to have lower rates of recommended molecular monitoring.29 Similarly, the SIMPLICITY study demonstrated that patients treated by community providers were less likely to have molecular monitoring when compared with patients treated at academic centers.21 These data serve as a call to action for better provider education to align real-world patient care with that recommended by consensus guidelines and appropriate care for a highly treatable disease.
The consequences of decreased molecular monitoring have previously been shown to be neither nominal nor benign. Prior analyses of younger patients (median age, <60 years) have shown a greater frequency of molecular monitoring to be associated with an increased rate of achieving major molecular remission, a lower risk of progression to more advanced phases of CML, and improved progression-free survival.9,29 Our study reaffirmed the impact of molecular monitoring on outcomes and demonstrated that 5-year survival was negatively affected by the absence of molecular monitoring during the first year of frontline TKI therapy. Similarly, lesser TKI adherence has been associated with substantially lower rate of complete cytogenetic response and molecular response.5-7 Even more impactful is the observation that <80% imatinib adherence is associated with a 0% rate of complete molecular response5,6; likely owing to the more frequent all-cause inpatient admissions and more inpatient days with which these patients are burdened, less TKI adherence has also been linked to increased health care costs, after controlling for relevant covariates and other likely confounders.30,31
Like other claims-based analyses, our study was subject to limitations, including the lack of detailed clinical, laboratory, and pathologic information necessary to assess patient disease risk or phase and to understand the reason for lack of qPCR monitoring or TKI adherence. Furthermore, our study sample was limited to patients with continuous Medicare fee-for-service coverage, and, consequently, the results from our analyses may not be generalizable to Medicare beneficiaries enrolled in Medicare advantage plans. Despite these potential limitations, our study sample was large and population-based, and we used data of a wide array of patient characteristics, including sociodemographic factors, comorbidities, and measures of health care access. Although we could not confirm disease phase for patients, it is most likely that the majority of patients (∼90%) had chronic phase disease at the time of TKI initiation and that patients with more advanced disease (eg, accelerated or blast phase) would need to have more frequent molecular monitoring than their chronic phase counterparts, further accentuating the critical gap in CML care for older patients.
In conclusion, using data from a large, population-based cohort of older patients with CML, we illustrated the contemporary, real-world patterns of molecular monitoring and TKI adherence during their first year of CML treatment. An unacceptable proportion of older patients have neither guideline-recommended molecular monitoring nor sufficient adherence to critical TKI therapy, and ultimately, the absence of molecular monitoring negatively affects patient survival. Interventions to improve suboptimal molecular monitoring and TKI adherence are imperative to allow patients the full benefits of effective CML management.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
This research was supported by the Frederick A. Deluca Foundation.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s SEER Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Authorship
Contribution: R.M.S., R.W., X.M., and N.A.P. conceptualized and designed the study and wrote the manuscript; R.W. and X.M. provided study material, recruited patients, and collected and assembled data; and all authors performed data analysis and interpretation, critically reviewed the manuscript, approved the final version of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: A.M.Z. has served in a consulting or advisory role for AbbVie, Acceleron, Agios, Amgen, Aprea, Astellas, AstraZeneca, BeyondSpring, Boehringer Ingelheim, Cardiff Oncology, Bristol Myers Squibb, Daiichi Sankyo, Epizyme, Genentech, Gilead, Kura, Incyte, Ionis, Loxo Oncology, Janssen, and Novartis; served on clinical trial committees for AbbVie, BioCryst, Bristol Myers Squibb, Geron, Gilead, Kura, Loxo Oncology, and Novartis; and has received research funding from AbbVie, Acceleron, ADC Therapeutics, Amgen, Aprea, Astex, Boehringer Ingelheim, Cardiff Oncology, Bristol Myers Squibb, Incyte, Jasper, Jazz, Novartis, and Pfizer. N.A.P. received consulting fees from Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene/Bristol Myers Squibb, CTI BioPharma, PharmaEssentia, Constellation Pharmaceuticals, and AbbVie and other financial support for serving on an independent data review committee for Cogent Biosciences. R.M.S. has served as a member of an advisory board for Bristol Myers Squibb and Gilead Sciences Inc. X.M. received research funding (institutional) from Celgene Corporation/Bristol Myers Squibb Inc. and consults for Bristol Myers Squibb Inc. None of these interests were related to the development of this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Nikolai A. Podoltsev, Section of Hematology, Department of Internal Medicine, Yale School of Medicine, 333 Cedar Street, PO Box 208028, New Haven, CT 06520-8028; e-mail: nikolai.podoltsev@yale.edu.
References
Author notes
∗R.M.S. and R.W. are joint first authors.
Preliminary results were presented at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2021.
The data used in this manuscript were provided through a collaboration of the National Cancer Institute and the Centers for Medicare and Medicaid Services. The data use agreement precludes us from sharing these data.