Key Points
Patterns of endothelial injury after allo-HCT differ between transplantation platforms.
Compared with pre-HCT, post-HCT dynamic EASIX scores may better predict NRM as patients acquire additional endothelial injury and toxicities.
Abstract
Endothelial activation and stress index (EASIX) predicts nonrelapse mortality (NRM) when assessed before hematopoietic cell transplantation (HCT). We sought to determine whether changes in EASIX after HCT may be an informative marker of NRM. We evaluated 509 adults who underwent reduced intensity, unmodified (N = 149, 29%), or myeloablative ex vivo CD34+-selected allogeneic HCT (allo-HCT) (N = 306, 71%) between 2008 and 2016. Patients who underwent unmodified allo-HCT received tacrolimus-based graft-versus-host disease (GVHD) prophylaxis, whereas CD34+-selected patients received no planned immunosuppression. EASIX (lactate dehydrogenase × creatinine/platelet count) was calculated continuously until 1-year after HCT. Log transformation using base 2 (log2) was applied to all EASIX variables to reduce skew. In total, 360 patients (71%) received CD34+-selected and 149 (29%) unmodified allo-HCT. Among all patients, EASIX scores increased rapidly, peaked at day +8, then declined rapidly until day +33. Thereafter, scores declined gradually but remained above the pre-HCT baseline. In unmodified HCT, scores appeared higher over time than in CD34+-selected patients. EASIX discrimination of NRM was highest around day +180 (concordance index = 0.85) in both platforms, but the prognostic impact of EASIX across time points differed between the 2 platforms. Mean EASIX scores were higher in men (mean log2 +0.52) and in patients who developed grade 2 to 4 GVHD (+0.81) and lower in patients who received matched vs mismatched donors (−0.81, all P < .01). EASIX scores are dynamic and variably concordant with NRM when analyzed longitudinally, and patterns differ between HCT platforms. Compared to pre-HCT evaluation, post-HCT EASIX scores may better predict risk of NRM as patients acquire additional endothelial injury and toxicities.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for many malignant and nonmalignant hematologic diseases but is associated with several unique complications related to endothelial dysfunction, including sinusoidal obstruction syndrome, transplant-associated microangiopathy, graft-versus-host disease (GVHD), and sepsis among others.1,2 The Endothelial Activation and Stress Index (EASIX) score was developed as a prognostic marker and simple surrogate of endothelial dysfunction.3 The EASIX score is based on routine laboratory parameters and correlates closely with serum markers of endothelial dysfunction.4 In a retrospective study of patients who had undergone unmodified allo-HCT with reduced intensity conditioning, EASIX enabled easy and early identification of patients likely to suffer steroid-refractory GVHD and related mortality.3
Our group and others have found that EASIX is a strong predictor of nonrelapse mortality (NRM) when evaluated before allo-HCT and at specific landmarks after HCT.4-7 In this analysis, we evaluated EASIX as a continuous variable over time in 2 distinct transplantation platforms: unmodified allografts in patients receiving nonmyeloablative or reduced intensity conditioning (NMA/RIC) and ex vivo, CD34+-selected allografts in patients receiving myeloablative conditioning (MAC). These platforms differ in the use of post-HCT immunosuppression, intensity of the conditioning regimen, and the inherent risk of GVHD, factors thought to influence the development of endothelial injury after HCT.8-13
We hypothesized that defining the natural history of EASIX scores after HCT may help identify an optimal time point at which EASIX has the highest discrimination for NRM. We also sought to determine whether changes in EASIX over time could serve as a prognostic biomarker for outcomes after allo-HCT. Finally, we sought to compare patterns of endothelial damage over time between 2 distinct HCT platforms using EASIX.
Methods
Study population and transplantation procedures
This is a single-center retrospective analysis of adult patients aged ≥18 years who underwent their first allo-HCT. Patients were divided into 2 distinct cohorts for analysis. Cohort 1 included patients who underwent unmodified allo-HCT with NMA/RIC. These regimens included melphalan/fludarabine, busulfan/fludarabine, and low-dose total body irradiation (TBI)-based regimens with fludarabine/cyclophosphamide with or without thiotepa. Cohort 2 included patients who underwent ex vivo CD34+-selected, calcineurin inhibitor (CNI)-free allo-HCT with myeloablative conditioning (MAC). These regimens included busulfan/fludarabine/melphalan and clofarabine/thiotepa/melphalan, and high-dose TBI-based conditioning (1375 cGy) with thiotepa and with cyclophosphamide or fludarabine. All patients underwent allo-HCT between July 2006 and May 2017.
