TO THE EDITOR:
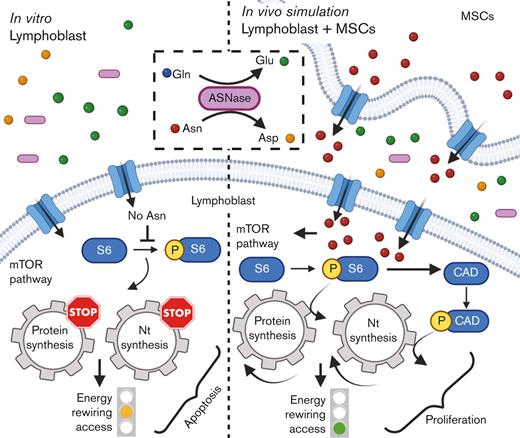
L-asparaginase (ASNase), the drug included on the World Health Organization's list of essential medicines, is irreplaceable in the front-line treatment of childhood acute lymphoblastic leukemia (ALL).1 However, the relapse of ALL2,3 is often associated with resistance4 to ASNase and its mechanisms are not fully understood. The cytotoxic effect of ASNase relies on depleting exogenous asparagine (Asn) and glutamine (Gln), inducing apoptosis in leukemic cells because of their reduced capability of Asn synthesis.5 Our previous in vitro data demonstrated that ASNase triggers metabolic reprogramming of leukemic cells, which impedes the anti-leukemic effect.6 Metabolic processes of leukemic cells have been shown to be altered by the environment of the bone marrow (BM), which may contribute to chemoresistance.7-12 Herein, we investigated the impact of BM attributes on cellular metabolic processes of leukemic cells in order to demonstrate the more complex picture13 of ASNase-driven metabolic rewiring and its role in the mechanism of resistance.
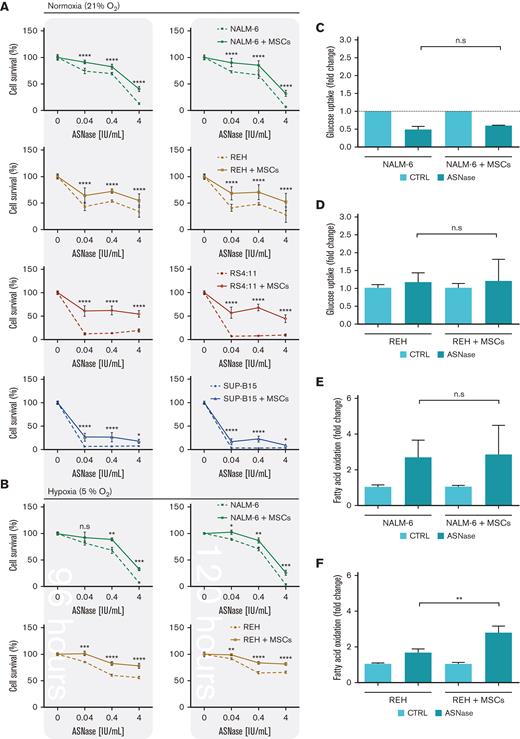
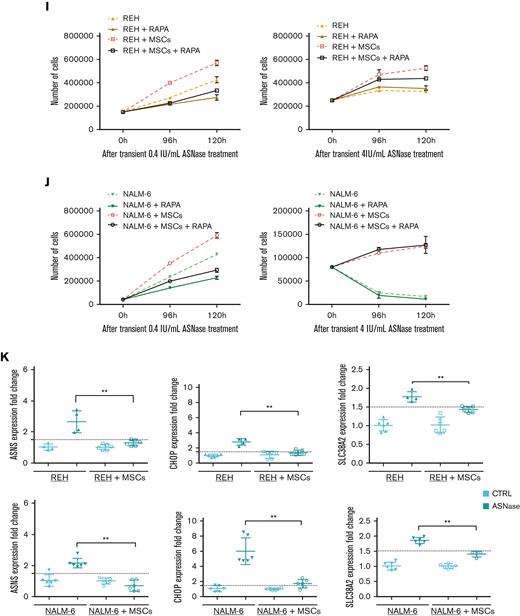
First, we explored the effect of ASNase on leukemic cell survival in the experimental model established in this study. The model mimics the BM environment of patients with ALL undergoing ASNase treatment, which was achieved using transient ASNase treatment that resembles an inherent elimination of the drug in vivo, a coculture with mesenchymal stromal cells (MSCs) that partially simulates the BM matrix (supplemental Figure 1A-E)14,15 and hypoxic conditions.16,17 Four B-cell precursor (BCP)-ALL cell lines (NALM-6, REH, RS4-11, and SUP-B15) in the presence and absence of MSCs were treated with different concentrations of transient ASNase treatment. After 96 and 120 hours of ASNase treatment, the survival of all tested leukemic cell lines in coculture was significantly increased compared with that in monoculture (Figure 1A; supplemental Figure 1F). Interestingly, the survival of the cocultured leukemic cells following ASNase treatment in hypoxia was comparable to the effect under normoxic conditions (Figure 1B; supplemental Figure 1G-H). The prosurvival effect of MSCs on leukemic cells after ASNase treatment was also recapitulated in primary cells, observed in 4 of 7 patients whose cells were isolated from BCP-ALL diagnosis (supplemental Figure 1I). In addition, the protective effect of MSCs was also observed when using alternatives for the BM scaffold as HS-5, a fibroblast-like cell line with similar progenitor potential,18 and primary MSCs, isolated from pediatric patients with BCP-ALL (supplemental Figure 1J-K).
