Key Points
One hundred percent of patients remain alive without relapse following sequential pembrolizumab and AVD after nearly 3 years of follow-up.
PD-1 pathway correlatives were not associated with the depth of response to PD-1 blockade.
Abstract
In a multicenter, phase 2, investigator-initiated trial of sequential pembrolizumab and AVD (doxorubicin, vinblastine, and dacarbazine), nearly two-thirds of patients with untreated, unfavorable, or advanced-stage classic Hodgkin lymphoma (cHL) achieved positron emission tomography (PET)–defined, complete or near-complete metabolic responses (CMRs), following pembrolizumab monotherapy. Furthermore, all patients achieved CMR after 2 cycles of AVD, with 100% of patients alive and without relapse at initial publication. We now report long-term follow-up, including the 3-year overall survival (OS) and planned correlative analyses. Thirty patients received 3 cycles of single-agent pembrolizumab, followed by AVD chemotherapy for 4 to 6 cycles depending on the stage and bulk. PET/computed tomography scan was performed after pembrolizumab monotherapy, 2 cycles of AVD, and at the end of therapy. Baseline biopsy samples were analyzed for genomic alterations of chromosome 9p24.1 and programmed cell death protein 1 (PD-1) pathway markers. At a median follow-up of 33.1 months (range, 26.0-43.0), progression-free survival and OS remained 100%. All patients had genomic alterations in 9p24.1 and were positive for programmed death ligand 1 (PD-L1) by immunohistochemistry. There was no relationship between depth of response to single-agent pembrolizumab and 9p24.1 alterations or PD-1 pathway H-scores. After additional follow-up, sequential pembrolizumab and AVD remained highly effective. The high response rates observed at all PD-L1 levels suggest that even low levels of PD-L1 expression are sufficient for response to PD-1 blockade in untreated cHL. An international phase 2 trial (registered at www.clinicaltrials.gov as #NCT03226249) is ongoing to confirm our findings.
Introduction
Genomic alterations of chromosome 9p24.1 characterize classic Hodgkin lymphoma (cHL) leading to increased expression of programmed death ligand 1 (PD-L1) and PD-L2.1,2 Amplifications and high-level copy number gains (CNGs) are associated with advanced-stage cHL and inferior outcomes with standard chemotherapy.1 Clinical trials of programmed cell death protein 1 (PD-1) blockade have noted high frequencies of PD-L1/PD-L2 copy number alterations, increased PD-L1 and STAT3 expression by the Hodgkin Reed-Sternberg (HRS) cells, and decreased major histocompatibility complex 1 expression.3-6 Whereas PD-L1 expression on malignant Reed-Sternberg cells was associated with response to PD-1 blockade in relapsed/refractory cHL, neither 9p24.1 CNG nor PD-L1 or major histocompatibility complex I and II expression were associated with response to PD-1 blockade in the first-line setting.3,7 In contrast to the mechanism of PD-1 blockade in solid tumors, which relies on activation of an antitumor cytotoxic T-cell response, responses in cHL appear to be due to a combination of early disruptions in the tumor microenvironment (TME).8,9
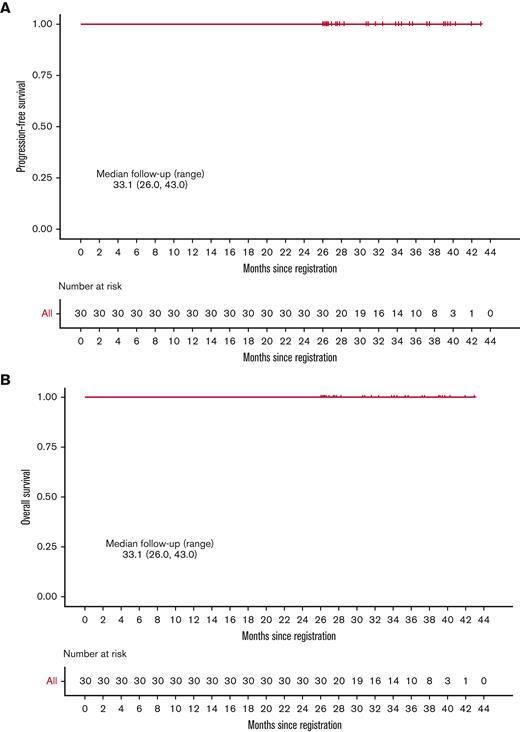
We conducted a phase 2 clinical trial of sequential pembrolizumab followed by AVD (doxorubicin, vinblastine, and dacarbazine) chemotherapy in newly diagnosed cHL.10 We demonstrated a complete metabolic response (CMR) rate of 37% to pembrolizumab monotherapy and progression-free survival (PFS) and overall survival (OS) of 100% at a median follow-up of 22.5 months (range, 14.2-30.6). The greatest risk of relapse occurs within the first 2 years after therapy, with a <5% risk of relapse in patients who are relapse-free at 24 months.11 However, it is not known whether later relapses may be observed following chemoimmunotherapy for Hodgkin lymphoma. Herein, we report updates at a minimum follow-up of 26 months and a median follow-up of 33.1 months (range, 26.0-43.0). We also report the results of correlative studies analyzing 9p24.1 alterations and PD-1 pathway expression.