In cohort 1, GVHD prophylaxis consisted of low-dose methotrexate (5 mg/m2 on days +1, +3, +6) and tacrolimus with or without sirolimus. Recipients of allografts from HLA-matched unrelated donors or from mismatched related or unrelated donors received equine antithymocyte globulin at 30 mg/kg for 2 doses.14 Patients in cohort 2 received no planned pharmacologic GVHD prophylaxis. CD34+-stem cell selection was achieved using a CliniMACS CD34 Reagent System (Miltenyi Biotec, Gladbach, Germany), as previously described.15,16 All patients in cohort 2 received rabbit antithymocyte globulin at a dose of 2.5 mg/kg per day on days −3, −2, ±1 as part of the conditioning regimen to prevent graft rejection.
Statistical analysis
Overall survival (OS) was defined as the time from allo-HCT to death from any cause. Kaplan-Meier methods were used to estimate OS are defined time points. NRM was defined as the time from allo-HCT to death in the absence of relapse. Cumulative incidence function was used to estimate the incidence of NRM and the analysis treated relapse as a competing event. We calculated the EASIX score (lactate dehydrogenase [LDH] [U/L] × creatinine [mg/dL]/platelet count [109 cells per L]) at continuous time points from baseline (day −30 to day −10) until 1 year after HCT. A log transformation using base 2 (log2) was applied to all EASIX variables to reduce skewness. A 1-unit increase in log2 EASIX is associated with a doubling (onefold increase) of EASIX on the original scale. In addition to the evaluation of log2 EASIX, a dichotomous “high” EASIX variable was also defined using log2 EASIX >2 (supplemental Figure 1). A cubic smoothing spline was used to estimate the average EASIX, creatinine, LDH, and platelet count over time. To estimate the discrimination of EASIX for subsequent NRM events, defined as events occurring within the subsequent 180 days, consecutive landmark analyses were conducted. Patients who relapsed before the start of the landmark analysis were excluded from the NRM analysis but were included in the mortality analysis. A cause-specific concordance index using inverse probability of censoring weights was estimated for each landmark time. The concordance index ranges from 0.5 to 1.0, where 1.0 indicates a biomarker able to perfectly discriminate survival times based on the biomarker value and 0.5 indicates no discriminatory ability. The index is analogous to the area under the receiver operating characteristic curve (AUROC); however, instead of a binary disease state, the ordering of survival times is examined and an inverse probability of censoring weights weighting is added to test statistic to account for censored observations. All analyses were conducted in R v4.0.5 (Vienna, Austria).
Results
Patient and HCT characteristics are detailed in Table 1. Cohorts 1 (unmodified) and 2 (CD34+-selected) included 149 and 360 patients, respectively. In cohort 1, 123 patients (82%) underwent allo-HCT for lymphoma, and in cohort 2, 318 patients (89%) underwent allo-HCT for acute leukemia or myelodysplastic syndrome. Most patients in cohort 1 received low-dose TBI-based conditioning (117 patients, 79%), whereas most patients in cohort 2 received chemotherapy-based conditioning (244 patients, 68%). The 2 cohorts were similar in age and balanced with regard to comorbidities. All patients except for 2 in cohort 1 received peripheral blood mobilized allografts.
Combined patients from EASIX unmodified and CD34+-selected datasets
| Patient characteristics . | Total . | Cohort 1 (Unmodified) . | Cohort 2 (CD34) . |
|---|---|---|---|
| Sample size, n (%) | 509 (100) | 149 (29) | 360 (71) |
| Female, n (%) | 209 (41) | 45 (30) | 164 (46) |
| Median age at transplant, y (range) | 55.6 (19.6-78.7) | 55.0 (23.6-78.7) | 55.8 (19.6-73.3) |
| Disease group, n (%) | |||
| Acute leukemia | 222 (44) | 4 (3) | 218 (61) |
| Chronic leukemia | 41 (8) | 22 (15) | 19 (5) |
| MDS | 100 (20) | 0 | 100 (28) |
| HL | 20 (4) | 20 (13) | 0 |
| NHL | 108 (21) | 103 (69) | 5 (1) |
| MPD | 18 (4) | 0 | 18 (5) |
| HCT-CI score, n (%) | |||
| 0 | 121 (24) | 48 (32) | 73 (20) |
| 1-2 | 165 (32) | 40 (27) | 125 (35) |
| ≥3 | 223 (44) | 61 (41) | 162 (45) |
| Donor type, n (%) | |||