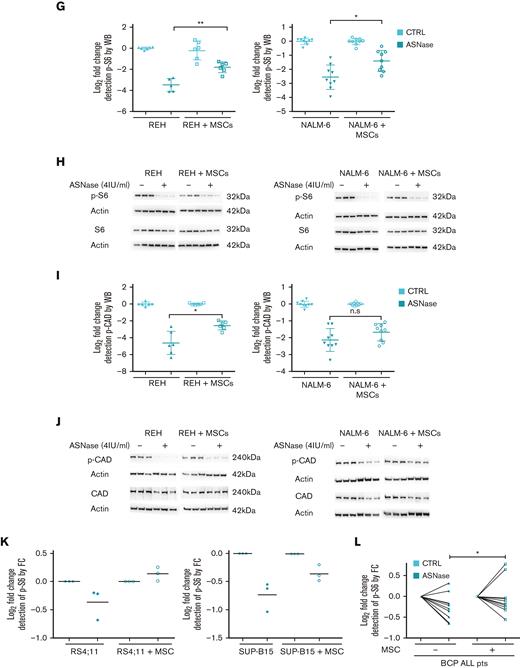
Next, we investigated the changes in bioenergetic and biosynthetic pathways after 48 hours of ASNase treatment.19 We previously showed a metabolic rewiring of leukemic cells after ASNase treatment, resulting in glycolysis reduction (via glucose uptake measurement [GU]) and fatty acid oxidation (FAO) elevation.6 Herein, we showed that ASNase-induced changes in bioenergetic metabolism persist in the presence of MSCs. Measuring GU, we detected similar changes in the mono and cocultures of NALM-6 cell line (Figure 1C). REH cells did not decrease GU in either presence or absence of MSCs, in line with previously published data showing milder changes in GU in this cell line6 (Figure 1D). FAO significantly increased in both cell lines independent of MSCs’ presence, having significantly higher levels in REH cells in the coculture than those in the monoculture (Figure 1E-F). We assume it is caused by the higher energy demand required to sustain the increase of biosynthetic processes that depend uniquely on FAO because other bioenergetic sources (glucose, Gln) are shut down.20 Biosynthetic pathways were explored by analyzing mTOR, the mechanistic target of rapamycin (RAPA) pathway, as the major nutrient-sensitive regulator.21 We previously showed that ASNase in vitro inhibits the mTOR signaling pathway in leukemic cells and its downstream targets.6 Herein, we observed the inhibition of p-S6 in leukemia cells after the ASNase treatment in both cultures. However, the inhibition was significantly less prominent in the coculture model (Figure 1G-K; supplemental Figure 1L). Partial reactivation in the presence of MSCs was also detected in the phosphorylation of CAD (p-CAD), another downstream protein of mTOR complex 1 (mTORC1)/S6K1 (prevalent in REH cells; Figure 1I-J).22,23 Furthermore, we also observed the partial restoration of p-S6 induced by MSCs in primary leukemic cells upon ASNase treatment, detected in 9 of 11 analyzed patients with statistical significance (Figure 1L). These results suggested the contribution of MSCs in the reactivation of protein (p-S6) and nucleotide (p-CAD) synthesis, regulated by mTOR.
