Key Points
Patients with DLBCL Richter transformation of CLL have a poor prognosis, especially if they have had prior BTKi exposure.
Nivolumab and ibrutinib combination therapy is a safe and potential treatment option in patients with DLBCL Richter transformation.
Abstract
Richter transformation (RT) is a rare complication of chronic lymphocytic leukemia (CLL) that has dismal outcomes. Upregulation of PD-1/PD-L1 drives immunological evasion in patients with RT. We hypothesized that combining nivolumab, a PD-1 blocking antibody, with the BTK inhibitor (BTKi) ibrutinib could potentiate tumor-cell killing. We conducted an investigator-initiated phase 2 clinical trial to assess the efficacy of combined nivolumab and ibrutinib in patients with diffuse large B-cell lymphoma (DLBCL) RT and CLL. Patients included were ≥18 years of age with adequate hepatic and renal function. Patients received nivolumab every 2 weeks of a 4-week cycle for a maximum of 24 cycles. A standard dose ibrutinib was initiated from cycle 2 onward and continued daily until progression. For patients who were already on ibrutinib at the time of study entry, the same was continued while nivolumab was initiated. A total of 24 patients with RT with a median age of 64.5 years (range, 47-88) were enrolled. Ten patients (42%) had received prior treatment for RT and 13 patients (54%) had received a prior BTKi. A total of 10 patients (42%) responded with a median duration of response of 15 months. The median overall survival was 13 months. Four of 24 (17%) patients had checkpoint inhibition–related immunological toxicities. In the CLL cohort, 10 patients were enrolled, of whom 3 patients converted from partial to complete remission; 1 patient had a grade 2 immunological toxicity. Combined nivolumab and ibrutinib is an active regimen for patients with DLBCL RT with an overall response rate of 42%. Given the limited treatment options for patients with RT, checkpoint inhibition provides a potential therapeutic option. This trial is registered at www.clinicaltrials.gov as #NCT02420912.
Introduction
The treatment of patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) has significantly improved over the last decade with better understanding of disease biology and the availability of multiple targeted therapies.1,2 Despite these improvements, about 2% to 10% of patients with CLL develop a diffuse large B-cell lymphoma (DLBCL) transformation over the duration of their CLL diagnosis, known as Richter transformation (RT), resulting in median overall survival (OS) of less than 12 months.3-8 Chemoimmunotherapy is the standard first-line therapy for patients with RT, resulting in objective responses in ∼40% to 50% of the patients.3,9 Targeted therapies, such as Bruton tyrosine kinase inhibitor (BTKi) and BCL2 inhibitor (BCL2i) venetoclax, have shown modest responses in RT.10-13
Immune dysfunction is common in CLL with increased PD-1 expression on T cells and PD-L1 and PD-L2 on CLL cells; blocking PD-1/PD-L1 activates T cells and promotes immunological synapse formation.14-18 However, checkpoint inhibitors have shown limited clinical activity in patients with CLL.19 In patients with RT, PD-1 is expressed on the neoplastic B cells but not on the CLL cells.20-23 In addition, there is expression of PD-L1 by histiocytes and dendritic cells in the tumor microenvironment and higher infiltration of FOXP3-positive T cells.23 In consequence, we anticipate that the use of PD-1/PD-L1 pathway inhibitors would reduce the immunological escape and potentially improve outcomes in patients with RT.
The use of the PD-1 monoclonal antibody pembrolizumab as monotherapy led to an overall response rate of 44% (4 of 9 patients responded with 1 complete response [CR] and 3 partial responses [PRs]) in patients with RT, with a median progression-free survival (PFS) of 5.4 months and median OS of 10.7 months.19 In the KEYNOTE-170 study, Armand et al showed partial remission in only 1 of 18 (6%) patients with DLBCL RT when treated with pembrolizumab.24 Given the role of ibrutinib in reversing immune exhaustion and preclinical data reporting synergy between PD-1/PD-L1 blockade and ibrutinib,25,26 we designed an investigator-initiated phase 2 clinical trial to study the efficacy and safety of combined ibrutinib and nivolumab therapy in patients with RT. This study also included a cohort of patients with CLL which was closed early due to slow accrual and availability of additional novel therapeutic agents for patients with CLL.