| Matched related | 189 (37) | 61 (41) | 128 (36) |
| Mismatched related | 1 (0.2) | 0 | 1 (0.3) |
| Matched unrelated | 246 (49) | 74 (50) | 172 (48) |
| Mismatched unrelated | 72 (14) | 14 (9) | 58 (16) |
| Conditioning intensity, n (%) | |||
| Ablative | 357 (70) | 0 | 357 (99) |
| Reduced | 41 (8) | 38 (26) | 3 (1) |
| Nonmyeloablative | 111 (22) | 111 (74) | 0 |
| Conditioning regimen, n (%) | |||
| Myeloablative, chemotherapy-based | 244 (68) | ||
| Myeloablative, high-dose TBI-based | 116 (32) | ||
| Melphalan/fludarabine | 27 (18) | ||
| Low-dose TBI | 117 (79) | ||
| Other | 5 (3) | ||
| Graft source, n (%) | |||
| Bone marrow | 2 (0.4) | 2 (1) | 0 |
| Peripheral blood stem cell | 507 (99.6) | 147 (99) | 360 (100) |
| Patient characteristics . | Total . | Cohort 1 (Unmodified) . | Cohort 2 (CD34) . |
|---|---|---|---|
| Sample size, n (%) | 509 (100) | 149 (29) | 360 (71) |
| Female, n (%) | 209 (41) | 45 (30) | 164 (46) |
| Median age at transplant, y (range) | 55.6 (19.6-78.7) | 55.0 (23.6-78.7) | 55.8 (19.6-73.3) |
| Disease group, n (%) | |||
| Acute leukemia | 222 (44) | 4 (3) | 218 (61) |
| Chronic leukemia | 41 (8) | 22 (15) | 19 (5) |
| MDS | 100 (20) | 0 | 100 (28) |
| HL | 20 (4) | 20 (13) | 0 |
| NHL | 108 (21) | 103 (69) | 5 (1) |
| MPD | 18 (4) | 0 | 18 (5) |
| HCT-CI score, n (%) | |||
| 0 | 121 (24) | 48 (32) | 73 (20) |
| 1-2 | 165 (32) | 40 (27) | 125 (35) |
| ≥3 | 223 (44) | 61 (41) | 162 (45) |
| Donor type, n (%) | |||
| Matched related | 189 (37) | 61 (41) | 128 (36) |
| Mismatched related | 1 (0.2) | 0 | 1 (0.3) |
| Matched unrelated | 246 (49) | 74 (50) | 172 (48) |
| Mismatched unrelated | 72 (14) | 14 (9) | 58 (16) |
| Conditioning intensity, n (%) | |||
| Ablative | 357 (70) | 0 | 357 (99) |
| Reduced | 41 (8) | 38 (26) | 3 (1) |
| Nonmyeloablative | 111 (22) | 111 (74) | 0 |
| Conditioning regimen, n (%) | |||
| Myeloablative, chemotherapy-based | 244 (68) | ||
| Myeloablative, high-dose TBI-based | 116 (32) | ||
| Melphalan/fludarabine | 27 (18) | ||
| Low-dose TBI | 117 (79) | ||
| Other | 5 (3) | ||
| Graft source, n (%) | |||
| Bone marrow | 2 (0.4) | 2 (1) | 0 |
| Peripheral blood stem cell | 507 (99.6) | 147 (99) | 360 (100) |
Percentages may add up to greater than 100 due to rounding.
HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; NHL, non-Hodgkin lymphoma; TBI, total body irradiation.
Outcomes
The median follow-up time among survivors was 144 months (range 20-300 months). Among all patients, 1- and 3-year overall (OS) rates were 80% (95% CI, 76%-83%) and 63% (95% CI, 59%-67%), respectively, and 1- and 3-year NRM rates were 13% (95% CI, 10%-16%) and 22% (95% CI, 18%-26%), respectively. In cohort 1, 1- and 3-year OS rates were 84% (95% CI, 78%-90%) and 68% (95% CI, 60%-75%), respectively, and 1- and 3-year NRM rates were 8% (95% CI, 4%-12%) and 16% (95% CI, 10%-23%), respectively. In cohort 2, 1- and 3-year OS rates were 78% (95% CI, 74%-83%) and 61% (95% CI, 56%-67%), respectively, and 1- and 3-year NRM rates were 15% (95% CI, 12%-19%) and 25% (95% CI, 20%-29%), respectively (Table 2).
Post-HCT outcomes combined and by cohort (unmanipulated vs CD34+-selected)
| . | 1-year OS (IQR), % . | 3-year OS (IQR), % . | 1-year NRM (IQR), % . | 3-year NRM (IQR), % . | CI G2-4 aGVHD, day 100 (IQR), % . | CI G2-4 aGVHD, day 365 (IQR), % . |
|---|---|---|---|---|---|---|
| Total | 80 (76-83) | 63 (59-67) | 13 (10-16) | 22 (18-26) | 19 (16-23) | 33 (29-37) |
| Cohort 1 (unmodified) | 84 (78-90) | 68 (60-75) | 8 (4-12) | 16 (10-23) | 23 (16-30) | 41 (33-49) |
| Cohort 2 (CD34) | 78 (74-83) | 61 (56-67) | 15 (12-19) | 25 (20-29) | 18 (14-22) | 30 (25-34) |
| . | 1-year OS (IQR), % . | 3-year OS (IQR), % . | 1-year NRM (IQR), % . | 3-year NRM (IQR), % . | CI G2-4 aGVHD, day 100 (IQR), % . | CI G2-4 aGVHD, day 365 (IQR), % . |
|---|---|---|---|---|---|---|
| Total | 80 (76-83) | 63 (59-67) | 13 (10-16) | 22 (18-26) | 19 (16-23) | 33 (29-37) |
| Cohort 1 (unmodified) | 84 (78-90) | 68 (60-75) | 8 (4-12) | 16 (10-23) | 23 (16-30) | 41 (33-49) |
| Cohort 2 (CD34) | 78 (74-83) | 61 (56-67) | 15 (12-19) | 25 (20-29) | 18 (14-22) | 30 (25-34) |
aGVHD, acute graft-versus-host disease; NRM, nonrelapse mortality; OS, overall survival; CI, cumulative incidence.