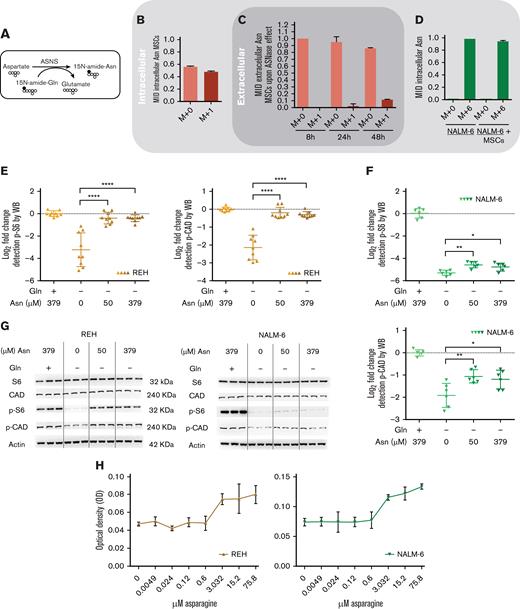
Downstream signaling of mTORC1 responds to extracellular Asn,6,24 which could be released from MSCs. To elucidate the hypothesis, we measured the flux of Asn using stable isotope tracing and assessed the activity of de novo Asn synthesis in MSCs followed by the efflux of Asn to culture media (Figure 2A). MSCs were first cultivated without Asn and with 15N-Gln for 7 days, resulting in 50% labeled intracellular Asn (Figure 2B). Next, we transferred MSCs (with labeled intracellular Asn) to a medium simulating the ASNase effect and detected extracellular Asn from which 11.67% ± 0.57 was labeled (M+1) after 48 hours of incubation, confirming the efflux of Asn from MSCs (Figure 2C). We also verified 94% of intracellular Asn (M+6) were taken in by leukemic cells in both monoculture and coculture using 15N2-13C4-Asn in culture media (Figure 2D). These data confirmed that Asn is replenished in the presence of MSCs, which are supported by the increased levels of p-S6 and p-CAD proteins in leukemic cells incubated with increasing Asn concentrations and without Gln (Figure 2E-G). Moreover, increased cell proliferation correlated with increased levels of Asn (Figure 2H). Importantly, the extent of the described phenomenon is different for each cell line because of their specific intrinsic factors (cell proliferation, doubling time, and basal metabolic profile).
In order to test if Asn supplementation from MSCs hinders ASNase sensitivity via mTOR restoration, we treated leukemic cells in monoculture and coculture with a combination of either 0.4 IU/mL or 4 IU/mL of ASNase and 25nM of RAPA (supplemental Figure 1M), an mTOR inhibitor.25 The combination of RAPA and 0.4 IU/mL of ASNase diminished the prosurvival effect of MSCs in both NALM-6 and REH cell lines. REH cells also showed similar results using 4 IU/mL of ASNase and RAPA. This effect was not observed in NALM-6, suggesting a more complex mechanism given its resistant phenotype to ASNase. Moreover, in lower concentrations of ASNase, RAPA decreased the survival of NALM-6 and REH in monoculture as well (Figure 2I-J). These results confirmed that the mTOR pathway plays a crucial role in the rescue mechanism induced by MSCs on leukemic cells upon ASNase treatment.
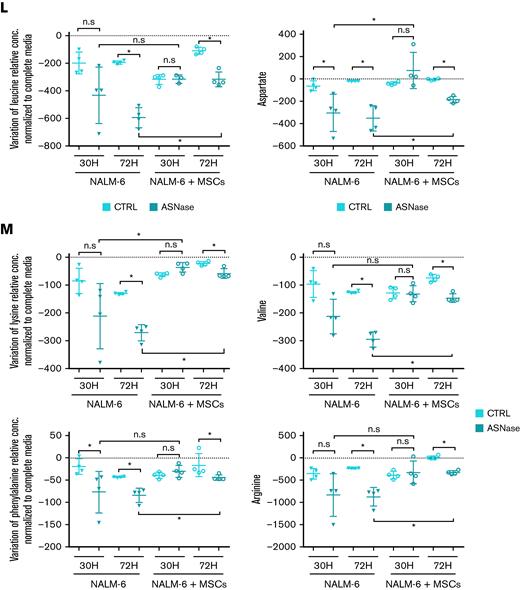
Because Asn deficiency is reflected in the induced mRNA levels of ASNS, we assessed ASNS gene expression in the presence and absence of MSCs as well as the expression of CHOP, a product of amino acid stress response, and the amino acid transporters SLC38A2, SLC7A1, and SCL1A4.26,27 After ASNase treatment, the expression of all studied genes was induced only in the monoculture of both leukemic cell lines. In contrast, the gene expression was unchanged in the coculture, confirming the amino acids deficit in the monoculture (Figure 2K; supplemental Figure 2A-B). Furthermore, we asked how Asn deficiency influenced the transport of other amino acids. Amino acids flow analysis showed a significant (mostly gradual) increase in the uptake of most of the amino acids after ASNase treatment in monoculture. The increase in amino acid uptake was less prominent in cocultured leukemic cells following ASNase treatment (Figure 2L; supplemental Figure 2C).