Methods
The study was approved by the Institutional Review Board, registered on ClinicalTrails.gov (#NCT02420912), and conducted in accordance with the Declaration of Helsinki. All patients were recruited and treated at The University of Texas MD Anderson Cancer Center (MDACC), Houston, Texas.
Patient population
DLBCL RT
Patients had to have a confirmed diagnosis of DLBCL RT from either a lymph node/tumor site biopsy and/or bone marrow biopsy and had relapsed or were refractory to at least 1 prior line of therapy for CLL or RT. Patients with del(17p) were eligible even if they had no prior therapy for CLL or RT. Eligible patients had to be ≥18 years of age, have an Eastern Cooperative Oncology Group performance status of ≤2, and have adequate renal (serum creatinine ≤1.5 × upper limit of normal) and hepatic function. Detailed eligibility criteria are listed in the supplemental Appendix. Patients with prior exposure to ibrutinib were allowed to enroll, whereas those with autoimmune diseases were excluded. Patients with prior allogeneic stem cell transplant (allo-SCT) within 6 months or with active acute or chronic graft-versus host disease (GVHD) were excluded.
CLL
Patients enrolled had to have CLL/SLL which was relapsed or refractory to at least 1 prior line of standard therapy or untreated del(17p) confirmed by fluorescence in-situ hybridization (FISH) and an indication for treatment by International Workshop on CLL (iwCLL) 2008 criteria. Patients were also eligible if they had been on ibrutinib for at least 9 months for CLL/SLL with measurable persistent disease (absolute lymphocyte count >4000/μL, any lymph node >1.5 cm by CT scan, or >30% lymphocytes on bone marrow aspirate differential).
Treatment plan and response assessments
Nivolumab was administered as a 3 mg/kg IV infusion over 1 hour every 2 weeks each 4-week cycle, starting cycle 1 day 1 for a total of 24 cycles, in the absence of disease progression or significant toxicities. Ibrutinib was given 420 mg once daily starting cycle 2 day 1 and continued until disease progression or unacceptable toxicities; ibrutinib could be initiated during cycle 1 for patients with worsening disease. In both the RT and CLL arms, for patients who were already on ibrutinib, the same was continued while nivolumab was introduced. After the first 3 cycles, the frequency of nivolumab administration could be reduced to once every 4 weeks at 3 mg/kg as per the discretion of the investigator.
Response assessments were done by positron emission tomography-computed tomography (PET-CT) scan and bone marrow aspiration/biopsy after cycles 1, 3, 6, 9, 12, 18, and 24 for all patients in the study. For patients with RT, the imaging was graded as complete metabolic response (CMR), partial metabolic response (PMR), no response, or progressive disease, as per the Lugano classification.27 Responses for CLL were graded as per the iwCLL 2008 modified response criteria.28
Adverse events were reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3. Special attention was given to monitoring for immunological adverse events such as colitis, pneumonitis, and endocrinopathy. Further details of response assessment, toxicity monitoring, dose modifications of study drug, and therapy interruptions or discontinuation are present in the supplemental Appendix.
Statistical analysis
DLBCL RT
The primary objective was to evaluate the best response (BR) during the first 12 months of therapy. The primary efficacy end point, BR, is defined as CMR or PMR occurring during the first 12 months of treatment. The optimal 2-stage design proposed by Simon was implemented.29 We assumed a target CMR/PMR rate of 20% and a response rate of 5% or lower was considered not desirable. With a type 1 error rate of 10% and 80% power, we planned to enroll 9 patients in the first stage. If no patients achieved CMR/PMR, the trial had to be stopped. If 1 or more of the first 9 patients attained CMR/PMR, accrual had to be continued until a total enrollment of 24 patients was reached. At the end of the study, if ≥3 of the 24 patients achieved CMR/PMR, the combination treatment was considered efficacious and worthy of further investigation.