Methods
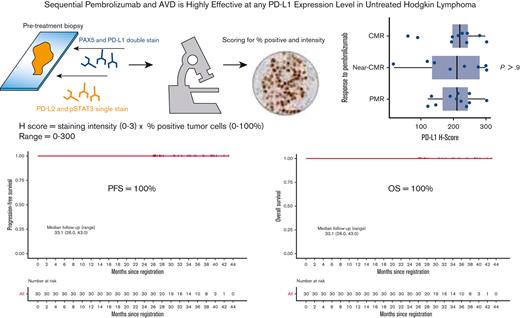
Patients with newly diagnosed cHL were treated sequentially with pembrolizumab for 3 cycles followed by AVD chemotherapy for 4 to 6 cycles as previously described.12 The primary end point of the trial was the single-agent metabolic response to pembrolizumab according to the Lugano 2014 criteria.13 Response by Lymphoma Response to Immunomodulatory Therapy Criteria was assessed secondarily.14 The secondary exploratory end point included assessing positron emission tomography (PET) responses by the percent decline in metabolic tumor volume (MTV) following pembrolizumab monotherapy and AVD chemotherapy. To capture the depth of response not meeting a CMR according to Lugano criteria, we defined “near-CMR” as ≥90% reduction in MTV, whereas partial metabolic response (PMR) included patients with >50% by <90% reduction by MTV.
Pretreatment diagnostic biopsy specimens were double stained for PD-L1 (E1L3N, XP monoclonal antibodies, Cell Signaling Technology) and PAX5, single stained for PD-L2 and pSTAT3, and scored by 2 expert hematopathologists (Q.C., L.B.S.) for the percentage of positive cells and intensity of staining. A modified H score was calculated as the product of staining intensity (0-3) and the percentage of positive tumor cells (0%-100%), ranging from 0 to 300. Fluorescence in situ hybridization (FISH) was performed to assess chromosome 9p24.1 copy number variants by cohybridizing PD-L1/PD-L2 probes (target) with the centromeric 9 probe (control). In each case, the percentage and magnitude of 9p24.1 copy number variants were evaluated. Four FISH categories were defined based on the target: control ratio and the total copy numbers (CNs) of the target per HRS cell to include amplification (ratio ≥3), CNGs (1 ≤ ratio < 3), polysomy (ratio ∼1; CNs = 3-5), and disomy (ratio = 1; CNs = 2). The patients were categorized according to the highest level of 9p24.1 alteration. The relationships between PD-1 pathway markers, genomic alterations, and response to single-agent pembrolizumab by MTV were assessed statistically using Fisher exact test, Kruskal-Wallis test, and Spearman rank correlation, as appropriate. PD-L1 H scores were grouped into terciles of approximately equal size for categorical analysis. This study was conducted following the Declaration of Helsinki. The investigators obtained informed consent from each participant after approval from the local institutional review board.
Results
Thirty treatment-naïve patients were enrolled between September 2017 and 1 August 2019. The response to single-agent pembrolizumab was CMR in 11 patients (36.7%) and near-CMR in 8 patients (26.7%). Following 2 cycles of AVD, the CMR rate was 100% and remained at 100% at the end of treatment. At a median follow-up of 33.1 months (range, 26.0-43.0), PFS and OS remained at 100% (Figure 1). There were no deaths and none of the patients discontinued therapy early because of toxicity. The most common nonhematological adverse events were elevated liver enzymes and infusion reactions in 23.3% and 16.7% of patients, respectively. One patient had grade 4 elevation in liver enzymes during pembrolizumab treatment successfully treated with corticosteroids, 1 patient had grade 3 Bell palsy in the setting of a viral infection, and 1 patient had grade 3 diarrhea. All other nonhematologic adverse events were of grade 2 or lower.