Our previously published results on metabolic rewiring in leukemic cells after ASNase treatment were obtained in purely in vitro conditions in which ASNase was constantly present in the culture and did not reflect the pharmacokinetics of the in vivo administration. Additionally, leukemic cells were cultured without any scaffold characteristic of the BM environment.6 In this study, we used a more relevant in vitro model considering the main aspects of the BM environment. Using this model, we confirmed the survival advantage of leukemic cells in the presence of MSCs when comparing proliferation and viability with those of common in vitro cultures. Additionally, culturing the cells at different oxygen concentrations did not affect survival. Surprisingly, the change in bioenergetic pathways was similar to that in monoculture, supporting the relevance of our previous findings and showing that the presence of MSCs does not affect ASNase-driven metabolic reprogramming.6 Furthermore, we showed that Asn was de novo synthesized in MSCs and released into the Asn-free culture media, reactivating p-S6 and p-CAD. This event prompted leukemic cells to proliferate even in the presence of ASNase, the effect that was diminished using an mTOR inhibitor, RAPA. The amino acid stress caused by Asn depletion, represented by elevated levels of ASNS, SLC38A2, CHOP, SLC7A1, and SLC1A4 and an increased amino acid uptake was compensated in coculture.
To our knowledge, this study is the first to demonstrate that protein and nucleotide synthesis participate in the MSCs-mediated chemoresistance to ASNase treatment. Our results show that MSCs sustain biosynthetic pathways in leukemic cells, making them more accessible to bioenergetic rewiring, which may counteract ASNase cytotoxicity. These results bring us closer to a better understanding of the mechanism of action of common cytostatic drugs and unravel survival mechanisms that could help to develop novel treatment options to improve the outcome for patients with ALL.
Acknowledgments: This work was supported by the Grant Agency of Czech Republic (GAČR no: 20-27132S), the project National Institute for Cancer Research (Programme EXCELES, ID project number LX22NPO5102), funded by the European Union NextGenerationEU and by the Ministry of Health, Czech Republic, and the conceptual development of research organization, Motol University Hospital, Prague, Czech Republic 00064203. N.A.-A. was supported by the grant agency of Charles University, nr.794218. A.P. was supported by the Grant Agency of Czech Republic (GAČR no: 21-18993S). The figures were created with BioRender.com.
Contribution: N.A.A. optimized and performed cell cultivation models and flow cytometry; J.R., K.H., and D.A.T. designed and performed stable isotope tracing; N.A.A. and D. Kuzilkova performed assays with patients with ALL; N.A.A., I.H., A.P., P.P., and T.M. optimized and performed glucose uptake and FAO; N.A.A. and E.P. performed protein isolation and western blot; N.A.A. and M. Zwyrtkova performed RNA isolation and qPCR; D. Kavan and P.N. performed high-performance liquid chromatography analysis; L.S. and J.T. were responsible for collection of patient samples and isolation of primary leukemic cells; J.S. designed the project and coordinated the study; J.S., N.A.A., K.H., J.T., and M. Zaliova wrote the manuscript and analyzed the data; and all the authors revised the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julia Starkova, Second Faculty of Medicine, Dept.of Pediatric Hematology and Oncology, Charles University, V Uvalu 84, 15006 Prague, Czech Republic; e-mail: julia.starkova@lfmotol.cuni.cz.
References
Author notes
Data are available on request from the corresponding author, Julia Starkova (julia.starkova@lfmotol.cuni.cz).
The full-text version of this article contains a data supplement.