Abstract
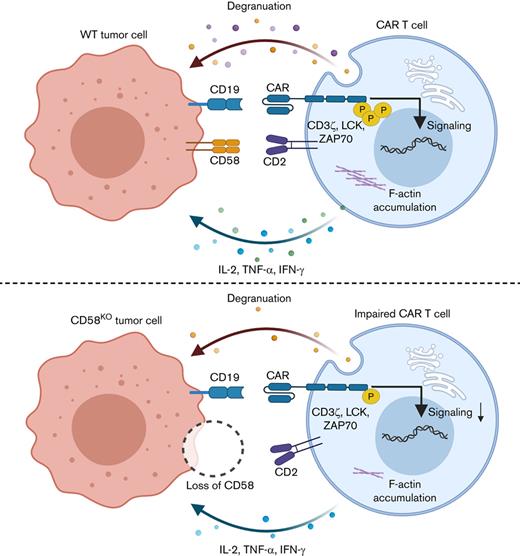
Chimeric antigen receptor (CAR) T-cell therapy has achieved significant success in treating a variety of hematologic malignancies, but resistance to this treatment in some patients limited its wider application. Using an unbiased genome-wide clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) screening, we identified and validated loss of CD58 conferred immune evasion from CAR T cells in vitro and in vivo. CD58 is a ligand of the T-cell costimulatory molecule CD2, and CD58 mutation or downregulated expression is common in hematological tumors. We found that disruption of CD58 in tumor cells induced the formation of suboptimal immunological synapse (IS) with CAR T cells, which conferred functional impairment of CAR T cells, including the attenuation of cell expansion, degranulation, cytokine secretion, and cytotoxicity. In summary, we describe a potential mechanism of tumor-intrinsic resistance to CAR T-cell therapy and suggest that this mechanism may be leveraged for developing therapeutic strategies to overcome resistance to CAR T-cell therapy in B-cell malignancies.
Key Points
We emphasized the potential critical role of CD58 loss in resistance to CAR T-cell therapy.
Loss of CD58 caused inefficient IS formation with CAR T cells, impairing the activation and cytotoxic function of CAR T cells.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has demonstrated unprecedented success in the treatment of B-cell malignancies, especially CD19-targeted CAR T-cell therapy for acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).1-5 Notwithstanding some progress achieved, primary or acquired resistance to the treatment still occurs.1,6 A deeper exploration for resistance mechanisms to CAR T-cell therapy may provide diverse rationales for patient selection or potential strategies.
Target antigen evasion has been confirmed as a mechanism for acquired resistance to CAR T-cell therapy.7-10 Increasing evidence has suggested that the mechanisms of primary resistance to CAR T-cell therapy involve CAR T-cell defects, including impaired proliferative capacity, an exhaustion phenotype, and attenuated T-cell–mediated cytotoxicity.2,11,12 Nevertheless, intrinsic mechanisms of primary resistance of tumor cells to the treatment remain largely elusive.
High-throughput clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-based screening is a powerful tool that provides unbiased critical genetic data to exploit for the reasons for resistance to CAR T-cell therapy or to find new biomarkers for stratifying patients. To identify tumor cell–intrinsic factors that determine resistance to CAR T-cell cytotoxicity, we performed unbiased genome-wide CRISPR/Cas9 screening with a coculture model consisting of Nalm6 cells and CD19 CAR T cells. We revealed that CD58, a ligand for the CD2 receptor expressed on T cells,13 plays a key role in resistance to CAR T-cell therapy in preclinical tests. Disruption of CD58 in tumor cells impaired immunological synapse (IS) formation with CAR T cells, which led to the dysfunction of CAR T cells, including attenuated CAR signal transduction, CAR T-cell expansion, and cytotoxicity. Taken together, these findings suggest a potential mechanism for resistance to CAR T-cell therapy.
Materials and methods
Additional materials and methods are provided in supplemental Materials.
CRISPR/Cas9 screening
The process of CRISPR/Cas9 screening has been described in our previous report.14 Briefly, Nalm6 cells were transfected with lentivirus carrying the Brunello library,15 and cells exhibiting stable lentivirus integrations were selected with puromycin. Transduced Nalm6 cells were cultured with CD19 CAR T cells or control T cells at a 1:50 effector:target (E:T) ratio. Control T or CD19 CAR T cells were added to the culture at a 1:50 E:T ratio every 3 days. The cells were collected using a death cell removal magnetic bead kit (Miltenyi) for genomic DNA analysis on day 15. After removing low-quality reads from the original sequencing data, the reads were mapped to single-guide RNA (sgRNA) sequence, and each sgRNA read was counted to generate a sgRNA count table. sgRNA data in the sgRNA table was normalized and used for significance analysis. sgRNA read counts were analyzed with the MAGeCK v0.5.7 algorithm. Genes with significantly enriched sgRNAs were identified based on a log2 fold change and P value criteria. Hypergeometric distribution statistics were used to identify gene sets that overlapped with candidate genes (log2 fold change > 2 and P < .05).
Blocking experiment
Cells were pretreated with blocking monoclonal antibody (mAb) against CD58 (clone TS2/9, 8 μg/mL, BioLegend) or CD2 (clone RPA-2.10, 10 μg/mL, eBioscience) or with isotype-matched control mAbs for 30 minutes. The blocking effect was detected by staining for anti–CD58-PE (BioLegend) or anti–CD2-APC-Cy7 (BioLegend). Tumor cells blocked by anti-CD58 mAbs or CAR T cells blocked by anti-CD2 mAbs were subjected to subsequent experiments, including cytotoxicity assays and degranulation assays, according to the methods described above.