CLL
Patients with CLL were included in 2 cohorts: (1) those who were to be initiated on ibrutinib along with the nivolumab on trial and (2) those who had already been on ibrutinib for >9 months and nivolumab was introduced as part of the trial therapy.
In the first cohort, the primary objective was to evaluate BR during the first 12 months of therapy. The primary efficacy end point, BR, was defined as CR or CRi (complete remission with incomplete count recovery) that occurred during the first 12 months of treatment. For the second cohort, the primary objective was to evaluate the conversion rate during the first 12 months of therapy. The primary efficacy end point, the conversion rate, was defined as the conversion from PR to CR/CRi that occurred during the first 12 months of treatment.
For both CLL cohorts, like the DLBCL RT cohort, the optimal 2-stage design proposed by Simon was implemented. We assumed a target BR (attainment of CR/CRi for the first cohort or conversion to CR/CRi from PR for the second cohort) of 20% and a BR of 5% or lower was considered not desirable. With a type 1 error rate of 10% and 80% power, we had to enroll 9 patients in the first stage. If no patients achieved BR, the trial had to be stopped. If 1 or more of the first 9 patients attained BR, accrual had to continue until a total of 24 patients had been enrolled. We had to suspend accrual if, at the end of the first stage, all 9 patients had been enrolled and no response had been observed. At the end of the study, if 3 or more of the 24 patients achieved BR, the combination treatment would be considered efficacious and worthy of further investigation.
All patients who received at least 1 dose of any of the study drugs constituted the efficacy and safety population. For both DLBCL RT and CLL groups, toxicity monitoring was performed in cohorts of 6 patients using a Bayesian approach and the trial had to be stopped early if, at any point, there was an 80% possibility of toxicity (defined as any grade 3 or higher nonhematologic toxicity, which is at least possibly related to the treatment) rate being >30%. Summary statistics are provided for continuous variables. Frequency tables were used to summarize categorical variables. OS and duration of response (DOR) probabilities were calculated using the Kaplan-Meier method. DOR was calculated from the time of response (CMR/PMR for DLBCL RT; CR/CRi or conversion from PR to CR/CRi for CLL) to relapse/progression or death from any cause. Statistical analyses were performed using GraphPad Prism version 9, GraphPad Software, San Diego, CA.
Results
DLBCL RT
From March 2016 to August 2018, 24 patients with a confirmed diagnosis of DLBCL RT were enrolled and initiated into therapy. Baseline disease characteristics and treatment history are detailed in Table 1 and supplementary Table 1. The median age was 64.5 years (range, 47-88 years). The median number of prior therapies received for CLL and/or RT was 3 (range, 0-10). Only 1 patient had not received any prior therapy for CLL and/or RT and was enrolled with high-risk genetics [del(17p)], per protocol. A total of 14 of 24 (58%) were previously untreated for RT and had received a median of 2 (range, 0-5) prior therapies for CLL. The remaining 10 of 24 (42%) patients had received a median of 1 prior therapy for RT (range, 1-5) and a median of 4.5 prior therapies for both CLL and/or RT (range, 1-10). Three patients (13%) had undergone prior stem cell transplantation for their RT (2 allo- and 1 autologous SCT) and 1 patient had an allo-SCT for CLL. Thirteen patients (54%) had prior exposure to BTKi (ibrutinib, n = 12; acalabrutinib, n = 1). No patient had received prior PD-1/PD-L1 inhibitor for CLL and/or RT. A total of 6 of the 24 (25%) patients had bone marrow involvement with RT. Of the 24 patients in the study, 5 were already on ibrutinib at the time of trial therapy initiation and ibrutinib was continued with the first cycle of nivolumab as per trial protocol. Of the remaining 19 patients who were not on ibrutinib at the time of trial therapy initiation, ibrutinib was initiated in 18 (1 patient never received ibrutinib and came off study during cycle 1) during cycle 1 (n = 12) or cycle 2 (n = 6). The median follow-up is 46.3 months (range, 1.2-61.3 months).