In total, 211 patients died during follow-up: 79 of relapse and 132 of NRM. NRM causes of death were GVHD in 55 patients (42%), infection in 42 patients (32%), toxicity/organ failure in 21 patients (16%), and other causes in 14 patients (11%).
EASIX scores over time
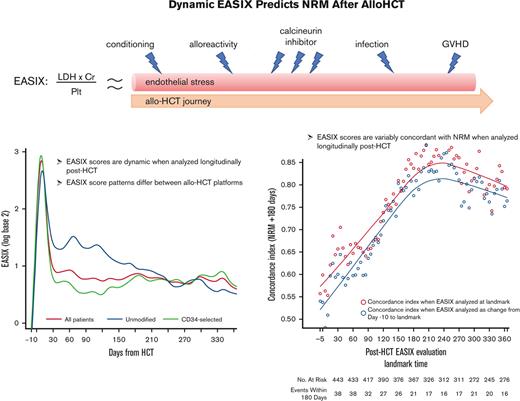
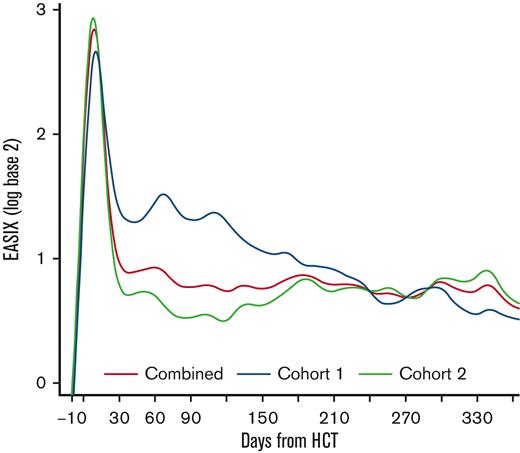
Among all patients, EASIX scores increased rapidly in the early post-HCT period and peaked at day +8 (median log2 3.07, interquartile range [IQR] 2.05-3.91) followed by a sharp decline until day +33 (median log2 0.59, IQR 0.01-1.55) (Figure 1). Thereafter, EASIX scores declined gradually but remained above the pre-HCT baseline for the duration of the first year after HCT. We then analyzed cohorts 1 and 2 separately, revealing different patterns in EASIX scores over time in the 2 populations. Patients in cohort 1 had higher EASIX scores over time than patients in cohort 2. In cohort 2, scores rapidly decreased until plateauing around 2 months after HCT (Figure 1).
EASIX scores over time post-HCT. Estimated average EASIX scores post-transplantation on a log2 scale for all patients, as well as for the individual cohorts (cohort 1 = unmodified; cohort 2 = ex-vivo CD34+-selected).
EASIX scores over time post-HCT. Estimated average EASIX scores post-transplantation on a log2 scale for all patients, as well as for the individual cohorts (cohort 1 = unmodified; cohort 2 = ex-vivo CD34+-selected).
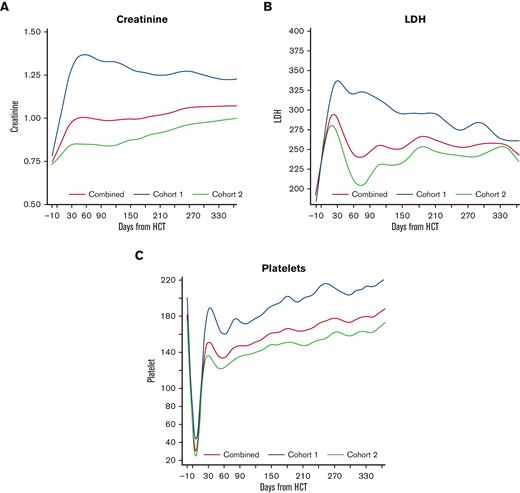
To estimate the trend of the individual EASIX components, we plotted LDH, creatinine, and platelet count over time in each cohort (Figure 2). Patients in cohort 1 experienced a higher peak creatinine and higher platelet nadir compared to cohort 2, and these values remained higher in cohort 1 over time. LDH peaked early in both populations, reaching a higher peak in cohort 1, and remained above baseline for the remainder of the year.
