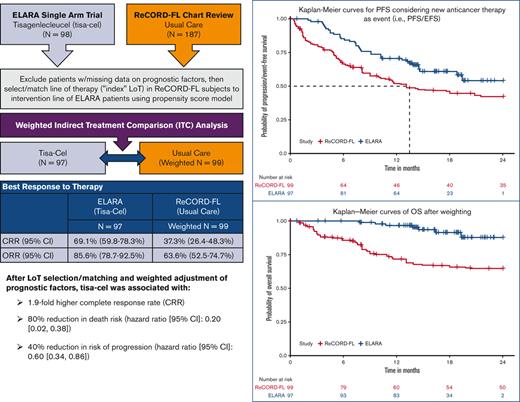
Abstract
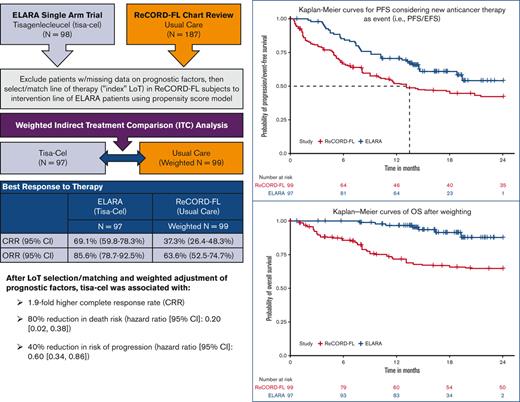
The ELARA trial indicates tisagenlecleucel (tisa-cel) is an effective anti-CD19 chimeric antigen receptor T-cell therapy for relapsed or refractory follicular lymphoma (r/r FL). As ELARA is a single-arm trial, this study compares tisa-cel outcomes from the ELARA trial with usual care from a real-world cohort. ELARA enrolled 98 patients as of 29 March 2021 (median follow-up: 15 months from enrollment). Usual care data were obtained from ReCORD-FL, a global retrospective study of patients with r/r FL, who met similar eligibility criteria to ELARA. With a data cutoff date of 31 December 2020, 187 patients with ≥2 preceding treatment lines were included in the ReCORD-FL (median follow-up: 57 months from third-line) study. An indirect treatment comparison was performed for 97 patients from the ELARA trial and 143 patients from the ReCORD-FL study with no missing data on baseline factors. The line of therapy for which outcomes were assessed was selected or matched between cohorts using propensity score modeling. After baseline factor adjustment via weighting by odds, complete response rate (CRR; 95% confidence interval) was 69.1% (59.8%-78.3%) for tisa-cel vs. 37.3% (26.4%-48.3%) for usual care; overall response rate was 85.6% (78.7%-92.5%) vs. 63.6% (52.5%-74.7%). Kaplan-Meier probability of being progression/event-free at 12 months was 70.5% (61.4%-79.7%) for tisa-cel vs. 51.9% (40.6%-63.3%) for usual care, with hazard ratio (HR)=0.60 (0.34-0.86); 12-month overall survival was 96.6% (92.9%-100%) vs. 71.7% (61.2%-82.2%), with HR=0.2 (0.02-0.38). In conclusion, tisa-cel was associated with a 1.9-fold higher complete response rate and a 1.4-fold higher rate of being progression or event free at 12 months vs usual care, as well as a death risk reduction of 80%. The findings provide additional evidence on the benefit of tisa-cel in patients with r/r FL after ≥2 treatment lines. This trial was registered at www.clinicaltrials.gov as NCT03568461
Key Points
Our study contextualizes the single-arm ELARA trial by providing historical control data from clinically similar patients on usual care.
Tisagenlecleucel was shown to have superior efficacy than usual care in patients with multiply relapsed or refractory follicular lymphoma.