Efficacy
A total of 10 of the 24 (42%) patients responded to combined nivolumab and ibrutinib at a median of 28 days (end of cycle 1) from trial therapy initiation (range, 25-85 days). Of these 10 patients, 8 had a CMR observed on PET scan, and 2 had a PMR. The median duration of treatment for patients who had a response was 8.4 months (range, 3.7-16 months) compared to 2.6 months (range, 1-3.6 months) for nonresponders. Supplemental Figure 1 shows the percentage change in lymphadenopathy as assessed by PET-CT imaging and supplementary Figure 2 shows the PET images of a heavily pretreated patient who attained CMR for RT. Two of the responding patients had bone marrow involvement by DLBCL at the time of study initiation; both patients cleared the marrow DLBCL component at the time of metabolic response. Among the RT responders, all 10 patients had evidence of CLL in the bone marrow at baseline. With the exception of 1 patient (patient #8, supplemental Figure 2) who achieved measurable residual disease-negative remission in bone marrow, the remaining 9 RT responders had no significant improvement in CLL bone marrow involvement.
A total of 3 of the 13 (23%) patients who had a prior exposure to BTKi had a response, compared to 7 of 11 (64%) who were BTKi-naïve. Importantly, the median number of prior therapies received by the BTKi-exposed patients was 4 compared to 1 in the BTKi-naïve group. Among the 14 patients who had not received prior therapy for RT, 7 of 14 (50%) responded, compared to 3 of 10 (30%) patients who had received prior therapy for RT. Five of 11 patients (45%) with del(17p) and/or TP53 mutation had a response (all CMR). Among the 10 responders, 6 patients initiated ibrutinib during cycle 1 (1 patient was already on ibrutinib at trial therapy initiation) and 4 patients initiated ibrutinib during cycle 2.
We assessed PD-1/PD-L1 expression in 10 patients (7 responders and 3 nonresponders) by immunohistochemistry in available baseline samples (lymph node, n = 9 and bone marrow, n = 1) (supplemental Figure 3). Among the 7 responders, 4 had PD-1 expression on large tumor cells; among the 3 nonresponders, 2 had PD-1 expression on large tumor cells.
Of the 10 patients who responded to the combination therapy, 4 (40%; 3 with CMR and 1 with PMR) proceeded to allo-SCT at a median of 6.7 months (range, 4.2-10.4 months) from initiation of treatment on the trial and at a median of 40 days (range, 28-56 days) from the last dose of nivolumab (Figure 1). Post allo-SCT data were available for 3 patients; 1 patient had extensive acute liver, gut, and skin GVHD and 2 patients had acute skin GVHD. One additional responding patient underwent allo-SCT after interval therapy with ibrutinib plus venetoclax for 3 months for CLL, while remaining in CMR for RT. Another responding patient underwent allo-SCT after receiving salvage therapy for relapse.
Swimmer’s plot of the study patients (N = 24). Patients 1 to 10 were responders; 11 to 24 were nonresponders. Patients alive at last follow-up are represented by arrowheads; patients who died are represented by crosses at the end. Time ‘0’ is C1D1 of trial therapy. Relapse has been denoted only for patients who responded to the trial therapy.
Swimmer’s plot of the study patients (N = 24). Patients 1 to 10 were responders; 11 to 24 were nonresponders. Patients alive at last follow-up are represented by arrowheads; patients who died are represented by crosses at the end. Time ‘0’ is C1D1 of trial therapy. Relapse has been denoted only for patients who responded to the trial therapy.