Creatinine, platelet count, and LDH over time post-HCT. Estimated average individual components of the EASIX score post-transplantation for all patients, as well as for the individual cohorts (cohort 1 = unmodified; cohort 2 = ex-vivo CD34+-selected).
Creatinine, platelet count, and LDH over time post-HCT. Estimated average individual components of the EASIX score post-transplantation for all patients, as well as for the individual cohorts (cohort 1 = unmodified; cohort 2 = ex-vivo CD34+-selected).
Varying association between EASIX and post-HCT outcomes over time
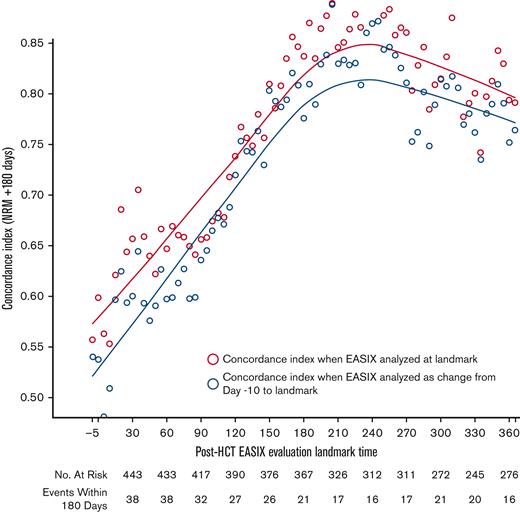
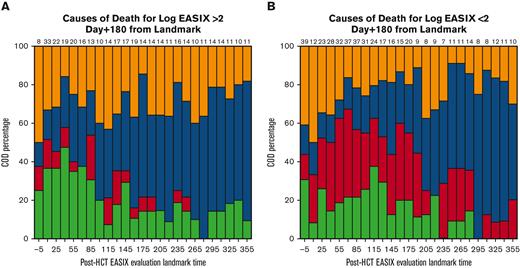
EASIX discrimination of NRM events within the subsequent 180 days increased from the time of allo-HCT until day +180 to +210, when concordance was highest (concordance index = 0.84) (Figure 3). At this time point, EASIX concordance with NRM was similar in cohort 1 (concordance index = 0.81) and cohort 2 (concordance index = 0.85). Patients with high EASIX at this landmark died of GVHD (6 patients), infection (3 patients), relapse (3 patients), and other causes (2 patients) in the subsequent 180 days. Relapse accounted for fewer causes of death among patients with high EASIX (Figure 4A), particularly at later time points, compared with patients with low EASIX (Figure 4B).
Concordance of EASIX with NRM over time. EASIX discrimination of non-relapse mortality (NRM) events within the subsequent 180 days. Gray circles demonstrate the concordance indices when EASIX is analyzed as a categorical variable at landmark timepoints; yellow circles demonstrate the concordance indices when EASIX is analyzed as change from pre-HCT baseline to landmark.
Concordance of EASIX with NRM over time. EASIX discrimination of non-relapse mortality (NRM) events within the subsequent 180 days. Gray circles demonstrate the concordance indices when EASIX is analyzed as a categorical variable at landmark timepoints; yellow circles demonstrate the concordance indices when EASIX is analyzed as change from pre-HCT baseline to landmark.
Causes of death within subsequent 180 days. Causes of death stratified by post-HCT timepoint in patients with low EASIX (Fig 4a) and high EASIX (Fig 4b) at the day 180 landmark, when EASIX concordance with NRM was highest.
Causes of death within subsequent 180 days. Causes of death stratified by post-HCT timepoint in patients with low EASIX (Fig 4a) and high EASIX (Fig 4b) at the day 180 landmark, when EASIX concordance with NRM was highest.
Overall, the ability of the EASIX score to discriminate NRM in the subsequent 180 days was similar when EASIX was analyzed as a categorical variable at landmark time points and as change from pre-HCT baseline EASIX scores (Figure 3).
We then analyzed the concordance of each EASIX component with NRM at day 180 to weigh each parameter’s relative prognostic value. Platelets were the most concordant with NRM in both cohorts, with a concordance index of 0.85 in the combined population at this time point. Creatinine and LDH both had a concordance index of 0.63 in the combined population at this time point.