Introduction
Patients with multiply relapsed or refractory follicular lymphoma (r/r FL) represent an area of high unmet need for which newer treatments with novel mechanisms of action are needed to offer long-term remissions.1,2 Tisagenlecleucel (tisa-cel) is an anti-CD19 chimeric antigen receptor T-cell therapy currently undergoing clinical study in the phase 2 ELARA (E2202) trial (NCT03568461) for adult patients with grade 1-3A r/r FL who meet the following inclusion criteria: (1) refractory to a second or later line of systemic therapy (including an anti-CD20 monoclonal antibody [mAb] and an alkylating agent) or relapsed within 6 months after completion of a second or later line of systemic therapy, (2) relapsed during anti-CD20 mAb maintenance (after at least 2 lines of therapies as mentioned earlier) or within 6 months after maintenance completion, or (3) relapsed any time after autologous hematopoietic stem cell transplantation (HSCT). In the ELARA trial, tisa-cel has demonstrated high response rates in patients with r/r FL, including those with high-risk disease, with an overall response rate (ORR) of 86% and a complete response rate (CRR) of 69%.3 As ELARA is a single-arm trial, the aim of this study was to perform a comparative effectiveness analysis using patient-level historical control data from a matched global retrospective cohort of patients with r/r FL receiving usual care. Results of this study will help to contextualize the efficacy outcomes of tisa-cel from the ELARA trial relative to usual care, which may help inform treatment decisions in the population with multiply r/r FL.
Methods
Patient population
ELARA is an ongoing, single-arm, global, multicenter, phase 2 trial evaluating the efficacy and safety of tisa-cel in adult patients with r/r FL.4 The study is now closed to enrollment, and as of 29 March 2021, a total of 98 patients were enrolled with a median follow-up of 15 months. Usual care data were obtained from ReCORD-FL, a global retrospective cohort study of clinical outcomes in patients who met the ELARA trial’s eligibility criteria.5 The ReCORD-FL study included 10 academic centers across North America and Europe; 7 ReCORD-FL sites are also participating in ELARA, but no patient is enrolled in both studies based on nonoverlapping case selection windows for the 2 studies. With a follow-up data cutoff date of 31 December 2020, in the ReCORD-FL study, a total of 187 patients with ≥2 lines of previous treatment were identified for inclusion, with a median follow-up from third-line therapy of 57 months. Full details on the ReCORD-FL study design and results are described in a recent publication.5 Multiple site-level and local or regional institutional review boards reviewed and approved this research. The research was conducted in accordance with the Declaration of Helsinki. Individual participant data will not be shared in order to maintain data privacy requirements prescribed by the various country-specific ethics approvals for study conduct,
Clinical end points
Clinical end points, including CRR, ORR, time to next treatment or death (TNT-D), progression- or event-free survival (PFS/EFS), and overall survival (OS) were evaluated for 1 eligible line of therapy (LoT) for each patient. In the ELARA cohort, the eligible (index) LoT was the one in which trial enrollment occurred after relapse or refractoriness to ≥2 prior lines of treatment, including both an anti-CD20 mAb and an alkylating agent. For the usual care cohort, the index LoT was selected using the statistical methods described later in the article.
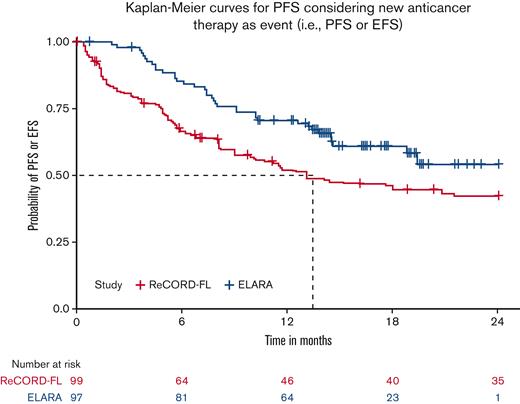
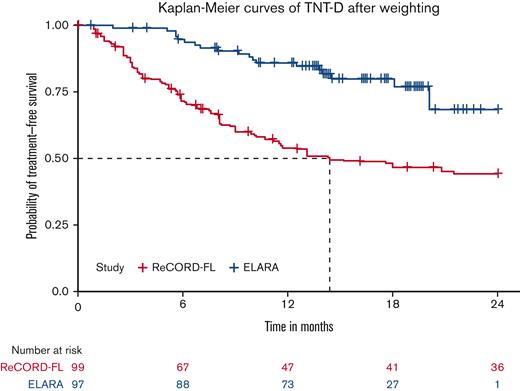
CRR was defined as the proportion of patients achieving a complete response to the index LoT. ORR was likewise defined as the proportion of patients achieving either a complete or partial response to the index LoT. In the ELARA cohort, responses were assessed as per the Lugano criteria, whereas in the ReCORD-FL cohort, responses were based on clinician judgment using retrospective information (eg, radiographic imaging, physical exam, and so on) collected from the patients’ charts. In the ReCORD-FL study, imposition of predefined response criteria such as the Lugano was not possible due to the retrospective, noninterventional design. TNT-D was defined as the time to the start of a new antilymphoma treatment (including HSCT) or death due to any cause. Patients without events through the course of follow-up were censored on the last contact date. In the ELARA cohort, PFS was defined as time to first documented disease progression or death due to any cause. In the usual care cohort, for cases in which clinical progression dates were missing or not recorded in the routine practice settings of the ReCORD-FL study, initiation of a new anticancer therapy was also considered to be a progression event. For consistency between cohorts, this additional criterion for a progression event was also applied to the ELARA cohort for the comparative analysis and therefore represents a composite end point of PFS or EFS. OS was defined as time to death due to any cause. If a death event was not observed, then OS was censored at the last date the patient was known to be alive.