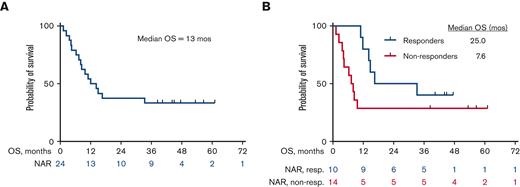
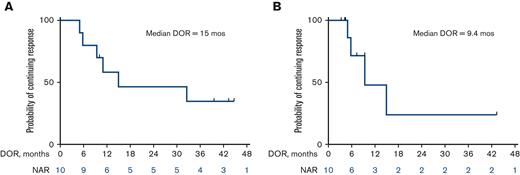
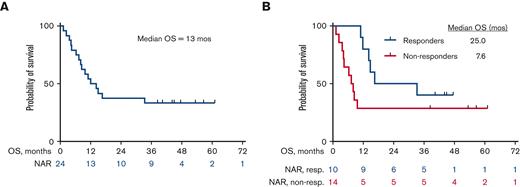
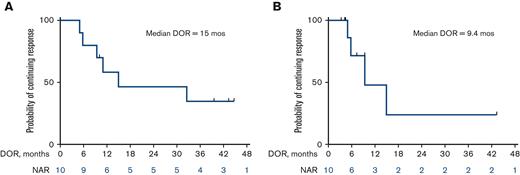
The median OS for all patients is 13 months (25 months for responders vs 7.6 months for nonresponders) (Figure 2A and B). Among the 10 patients who responded, the median DOR was 15 months, when not censored for allo-SCT, and 9.4 months, when censored for allo-SCT (Figure 3A and B). The median OS for the 14 patients who were treatment-naïve for RT was 24.1 months and 9.1 months for the 10 patients who had received prior therapy for RT (Figure 4). Supplemental Figure 4 shows the disposition of the patients treated on this study.
Kaplan-Meier estimates of OS (N = 24). (A) OS for the overall cohort, N = 24. The median OS was 13 months. (B) OS of responders (n = 10) vs nonresponders (n = 14). The median OS was 25 months for responders vs 7.6 months for nonresponders. NAR, Number at risk; non-resp, nonresponders; resp, responders.
Kaplan-Meier estimates of OS (N = 24). (A) OS for the overall cohort, N = 24. The median OS was 13 months. (B) OS of responders (n = 10) vs nonresponders (n = 14). The median OS was 25 months for responders vs 7.6 months for nonresponders. NAR, Number at risk; non-resp, nonresponders; resp, responders.
Kaplan-Meier estimates of DOR (n = 10). (A) Median DOR of 15 months when not censored for allo-SCT. (B) Median DOR of 9.4 months when censored for allo-SCT. NAR, number at risk.
Kaplan-Meier estimates of DOR (n = 10). (A) Median DOR of 15 months when not censored for allo-SCT. (B) Median DOR of 9.4 months when censored for allo-SCT. NAR, number at risk.
Kaplan-Meier estimates of OS in patients who had received prior treatment for RT (n = 10) and those who were treatment naïve for RT (n = 14). NAR, number at risk; Rx, therapy.
Kaplan-Meier estimates of OS in patients who had received prior treatment for RT (n = 10) and those who were treatment naïve for RT (n = 14). NAR, number at risk; Rx, therapy.
Safety
All patients were included in the safety analysis (Table 2). One patient with a prior history of allo-SCT had grade 3 transaminitis and features of GVHD reactivation for which further therapy with nivolumab was discontinued and the patient was taken off the protocol. Overall, 4 patients (17%) had immunological toxicities; 1 patient each had grade 4 lipase/amylase elevation, grade 3 transaminitis (mentioned above), grade 2 anterior uveitis and grade 2 pneumonitis. Except for the post allo-SCT patient who was taken off the protocol, the drug was reinitiated after resolution of immune toxicity with supportive care, including a short course of steroids in the patient with grade 2 pneumonitis and topical steroids for the patient with grade 2 anterior uveitis. There was no recurrence of immune toxicity on drug re-challenge.
CLL
From December 2015 to January 2017, 10 patients with CLL with a median age of 57 years (range, 42-70 years) were enrolled: 7 patients to the first cohort and 3 patients, already on ibrutinib, to the second cohort. Four patients (40%) had del(17p)/TP53 mutation and 4 patients (40%) had unmutated immunoglobulin heavy chain variable region gene. The median lines of prior therapy in the 7 patients in the first cohort was 1 (range, 1-3); 3 patients in the second cohort had only received prior ibrutinib for their CLL for 13, 26, and 32 months. Two patients (29%) in the first cohort had a protocol-specified response of CR after 9 and 24 cycles of therapy. One of 3 patients (33%) in the second cohort had a conversion to CR from PR after 3 cycles of nivolumab therapy. The median number of cycles on trial was 20 (range, 3-24). One patient in the second cohort developed grade 2 bilateral anterior uveitis after 21 cycles of therapy. The episode resolved on withholding nivolumab and treatment with topical steroids.