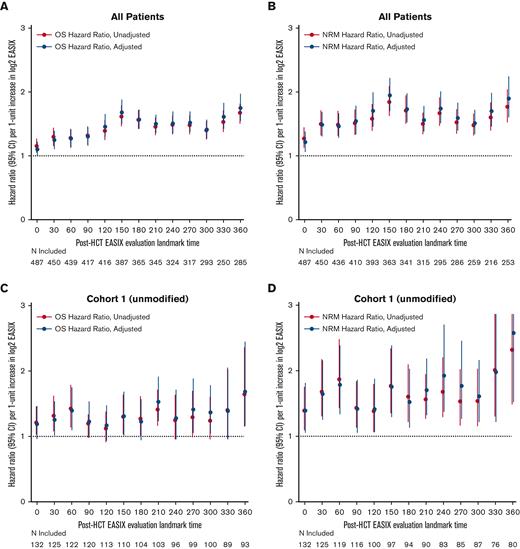
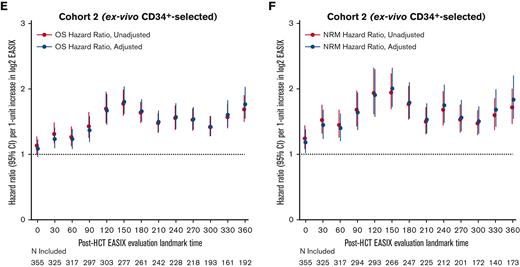
Next, we estimated the association between EASIX and post-HCT outcomes at given time points and evaluated how this association varied over time. The highest association between EASIX score and relative risk of death and NRM after HCT occurred at days 150 and 360 (Figure 5A and B), respectively. This held true when the analysis was adjusted for age, CD34+-selection, and HCT-specific comorbidity index. At day 150, the hazard ratio (HR) per 1-unit increase in log2 EASIX was 1.94 (95% CI, 1.70-2.22) for NRM and 1.67 (95% CI, 1.51-1.97) for death. At day 360, the HR per 1-unit increase in log2 EASIX was 1.89 (95% CI, 1.60-2.24) for NRM and 1.75 (95% CI, 1.55-1.97) for death. We then repeated this analysis in each cohort separately and found that the prognostic impact of EASIX at given time points appeared to differ based on cohort (Figure 5C-F); however, these analyses were limited by smaller numbers particularly at late post-HCT time points.
Varying association between EASIX and post-HCT outcomes over time. The hazard ratios for non-relapse mortality (NRM) and overall mortality (OM) events in the subsequent 180 days per 1-unit increase in log2 EASIX, and their variation over time post-transplantation. Adjustment factors include age, CD34-selection, and HCT-CI. Figures 5A-B include both cohorts; figures 5C-D include cohort 1 (unmodified) only; and figures 5E-F include cohort 2 (ex-vivo CD34+-selected) only.
Varying association between EASIX and post-HCT outcomes over time. The hazard ratios for non-relapse mortality (NRM) and overall mortality (OM) events in the subsequent 180 days per 1-unit increase in log2 EASIX, and their variation over time post-transplantation. Adjustment factors include age, CD34-selection, and HCT-CI. Figures 5A-B include both cohorts; figures 5C-D include cohort 1 (unmodified) only; and figures 5E-F include cohort 2 (ex-vivo CD34+-selected) only.
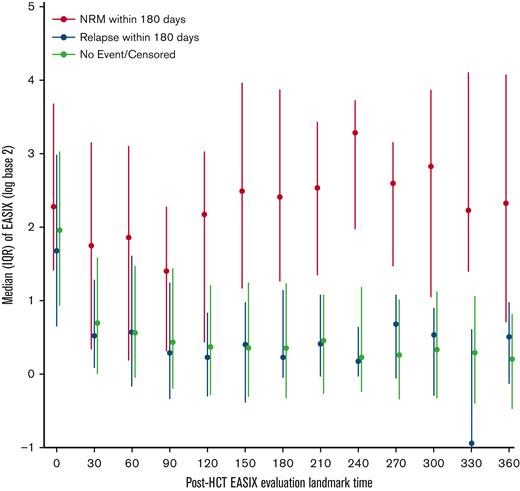
In the first 30 days after allo-HCT, EASIX values increased to a similar degree in patients regardless of whether they experienced NRM or relapse in the subsequent 180 days (Figure 6). After day +30, patients who did not experience NRM, including patients who relapsed, had consistently lower EASIX scores compared with those who experienced NRM in the subsequent 180 days.
Distribution of EASIX post-HCT and its association with NRM. Median EASIX scores post-transplantation stratified based on whether non-relapse mortality, relapse, or neither occurred within subsequent 180 days.
Distribution of EASIX post-HCT and its association with NRM. Median EASIX scores post-transplantation stratified based on whether non-relapse mortality, relapse, or neither occurred within subsequent 180 days.
Impact of baseline variables on EASIX
Mean EASIX scores at the day +180 landmark were higher in men (log2 difference 0.51, P < .005), in patients with an HCT-specific comorbidity index score of 2 or higher (log2 difference 0.53, P < .05), and in patients who had developed grade 2 to 4 acute GVHD by day +180 (log2 difference 0.78, P < .005) and were lower in patients who received matched unrelated vs mismatched donors (log2 difference −0.82, P <.005). Mean EASIX scores at day +180 did not differ based on age, performance score, disease, recipient cytomegalovirus serostatus, conditioning intensity, or graft manipulation (Table 3).