Statistical analyses
A complete-case indirect treatment comparison (ITC) analysis was performed for 97 patients who had undergone apheresis in the ELARA trial and 143 patients in the ReCORD-FL study, with complete data on relevant, predefined baseline variables and prognostic factors. For the usual care cohort, 1 LoT per patient was selected using a propensity score (PS) model to identify the LoT with the highest odds of being enrolled in the ELARA trial, conditional on baseline characteristics and prognostic factors at the start of the LoT. After LoT selection, an adjusted ITC was performed using the weighting by odds method.6 This method allowed for the use of data on all patients with nonmissing data on baseline factors from both the ELARA and usual care cohorts. This method also maintains the original composition of patients in the ELARA cohort (assigning each patient a weight of 1), whereas patients in the usual care cohort were weighted by the odds of meeting the ELARA inclusion criteria. The usual care sample size after weighting (ie, a sum of weights) was 99. To assess covariate balance, the distributions of baseline variables before and after weighting were summarized, with standardized mean differences (SMDs) reported for each variable. An absolute SMD threshold of <0.25 was used to define the sufficient balance between groups for each covariate.7 A subgroup analysis of usual care patients with ≥1 eligible LoT initiated from 2014 onward (coinciding with the introduction of the Lugano response criteria as well as approval of idelalisib) was performed for all end points.
CRR and ORR were analyzed using weighted (as described earlier) univariate statistics, whereas all time-to-event end points (TNT-D, PFS/EFS, and OS) were analyzed using weighted Kaplan-Meier analyses, and weighted Cox proportional hazards models were used to assess end point differences between the ELARA and usual care cohorts (ie, to assess the incremental effect on clinical outcomes of tisa-cel vs usual care). The weighted Cox models were implemented as univariate models, with each time-to-event end point as a function of the cohort (tisa-cel vs usual care). As the Cox models were weighted using weights from the PS model for LoT selection (ie, weighting by odds method), they were therefore adjusted for the same covariates used in LoT selection. Bootstrap methods were used to provide 95% confidence intervals (CIs) for all end point comparisons.
Results
Baseline characteristics
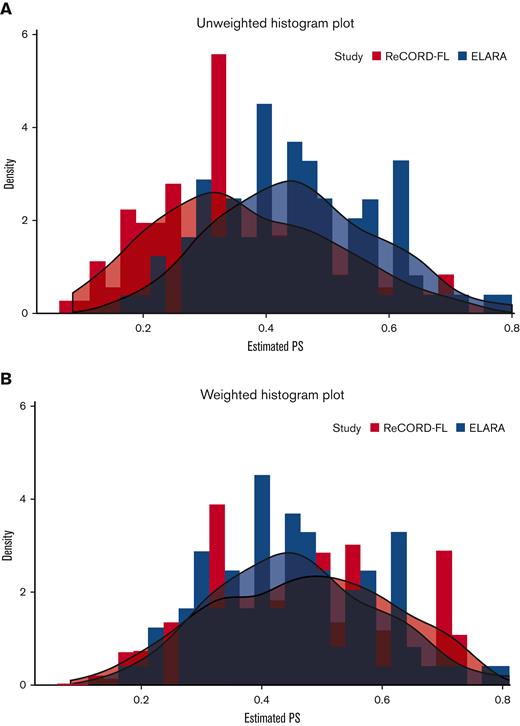
After weighting, baseline variables, including the number of previous lines of systemic therapy (median: 4 lines), and the proportion of patients receiving prior autologous HSCT (37%), were well balanced between the tisa-cel and usual care cohorts based on an absolute SMD of <0.25 for all variables (Table 1). Treatment regimens observed for the patients receiving usual care for the selected index LoT were as follows: (1) anti-CD20 mAb plus alkylator (31.5% of patients), (2) anti-CD20 mAb without alkylator (25.9%), (3) alkylator without anti-CD20 mAb (17.5%), and (4) regimens other than anti-CD20 mAb and alkylator (25.2%). In the preindex period (before the start of the selected index line for the ITC analysis), all patients (by study design) in both the ELARA trial and ReCORD-FL study received an anti-CD20 mAb and an alkylator, most often as a combination within the same regimen. Besides these therapies, other treatments commonly observed before the selected index line were PI3K inhibitors (14.0% of the ReCORD-FL patients and 21.4% of the ELARA enrollees), lenalidomide alone or in combination with other treatments (15.4% of the ReCORD-FL patients and 22.4% of the ELARA enrollees), and lenalidomide plus rituximab (9.8% of the ReCORD-FL patients and 17.3% of the ELARA enrollees). The weighted probability density function of the estimated propensity scores at the selected LoT provides additional assurance that the weighting by odds method adequately balances the PS distribution (and thus background covariates) between groups (Figure 1A-B).