Correlative analysis
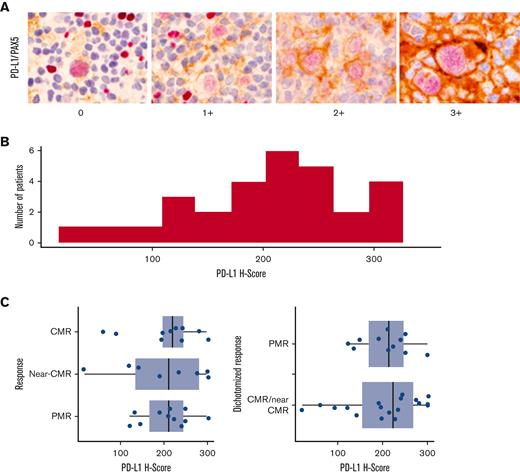
Twenty-eight patients and 29 patients had tissues available for FISH analysis and immunohistochemistry, respectively. All the patients had genomic alterations on chromosome 9p24.1. The highest level alteration was amplification in 14 patients (50%) and CNG in 14 patients (50.0%) (Table 1). There was no correlation between the response to single-agent pembrolizumab and 9p24.1 alteration, PD-L1, PD-L2, or STAT3 H scores, percent of residual HRS disomy, or Epstein-Barr virus–encoded small RNA (EBER) status (Table 1; Figure 2). Similarly, there were no relationship between PD-L1 H score and stage (P = .13), EBER positivity (P = .7), 9p24.1 alteration (P = .12), STAT3 H score (P = .3), or percent of HRS cells with polysomy/copy gain (P = .2) or amplification (P = .6). Following pembrolizumab monotherapy, 41% of patients with a CMR or near-CMR had 9p24.1 amplification, compared with 64% of patients with <90% reduction by MTV (P = .2). Of the 22 examined cases, 6 were EBER-positive. The depth of response to pembrolizumab monotherapy, measured by a decline in MTV, was not correlated with disease stage or EBER positivity (Table 1).
Baseline PD-L1 expression and correlation to single-agent pembrolizumab response. (A-C) PD-1 pathway correlates baseline PD-L1/PAX-5 staining by immunohistochemistry (A), PD-L1 H score distribution across a cohort of 29 patients (B), and association between PET responses to single-agent pembrolizumab and PD-L1 H score in baseline biopsy specimens (C).
Baseline PD-L1 expression and correlation to single-agent pembrolizumab response. (A-C) PD-1 pathway correlates baseline PD-L1/PAX-5 staining by immunohistochemistry (A), PD-L1 H score distribution across a cohort of 29 patients (B), and association between PET responses to single-agent pembrolizumab and PD-L1 H score in baseline biopsy specimens (C).
Discussion
Herein, we demonstrate the ongoing efficacy of sequential therapy consisting of 3 doses of pembrolizumab monotherapy followed by AVD chemotherapy. None of the patients relapsed or died after nearly 3 years of follow-up. In this previously untreated patient population, neither 9p24.1 alterations nor PD-L1/PD-L2 expression predicted outcomes, consistent with exquisite sensitivity to checkpoint inhibition in treatment-naïve patients.
Our study showed rapid responses to pembrolizumab monotherapy with a percent decline in MTV, but no relationship with PD-1 pathway markers. Voltin et al also noted rapid and deep responses to single-agent PD-1 blockade in the NIVAHL study, with a mean percentage decline of 93.3% following 4 doses of nivolumab, despite a CMR rate by Lugano criteria of only 51%.15 PET may not be the best strategy for response assessment in the presence of checkpoint blockade. Biomarkers such as circulating tumor DNA may more precisely predict response in the setting of immunotherapy or may complement PET-assessed response.16,17 Additionally, the high efficacy of our approach may impede the analysis of PD-1 biomarkers by eliminating comparative groups, as all patients in this study achieved and retained CMR following chemotherapy.
Monotherapy with checkpoint blockade has been assessed in the frontline setting as part of a sequential approach to therapy in 3 trials.4,5,10 Patients with newly diagnosed advanced-stage cHL treated on cohort D of the CheckMate 205 trial received single-agent nivolumab followed by a combination of nivolumab and AVD (N-AVD) chemotherapy. Single-agent nivolumab resulted in a CMR rate of only 18%, but with subsequent combination therapy, the PFS was 83% at 21 months, similar to that observed with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).4,5 NIVAHL assessed sequential or concurrent N-AVD in early unfavorable stage cHL. On interim PET, CMR was observed in 47 patients (87%) in the concurrent arm following 2 cycles of N-AVD compared with 26 patients (51%) treated with nivolumab monotherapy for 4 doses.4 The interim response measured by a change in MTV was similar in both groups, with near-CMR in 28 of 30 patients (93.3%) for concurrent N-AVD for 2 cycles vs 28 of 29 patients (96.6%) with nivolumab monotherapy for 2 cycles. At the end of treatment, the conventional complete response rate according to the Lugano criteria was identical, from 83% to 84%. Overall, there was no difference in outcomes with sequential vs concurrent approaches with 1-year PFS and OS of 100%.3,15,18
Preliminary results of a trial of concurrent pembrolizumab and AVD therapy in early-stage unfavorable and advanced-stage cHL were recently reported by Lynch et al16 with a short follow-up; the 1-year PFS and OS were 96% and 100%, respectively, similar to our study. The reasons for differences in outcomes across trials are not clear and may be related to patient selection, differences in central imaging review, small numbers of patients, and possible differences between pembrolizumab and nivolumab in untreated cHL.