Univariate linear regression for day 180 EASIX day (log base 2)
| . | Combined cohort . | |
|---|---|---|
| log2 Difference (95% CI) . | P . | |
| CD34+-selected graft | −0.15 (−0.52 to 0.23) | .445 |
| Age (per 10 years) | 0.01 (−0.13 to 0.15) | .899 |
| Male | 0.51 (0.17-0.86) | .004 |
| Donor Type | ||
| Mismatched | (reference) | |
| MRD | −0.44 (−0.97 to 0.08) | .098 |
| MUD | −0.82 (−1.33 to −0.31) | .002 |
| Conditioning intensity | ||
| MAC | (reference) | |
| NMA | 0.32 (−0.09 to 0.73) | .128 |
| RIC | −0.44 (−1.08 to 0.19) | .172 |
| Grade 2-4 aGVHD day 180 | 0.78 (0.41-1.16) | < .001 |
| Grade 3-4 aGVHD day 180 | 1.05 (0.49-1.62) | < .001 |
| Pre-EASIX (per unit) | 0.01 (−0.04 to 0.07) | .71 |
| HCT-CI | ||
| 0-1 | (reference) | |
| 2-3 | 0.53 (0.11-0.95) | .014 |
| 4+ | 0.52 (0.09-0.95) | .018 |
| KPS >80 | −0.05 (−0.41 to 0.31) | .798 |
| Patient CMV+ | −0.12 (−0.47 to 0.22) | .478 |
| Disease | ||
| Acute leukemia | (reference) | |
| Lymphoma | 0.27 (−0.14 to 0.68) | .2 |
| MDS | 0.17 (−0.3 to 0.64) | .473 |
| . | Combined cohort . | |
|---|---|---|
| log2 Difference (95% CI) . | P . | |
| CD34+-selected graft | −0.15 (−0.52 to 0.23) | .445 |
| Age (per 10 years) | 0.01 (−0.13 to 0.15) | .899 |
| Male | 0.51 (0.17-0.86) | .004 |
| Donor Type | ||
| Mismatched | (reference) | |
| MRD | −0.44 (−0.97 to 0.08) | .098 |
| MUD | −0.82 (−1.33 to −0.31) | .002 |
| Conditioning intensity | ||
| MAC | (reference) | |
| NMA | 0.32 (−0.09 to 0.73) | .128 |
| RIC | −0.44 (−1.08 to 0.19) | .172 |
| Grade 2-4 aGVHD day 180 | 0.78 (0.41-1.16) | < .001 |
| Grade 3-4 aGVHD day 180 | 1.05 (0.49-1.62) | < .001 |
| Pre-EASIX (per unit) | 0.01 (−0.04 to 0.07) | .71 |
| HCT-CI | ||
| 0-1 | (reference) | |
| 2-3 | 0.53 (0.11-0.95) | .014 |
| 4+ | 0.52 (0.09-0.95) | .018 |
| KPS >80 | −0.05 (−0.41 to 0.31) | .798 |
| Patient CMV+ | −0.12 (−0.47 to 0.22) | .478 |
| Disease | ||
| Acute leukemia | (reference) | |
| Lymphoma | 0.27 (−0.14 to 0.68) | .2 |
| MDS | 0.17 (−0.3 to 0.64) | .473 |
aGVHD, acute graft-versus-host disease; CMV, cytomegalovirus; HCT-CI: hematopoietic cell transplantation-specific comorbidity index; KPS, Karnofsky performance status; MAC, myeloablative conditioning; MRD, matched related donor; MUD, matched unrelated donor; NMA, nonmyeloablative conditioning; RIC, reduced intensity conditioning.
Discussion
In this analysis, we evaluated EASIX scores over time in 2 distinct HCT platforms: unmodified allografts in patients receiving NMA/RIC, and ex vivo CD34+-selected allografts in patients receiving MAC. To our knowledge, our data are the first to characterize dynamic EASIX scores after allo-HCT. We found different patterns of EASIX scores between the 2 HCT platforms, suggesting varying patterns of endothelial injury over time. In addition, we found that EASIX discrimination of NRM increased from the time of allo-HCT until day +180 to +210, when concordance was highest, and mean EASIX scores did not differ between the 2 cohorts at this time point. We speculate that high EASIX at this later time point more accurately reflects endothelial injury that has compounded over time since HCT, as common confounders of the EASIX score are less frequently encountered. For example, 6 months after transplantation, patients are generally no longer transfusion dependent and are exposed to fewer nephrotoxic and myelosuppressive drugs compared with earlier in the transplantation course.