Discussion
In our cohort of heavily pretreated patients with DLBCL RT, with a median number of prior therapies received for CLL/RT of 3, we noted an encouraging response rate of 42% with combined nivolumab plus ibrutinib. The median DOR was 15 months and OS was 25 months for the responding patients.
The standard first-line treatment for RT generally includes traditional chemoimmunotherapy regimens (such as R-CHOP, O-CHOP, R-DHAP, R-EPOCH, and OFAR) and these lead to responses in 30% to 60% of previously untreated RT with a median survival of less than 1 year.3,9,30-34 In a recent phase 2 study of venetoclax with dose-adjusted R-EPOCH (VR-EPOCH) in 26 patients with RT (24 of 26 were previously untreated for RT) with a median of 1 prior line of therapy for CLL, the overall response rate was 62% with a median PFS of 10.1 months and median OS of 19.6 months. In our study, we noted comparable results for the patients who were treatment-naïve for RT, without the use of intensive chemotherapy. We treated 14 patients who were previously untreated for RT with a median of 2 prior CLL therapies. We noted an overall response rate (ORR) of 50% (7 of 14) with a median DOR of 10.1 months and median OS of 24.1 months.
Ding et al reported the first study of checkpoint inhibition in patients with RT. Among the 9 patients with RT (6 relapsed/refractory RT; 3 treatment-naïve RT), 4 of 9 (44%) had a response (1 CMR, 3 PMR) to pembrolizumab monotherapy with a median PFS of 5.4 months and median OS of 10.7 months.19 Notably, all 4 responders had a prior exposure to ibrutinib. In contrast, in a retrospective study of PD-1 blockade for 10 patients with RT, all of whom were BTKi exposed, only 1 patient (10%) showed a response and the median OS from initiation of PD-1 inhibitor was just 2 months for the entire cohort.35 Younes et al reported a trial of combined nivolumab and ibrutinib in patients with RT with an overall response rate of 65% (10% CR and 55% partial response by CT assessments) in 20 patients with DLBCL RT; all patients, by study design, were BTKi naïve in this trial.36 In our study, we also saw the majority of responses in the BTKi-naïve group with 7 of 11 (64%) responses vs 3 of 13 (23%) responses among patients who had a prior exposure to BTKi. Our results are consistent with the results by Younes et al with both studies using combined nivolumab and ibrutinib for patients with RT and reporting ∼65% response rate in the BTKi-naïve cohort. To the best of our knowledge, our study is the largest cohort of patients with DLBCL RT treated with checkpoint inhibition. Based on these studies, PD-1 inhibition with or without ibrutinib is an appropriate treatment option for patients with RT and therefore included in the National Comprehensive Cancer Network guidelines.
Given that allo-SCT consolidation is the only potentially curative modality for patients with RT, notably 4 of 10 responding patients (40% of responders and 17% of all patients) were able to proceed directly to an allo-SCT after the treatment. A fifth patient underwent allo-SCT while maintaining response for RT with combined nivolumab and ibrutinib but received interim venetoclax due to CLL relapse. The use of checkpoint inhibitors like nivolumab before or after allo-SCT pose the risk for immunological toxicities, including higher risk of GVHD.37,38 In patients with Hodgkin lymphoma, around 30% of those who received nivolumab for disease relapse after allo-SCT had acute GVHD; notably all of these were reactivation of prior GVHD.39 In our study, 1 of 3 patients who proceeded to allo-SCT directly after the study experienced systemic acute GVHD and another patient who had a prior allo-SCT had reactivation of GVHD, causing study discontinuation for the patient. Though the risk appears low, it is important that patients who proceed to allo-SCT after nivolumab-based regimens or are offered the drug after a prior allo-SCT be closely monitored for the onset or reactivation of GVHD.40
Clonally-related RT is associated with worse outcomes than clonally-unrelated RT. Studies have shown that clonally-related DLBCL RT is more commonly associated with PD-1/PD-L1 expression than de novo DLBCL.20,21 Unfortunately, we do not have data for clonal relatedness of RT for our study patients; however, based on published literature, we expect >80% to 90% of the patients to have RT that is clonally-related to the underlying CLL. We assessed the PD-1/PD-L1 expression in 10 patients with available baseline samples, 7 of whom were positive for PD-1/PD-L1. Five patients positive for and 2 of 3 negative for PD-1/PD-L1 responded to the treatment. With the caveat of limited sample size, we are unable to establish response correlation with PD-1/PD-L1 expression. Correlative studies are ongoing in responders vs nonresponders to interrogate immune profile and activation in samples from patients treated in this trial.