PS distribution in original sample. (A) Before weighting PS. (B) After weighting PS. Usual care (ReCORD-FL) PS data are shown in orange; tisa-cel (ELARA) PS data are shown in blue.
PS distribution in original sample. (A) Before weighting PS. (B) After weighting PS. Usual care (ReCORD-FL) PS data are shown in orange; tisa-cel (ELARA) PS data are shown in blue.
ITC analysis
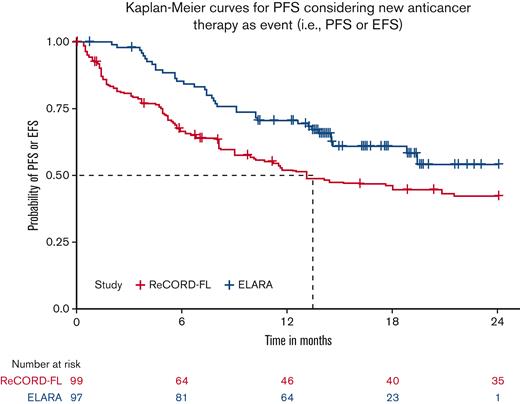
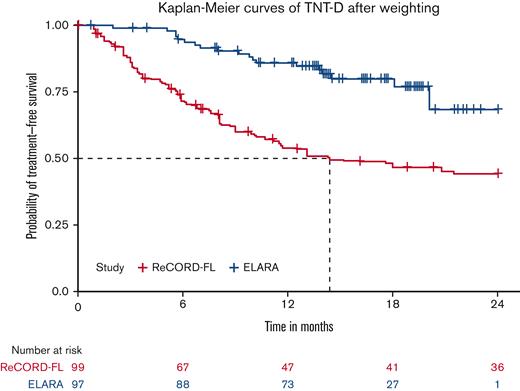
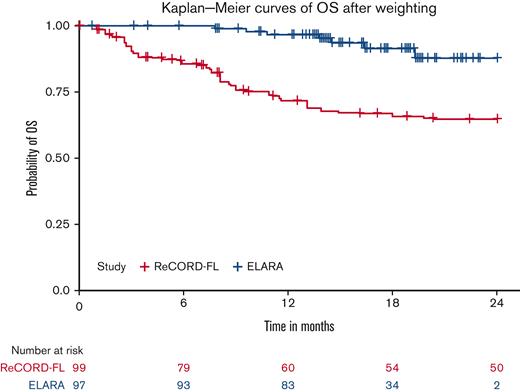
After LoT selection and weighted adjustment for differences in baseline variables, tisa-cel was associated with improvement over usual care in CRR (69.1%; 95% CI, 59.8%-78.3% vs 37.3%; 95% CI, 26.4%-48.3%) and ORR (85.6%; 95% CI, 78.7%-92.5% vs 63.6%; 95% CI, 52.5%-74.7%). The Kaplan-Meier median PFS or EFS was not reached for tisa-cel recipients in the ELARA trial but was reached at 13.1 (95% CI, 8.1; not reached) months for usual care recipients in the ReCORD-FL study after weighted adjustment. The estimated probability of being progression or event free at 12 months was 70.5% (95% CI, 61.4%-79.7%) for tisa-cel vs 51.9% (95% CI, 40.6%-63.3%) for usual care, with a corresponding hazard ratio (HR) of 0.60 (95% CI, 0.34-0.86) indicating an estimated 40% reduction in risk of progression, death, or starting a new anticancer therapy for tisa-cel recipients as compared with recipients of usual care. After weighted adjustment, the Kaplan-Meier estimate of OS rate at 12 months was 96.6% (95% CI, 92.9%-100%) for tisa-cel vs 71.7% (95% CI, 61.2%-82.2%) for usual care, with an estimated 80% risk reduction in favor of tisa-cel over usual care (HR, 0.2; 95% CI, 0.02-0.38). Additional efficacy results are presented in Table 2 and Figures 2-4. In the subanalysis of usual care, patients with lines of therapy initiated in or after 2014, the superiority of tisa-cel over usual care was confirmed in all the efficacy outcomes (CRR, 69.1% vs 30.5%; ORR, 85.6% vs 58.8%; HRs <1 for OS, PFS or EFS, and TNT-D). The effective sample size (due to weighting) in the subanalysis was substantially lower (n = 37) than the actual sample size (n = 95), indicating a greater loss of statistical precision for the subanalysis than for the main analysis (where actual and effective sample sizes were similar at n = 99 and 95, respectively). However, despite the lower effective sample size, nearly all end point differences remained statistically significant based on nonoverlapping 95% CIs.
Discussion
As the ELARA trial does not include a comparator arm, the aim of this study was to perform additional analyses to compare efficacy results of tisa-cel from the ELARA trial with a clinically similar group of patients with r/r FL receiving usual care in the real-world settings of the ReCORD-FL observational study. Based on the comparability of results with another similar observational study (SCHOLAR-58), ReCORD-FL presents a valid source of historical control data from a multinational population of patients with r/r FL. As documented in the ReCORD-FL study,5 patients with r/r FL have a high unmet medical need as evidenced by a long-term course of disease involving multiple therapy lines and varied treatment pathways, poorer outcomes in particular for patients with the double-refractory disease, and steadily worsening outcomes with successive lines of therapy. Because most trials in r/r FL are single arm, it is important to provide data from real-world cohorts as a means to establish the comparative effectiveness of novel therapies.