As anticipated, specimens from all patients demonstrated genomic alterations of 9p24.1 and expression of PD-L1/PD-L2, but neither the type of 9p24.1 alteration nor the level of PD-L1/PD-L2 expression correlated with the depth of response to single-agent pembrolizumab response measured by MTV. In the relapsed setting, the relationship between PD-L1 expression on HRS cells and response is weak at best.6-9 Although the KEYNOTE-013 study demonstrated immunologic changes with pembrolizumab treatment including increased T-cell and natural killer cell numbers and interferon γ in the blood with expanded immune-signaling gene signatures, none of these observations were correlated with response.8,9 Importantly, all studies to date have demonstrated clinical activity of PD-1 blockade in cHL, even in patients with low PD-L1 expression.9
The NIVAHL study also showed no correlation between baseline 9p24.1 CNG or PD-L1 expression and early responses to PD-1 blockade with nivolumab in a frontline setting.3 In addition to baseline samples, the NIVAHL trial analyzed paired biopsies and blood samples shortly after nivolumab induction. Similarly, we secured funding for paired biopsies; however, the rapidity of the responses precluded nonessential posttreatment biopsies in our study. The NIVAHL group reported several striking findings that signify alternative mechanisms of response to PD-1 blockade in cHL. First, they noted the disappearance of HRS cells within days of nivolumab, which was inconsistent with the immunologic response. Approximately 50% of repeat biopsy specimens had no HRS cells at all. They also noted alterations in TME, including a reduced number of PD-1+ tumor–associated macrophages (TAMs) and regulatory T cells. Surprisingly, clonal T-cell expansion was not seen. Among the relapsed patients who progressed while receiving PD-1 therapy, they noted decreased PD-L1 expression in TAMs, highlighting the essential role of TAMs for HRS survival. Overall, these studies suggest that the mechanism of PD-1 blockade in Hodgkin lymphoma is related to TME disruption, leading to the withdrawal of supportive factors rather than adaptive immune responses. These findings support our results and further highlight the difficulty in performing on-study paired biopsies during anti–PD-1 antibody therapy.
We demonstrated that with prolonged follow-up, sequential pembrolizumab and AVD chemotherapy remains a highly effective strategy, with 100% of the patients remaining alive without relapse. The high response rates observed at all programmed death ligand levels seen in this clinical study suggest that even low levels of programmed death ligand expression are sufficient for the response to PD-1 blockade in previously untreated cHL. KEYNOTE-C11, a large phase 2 trial (#NCT05008224), based upon this study with the addition of 4 doses of consolidative pembrolizumab, will provide more definitive evidence regarding the efficacy of this approach moving forward.19
Authorship
Contribution: P.B.A., A.M.E., and J.N.W. designed this study; P.B.A. wrote the protocol; J.N.W. edited the protocol; J.N.W., L.I.G., R.K., B. Pro, A.M.E., R.A., R.A.B., and E.M. accrued patients; X.L., Q.C., L.B.S., and M.S. performed the correlative analysis; G.D. and H.S. analyzed the radiographic images; K.O. and J.S.C. analyzed the data; P.B.A. and J.N.W. wrote the manuscript; and P.B.A., J.N.W., R.A., A.M.E., L.I.G., J.S.C., K.O., G.D., H.S., E.M., K.O., B. Pro, R.A.B., R.M.E., X.L., Q.C., B. Palmer, and L.B.S. reviewed the manuscript.
Conflict-of-interest disclosure: J.N.W. reports research funding from Merck, and an honorarium for an advisory board and for her spouse’s consultancy with an honorarium from Novartis, CVS Caremark, and Epizyme. L.I.G. reports honorarium from Janssen Data and advisory boards (Safety Monitoring Board) with Bristol Myers Squibb, Gilead/Kite, and Xylem Cofounder Inc. R.A. reports institutional research funding from Merck and advisory board and consultancy for Merck. A.M.E. reports honorarium for advisory boards with Seattle Genetics. B. Pro reports honorarium for advisory boards with Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Pamela B. Allen, Department of Hematology and Medical Oncology, Emory University, 1365 Clifton Rd NE, Atlanta, GA 30322; e-mail: pallen5@emory.edu.
References
Author notes
The primary end point was published in Blood: https://doi.org/10.1182/blood.2020007400.
The data are available on request from the corresponding author, Pamela B. Allen (pallen5@emory.edu).
Presented at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 to 10 December 2019, at the 62nd annual meeting of the American Society of Hematology, virtual conference, 5 to 8 December 2020, and at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 to 14 December 2021.