We found that EASIX scores peaked early in both cohorts, but scores decreased more quickly in patients receiving CD34+-selected grafts. This could be because the greatest threat to endothelium in this platform is the myeloablative conditioning regimen itself, whereas in cohort 2, CNI use and higher rates of GVHD may perpetuate endothelial injury for several months after HCT. Endothelial cell injury is thought to be triggered by many factors, including the conditioning regimen, use of CNI, infections, and GVHD.17,18 Our 2 cohorts differ in several ways but likely of most significance are the differences in conditioning intensity, graft composition, and the use of CNI-based GVHD prophylaxis in cohort 1. Also notable are the differences in patient and disease characteristics. Although our study cannot definitively determine which post-HCT clinical variables influenced the different EASIX patterns that we noted between the 2 cohorts, EASIX was concordant with NRM in both cohorts. This suggests that both HCT platforms share endothelial dysfunction as a major pathophysiology driving NRM. When our 2 cohorts were analyzed separately, the association between EASIX and post-HCT outcomes across time points appeared to differ between the 2 cohorts; however, these analyses were limited by smaller numbers. This highlights that the prognostic impact of EASIX may be context dependent.
Of the 3 EASIX laboratory components, we found platelets to be the most concordant with NRM in both cohorts when analyzed at the day +180 landmark. This is in contrast with the study by Luft et al, which found creatinine to be the most prognostic for NRM in a multivariate Cox regression model;4 though of note, this was analyzed at the pre-HCT time point. The mechanisms for the prognostic impact of platelet count are likely complex. Thrombocytopenia in patients with GVHD is among the most consistent and strongest predictors of poor survival across many studies19-22 and may be both cause and consequence of GVHD. Although GVHD has been linked to accelerated platelet destruction23-25 and decreased production, platelets have been shown to be important for the induction of immune tolerance and regulatory T-cell function either directly through cell-cell interactions or via release of transforming growth factor-B1.23,26 Furthermore, persistent thrombocytopenia after allo-HCT has been found to be a strong negative predictor of survival independent of GVHD,27 which is not surprising given that many common contributors to NRM, such as sepsis, thrombotic microangiopathy, veno-occlusive disease/sinusoidal obstruction syndrome, and so on, involve endothelial damage and platelet consumption or destruction. Finally, thrombocytopenia is likely also associated with NRM through endothelium-independent mechanisms; given the high incidence of infectious-related deaths in our cohorts, viral infection and their therapies may have been confounders contributing to the association of high EASIX with NRM.
Our study has some limitations. An inherent limitation of the EASIX score itself is related to potential confounders such as the use of platelet transfusions, underlying changes in hepatic and renal function, and other variables unrelated to endothelial stress that are known to impact these individual laboratory components.28-30 Also, given that our 2 cohorts are distinguished from one another based on conditioning intensity, use of CNI-based GVHD prophylaxis, and graft manipulation, it is difficult to statistically separate these factors, thereby limiting the ability to draw conclusions regarding the impact of each on EASIX scores. Finally, this study evaluated the discriminatory ability of EASIX at different times after transplantation. Before the biomarker can be used to intervene clinically for a specific patient, further study is required to identify the specific threshold to identify high-risk patients, a threshold which is likely to depend on the specific post-transplantation time point, the positive predictive value of this threshold, and, critically, further consideration of the potential harms vs benefit of the intervention. An important next step would be to first validate these findings in an independent cohort of patients.
In summary, EASIX scores are highly dynamic and have variable concordance with NRM when analyzed longitudinally after HCT. Although pre-HCT EASIX can be used to help guide allo-HCT treatment decisions before allo-HCT, evaluation of the dynamic changes in EASIX scores may be better associated with NRM over time as patients acquire additional endothelial injury and toxicities after HCT. Assessment of dynamic EASIX scores may be useful in anticipating complications related to endothelial injury along the post-transplantation course. This biomarker could guide risk-adapted, investigative interventions that aim to reduce or prevent the risk of these toxicities and NRM after transplantation.
Acknowledgments
This research was supported in part by National Institutes of Health (NIH) award numbers P01 CA23766 and NIH/NCI Cancer Center support grant P30 CA008748. M.S.-E. was supported by the Research Institute of Marques de Valdecilla (IDIVAL) Lopez-Albo-Wenceslao grant (WLA17/03).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: M.T.N., S.A.G., M.-A.P., C.S.S., M.S.-E., and M.S. contributed to the study proposal and design; M.T.N., M.S.-E., and M.A.M. contributed to data acquisition; S.M.D. contributed to data analysis and interpretation; M.T.N. and M.S. drafted the manuscript; and all authors critically revised and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariam T. Nawas, Hematopoietic Cellular Therapy Program, Department of Medicine, University of Chicago Medicine, 5841 S Maryland Ave, Chicago, IL 60637; e-mail: nawasm@bsd.uchicago.edu; and Michael Scordo, Adult Bone Marrow Transplant Service, Department of Medicine, Memorial Sloan Kettering Cancer, 1275 York Ave, New York, NY 10065; e-mail: scordom@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Mariam T. Nawas (nawasm@bsd.uchicago.edu).
The full-text version of this article contains a data supplement.