In the weighted analyses conducted here with adjustment for baseline prognostic factors, tisa-cel was associated with a 1.9-fold higher CRR and a 1.4-fold higher rate of being progression or event free at 12 months compared with the usual care in the ReCORD-FL study. A clinically meaningful and consistent improvement favoring tisa-cel was also observed for OS (80% reduction in death risk) and TNT-D (70% reduction in risk of starting new treatment or death)that are end points not subjected to heterogeneous interpretation or application of clinical response criteria. The efficacy benefits of tisa-cel vs usual care across all prespecified end points were consistent for both the main and sensitivity analyses in which patients’ LoT from the ReCORD-FL study for comparison with the ELARA trial was selected from 2014 or later when more patients could have been assessed with the Lugano response criteria as applied in the ELARA trial.
There were 3 major challenges in comparing efficacy data across the tisa-cel (ELARA) and usual care cohorts: (1) patients in the usual care cohort could meet the ELARA trial inclusion or exclusion criteria at the start of multiple lines of therapy, (2) differences in baseline prognostic factors between the ELARA and usual care cohorts introduced potential confounding on the study end points, and (3) heterogeneity in the measurement of treatment response and progression inherent in the routine practice settings of the ReCORD-FL study (specifically, in the ReCORD-FL study, clinical assessments of disease progression were not performed on a predetermined schedule or according to a predefined set of criteria as would typically be required in a prospective interventional trial). Multiple steps were taken to address these limitations through appropriate statistical methodologies as described earlier. Specifically, 1 eligible LoT per patient was systematically selected in the ReCORD-FL study based on the highest PS, and after the selection of 1 LoT for each patient in the ReCORD-FL study, indirect comparisons using the weighting by odds method were conducted to assess differences in clinical end points between tisa-cel recipients and patients receiving usual care.
In conclusion, this study provides further context to the results of the single-arm ELARA trial by providing needed historical control data from clinically similar patients receiving usual care in routine practice settings. The ITC results suggest that tisa-cel has superior efficacy over usual care in a clinically similar, matched group of patients with r/r FL for all evaluated end points. Taken together, the findings presented here provide a key benchmark and suggest that tisa-cel may be a valuable treatment option for consideration in patients with multiply r/r FL.