Check point inhibition adds to the emerging therapeutic landscape for patients with RT. Early results of noncovalent BTKi pirtobrutinib in patients with RT appear promising.41 Anti-CD19 chimeric antigen receptor T-cell therapy has also been investigated in patients with DLBCL RT with early positive results.42-45 However, these approaches need to be further studied to understand their optimum timing in the treatment schema, the need for subsequent allo-SCT and their effect on the underlying CLL compartment.
Of the 10 patients with CLL enrolled in the trial, 3 achieved CR/CRi. However, due to the changing landscape of CLL therapies with the introduction of novel BTK and BCL2 inhibitors, additional patients were not enrolled in this cohort. In the study by Ding and colleagues, of the 16 patients with CLL who received pembrolizumab monotherapy, none had a response.19 In the study by Younes and colleagues, the response rate in patients with CLL (n = 36) treated with ibrutinib and nivolumab combination therapy was similar to single agent ibrutinib.36
In conclusion, combined nivolumab and ibrutinib is an active and safe regimen for patients with DLBCL RT. Given the limited treatment options for patients with RT, checkpoint inhibition provides a potential therapeutic option for these patients.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the MDACC IND office for their oversight of the study, and the referring physicians. They also thank the entire clinical and research staff at the Department of Leukemia, MDACC.
This work was supported by Bristol Myers Squibb (BMS) and The University of Texas MD Anderson Cancer Center’s CLL Moon Shot program. This research was supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672.
BMS provided the study drug (nivolumab) and paid, in part, for conduct of the study protocol. They had no role in data curation and analysis, writing of the manuscript, or submission of the manuscript.
Authorship
Contribution: N.J. and W.W. designed and performed the research, analyzed data, and wrote the paper; J.S. analyzed data and wrote the paper; B.T., S.B., E.P., C.W., J.K., and C.B. performed research and analyzed the laboratory data; A.F., P.T., J.B., T.K., N.D., G.B., M. Konopleva., N.P., S.B., S.O., H.K., and M. Keating performed research and wrote the paper; J.A. and P.S. provided vital analytical tools; N.G., W.L., and A.A. performed the research; and X.W. analyzed the data.
Conflict-of-interest disclosure: N.J. has received research support from BMS, Pharmacyclics, AbbVie, Genentech, AstraZeneca, Pfizer, Servier, ADC Therapeutics, Cellectis, Adaptive Biotechnologies, Incyte, Precision Biosciences, Aprea Therapeutics, Fate Therapeutics, Kite/Gilead, Mingsight, Takeda, Medisix, Loxo Oncology, Novalgen, Dialectic Therapeutics, Newave, and TransThera Sciences, and has participated in advisory board meetings and received honoraria from BMS, Pharmacyclics, Janssen, AbbVie, Genentech, AstraZeneca, Adaptive Biotechnologies, Kite/Gilead, Precision Biosciences, Beigene, Cellectis, TG Therapeutics, MEI Pharma, Ipsen, and CareDX. P.T. has received research funding from AbbVie, Pharmacyclics, Lilly, Adaptive Biotechnologies and has participated in advisory boards/consultations with Janssen, AbbVie, Adaptive Biotechnologies, BeiGene, Lilly, and Genentech. A.F. has received research funding from BeiGene and AstraZeneca. J.B. has received honoraria from and participated in advisory boards/consultations with Janssen, has received research funding from AstraZeneca, BeiGene, and Pharmacyclics LLC, an AbbVie company, and has participated in speakers' bureau for and received travel expenses from Gilead, Janssen, Novartis, Pharmacyclics LLC, an AbbVie company, and TG Therapeutics. T.K. has received research grants from Amgen, Ascentage, Astellas, AstraZeneca, BMS, Cellenkos, Pulmotech, and Genfleet; personal fees from Agios, Cure, Daiichi Sankyo, Genzyme, Liberum, Novartis, and Sanofi-Aventis; and research grants and personal fees from AbbVie, Genetech, Jazz Pharmaceuticals, and Pfizer. N.D. has received research funding from Daiichi Sankyo, BMS, Pfizer, Karyopharm, Sevier, Genentech, Astellas, Sobi, Hanmi, Fate, Gilead, Forty-Seven, Trillium, KAHR, Kite, and ImmunoGen and has served in a consulting or advisory role for Daiichi Sankyo, BMS, Pfizer, Syndax, Astellas, Immunogen, Glycostem, Novartis, Celgene, AbbVie, Arch Oncology, Kite, Gilead, Servier, Trillium, Shattuck Labs, and Agios. G.B. has received research funding from AbbVie, Agensys, Arvinas, AstraZeneca, Bayer Healthcare AG, BioLineRx, BMS, Cantargia AB, Cyclacel, Eli Lilly and Company, Esai, GlaxoSmithKline, Incyte, Merck, Novartix, Oncoceutics, Polaris, Tetralogic Pharmaceuticals, and XBiotechUSA and personal fees from Argenx, BioLineRx, BioTheryX, Fate Therapeutics, NKarta, Strategia Therapeutics, and TPC Therapeutic. M. Konopleva received research funding from AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, AstraZeneca, Rafael Pharmaceutical, Sanofi and Forty-Seven; served as a consultant/advisory board member for AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji, and Janssen; and outside the submitted work has a patent US 7795305 B2 CDDO-compounds and combination therapies with royalties paid to Reata Pharm, a patent combination therapy with a mutant IDH1 inhibitor and a BCL-2 inhibitor licensed to Eli Lilly, and a patent 62/993 166 combination of an MCL-1 inhibitor and midostaurin, uses and pharmaceutical compositions thereof, pending to Novartis. N.P. has received research grants from Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectics, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon, and MustangBio; honoraria from Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer Science+Business Media LLC, Aptitude Health, NeoPharm, and CareDX; has participated in advisory boards/consultation with Blueprint Medicines, Pacylex Pharmaceuticals Inc, Immunogen, BMS, Clearview Healthcare Partners, Astellas Pharma US Inc., Protagonist Therapeutics, Triptych Health Partners, CTI BioPharma Corp; and received travel/accommodation expenses from Stemline Therapeutics, Celgene, AbbVie, DAVA oncology, and Mustang Bio. H.K. has received research grants and honoraria from AbbVie, Amgen, Ascentage, BMS, Daiichi Sankyo, Immunogen, Jazz, Novartis, Pfizer, and Sanofi, honoraria from Actinium (advisory board), Adaptive Biotechnologies, Aptitude Health, BioAscend, DeltaFly, Janssen Global, Oxford Biomedical, and Takeda Oncology. W.W. participated in advisory boards/consultation with Sanofi; and received research funding from GlaxoSmithKline/Novartis, AbbVie, Genentech, Pharmacyclics, Acerta Pharma, Gilead Sciences, Janssen, Juno Therapeutics, Kite, a Gilead company, Oncternal Therapeutics, Loxo, Xencor, miRagen, Sunesis Pharmaceuticals, and Cyclacel. The remaining authors declare no competing financial interests.
Correspondence: Nitin Jain, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: njain@mdanderson.org.
References
Author notes
Data are available on request from the corresponding author, Nitin Jain (njain@mdanderson.org).
The full-text version of this article contains a data supplement.