Acknowledgment
The research team for this study would like to acknowledge the critical contributions of Qiufei Ma, PhD, to the conception, design, and conduct of this study.
Authorship
Contribution: All authors provided critical review and revision of each manuscript draft and provided approval for submission of the final draft; G.S., J.Z., and B.L. provided additional contributions in the conception and design of the study; J.C. and K.D. provided additional contributions in the design of the study and conducted all data analyses; and G.S., S. Schuster, M. Dreyling, L.F., J.K., P.P., B.T., S. Smith, A.U., C.T., N.F., M. Dickinson, J.L., Y.W., and B.L. provided additional contributions in the collection and provision of study data.
Conflict-of-interest disclosure: G.S. provided consultancy and/or received honoraria from AbbVie, Allogene, Beigene, BMS/Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite-Gilead, Loxo, Miltneiy, MorphoSys, Novartis, Rapt, Regeneron, Takeda, and Velosbio and was also funded in part through the NIH/NCI Cancer Center support grant P30 CA008748. S. Schuster provided consultancy, received honoraria, patents and royalties, and/or research funding from Celgene, Nordic, Nanovector, Novartis, AbbVie, Acerta Pharma/AstraZeneca, Alimera Sciences, BeiGene, Juno Therapeutics, Loxo Oncology, Tessa Therapeutics, Genentech/Roche, Merck, Pharmacyclics, Adaptive Biotechnologies, lncyte, and TG Therapeutics. M. Dreyling provided consultancy, received research funding, and/or a part of speakers bureau of Bayer Healthcare Pharmaceuticals, AstraZeneca, BeiGene, Celgene, Amgen, Genmab, Gilead/Kite, Incyte, Janssen, Novartis, Roche, and AbbVie. L.F. received travel assistance from Roche. J.K. received honoraria and/or research funding from AbbVie, Amgen, Antengene, AstraZeneca, BMS, Gilead, Incyte, Janssen, Karyopharm, Medison Ventures, Merck, Novartis, Pfizer, Roche, Seattle Genetics, and TG Therapeutics. P.P. received honoraria and/or research funding from Roche, Gilead Sciences, AbbVie, AstraZeneca, Janssen, and Novartis. B.T. provided consultancy, received research funding, honoraria, and/or congress and travel support from AbbVie, Amgen, AstraZeneca, BMS-Celgene, Kite-Gilead, MSD, Novartis, Pentixafarm, Pfizer, Roche, and Takeda. S. Smith received research funding and other support from Adaptive, ADC Therapeutics, BMS, MorphoSys, Janssen, Karyopharm, Genentech, TGT, Bayer, Portola, Acerta, Pharmacyclics, Forty Seven/Gilead, Merck, and Novartis. A.U. has no relevant financial relationship to disclose. K.D. received research funding from Novartis, Vertex, Pfizer, Eisai, Eli Lilly and Company, and AstraZeneca. C.A. has current employment with Novartis. J.C. has current employment and is an equity holder with Novartis. J.Z. has current employment and is an equity holder with Novartis. C.B. has current employment and is an equity holder with Novartis, as well as has received travel support from Gilead. C.T. received honoraria from and has a board membership with Roche, AbbVie, Novartis, Gilead, Kyte, Incyte, Janssen, BMS/Celgene, and Takeda. N.F. has membership on board of directors and advisory committees and has received research funding from Bristol Myers Squibb, Hoffman-La Roche, TG Therapeutics, and Novartis, as well as is currently employed and an equity holder of Boston Gene. M. Dickinson offered consultancy, received honoraria, research funding, and/or a part of speakers bureau with Novartis, Roche, Gilead, and MSD. J.L. provided consultancy, received honoraria, research funding, and/or on speakers bureau with Novartis, Janssen, Incyte, Roche, and Astellas. Y.W. received research funding from Incyte, InnoCare, LOXO Oncology, Novartis, Genentech, and MorphoSys and board membership with Eli Lilly and Company, TG Therapeutics, LOXO Oncology, Incyte, and Kite. B.L. provided consultancy and/or received research funding from Genentech/Roche, MEI, Jannsen, and Novartis.
Correspondence: Keith L. Davis, Health Economics, RTI Health Solutions, 3040 E Cornwallis Road, Research Triangle Park, NC 27709; e-mail: kldavis@rti.org.