Imaging flow cytometry
The sorted CD19 CAR T cells were stained with 0.2 μM CellTrace Violet dye (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions. A total of 1 × 106 wild-type (WT) (mCherry+) Nalm6, 1 × 106 CD58 knockout(KO) (GFP+) Nalm6 and 1 × 106 CD19 CAR T cells (Violet+) were cocultured at 37°C for the indicated times, fixed, permeabilized, and then stained with phalloidin-AF647 (Thermo Fisher Scientific) for detecting F-actin. A total of 1 × 106 events were recorded, and samples were analyzed with an ImageStreamX MKII flow cytometer (Luminex). Image acquisition and data analysis16,17 was performed using IDEAS software version 6.2. The calculation formula for measuring IS is as follows: F-actin enrichment at IS (%) = 100× (intensity of phalloidin at IS)/(intensity of phalloidin-stained CAR T cells)
Xenograft mouse models
A total of 1 × 105 luciferase+ Nalm6 cells (WT or CD58KO) were IV transplanted into 4- to 6-week-old female NOD-Prkdc-scid-Il2rg-null mice (NPG/Vst, VITALSTAR). Purified CD19 CAR T cells were selected using magnetic beads (Miltenyi Biotec) 3 days post–lentiviral infection. Seven days after Nalm6 cell injection, the mice were IV injected with 1 × 106 CD19 CAR T or control T cells in 100 μl of phosphate-buffered saline (PBS) (n = 6 mice per group). Leukemia burden was monitored once per week by bioluminescence in vivo imaging (BLI) with an in vivo imaging system (IVIS) (PerkinElmer). The average flux (photons per second/area [mm2]) was used to evaluate the BLI signal. Mouse peripheral blood samples were collected through the orbital sinus and lysed using ammonium chloride-potassium (ACK) lysing buffer (Thermo Fisher Scientific). The remaining cells were stained with the indicated fluorochrome-conjugated antibodies. All studies were approved by the Institutional Animal Care and Treatment Committee of the Chinese People's Liberation Army General Hospital.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 6. Statistical tests were performed using a 2-tailed t test, 1-way analysis of variance (ANOVA) test, and 2-way ANOVA test with Bonferroni correction to compare the significant differences. Survival analysis was analyzed using the log-rank test. Unless otherwise indicated, P ≤ .05 was considered statistically significant for all analyses. All group values are represented as means plus or minus standard deviation (SD) if not stated otherwise.
Results
CRISPR screening reveals critical regulators that determine resistance to CAR T-cell therapy
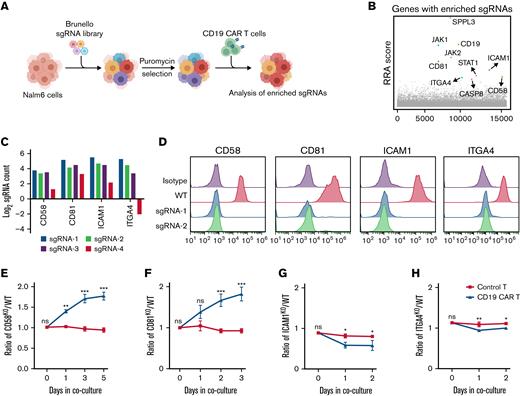
To systematically identify critical regulators that determine resistance to CAR T-cell therapy in tumor cells, we conducted genome-wide CRISPR/Cas9 screening with a coculture model containing Nalm6 cells, CD19+ human pre-B ALL cells, and CD19 CAR T cells (Figure 1A). The Nalm6 cells were transduced with a lentiviral CRISPR Brunello library targeting ∼19 000 genes and then selected under puromycin pressure for 2 days. To better reflect the long-term high tumor burden in vivo, Nalm6 cells were supplemented with CD19 CAR T cells every 3 days for 15 days at a 1:50 E:T ratio.18 To avoid contamination by the enrichment of sgRNAs that are associated with tumor cell survival but not with CAR T-cell therapy, we cocultured Nalm6 cells that had been transduced with the aforementioned CRISPR library with control T cells as the control condition. The composition of the sgRNAs in surviving tumor cells under CAR T-cell or control T-cell treatment conditions was evaluated by Illumina sequencing and analyzed by MAGeCK algorithm (supplemental Table 1).19 Our CRISPR screening identified expected candidates among the top 10 hits, namely CD19,7,8,20 JAK2,21 and CASP8,22 consistent with known mechanisms of resistance to CD19-targeted immunotherapies (Figure 1B). Interestingly, a cluster of membrane protein genes, including CD58, ICAM1, CD81, and ITGA4 were also positively selected (Figure 1C). In order to verify our screening findings, we generated stable KO cell lines of membrane proteins (CD58, CD81, ICAM1, and ITGA4) in Nalm6 cells by the CRISPR/Cas9 technology (Figure 1D). Growth competition assays were conducted for WT or KO target cell lines with different fluorescence labels in the presence of CAR T cells. The growth competition assays revealed that CD58KO and CD81KO cells conferred progressive enrichment in the presence of CD19 CAR T cells compared with WT cells (Figure 1E-F; supplemental Figure 1A-B). However, ICAM1KO and ITGA4KO cells did not show a significant increase (Figure 1G-H; supplemental Figure 1C-D). We identified that CD81 loss induced disruption of CD19 membrane trafficking (supplemental Figure 2). This finding is similar to previous reports in which downregulation of CD81 has been identified as a mechanism for resistance to CD19-targeted therapy.20,23 In the current study, we focused on the role of CD58 in CAR T-cell therapy.
Use of CRISPR/Cas9 screening to study intrinsic tumor resistance mechanisms to CAR T cells. (A) Schematic showing the CRISPR/Cas9 screening process. (B) The 10 genes with the most significant sgRNA enrichment. (C) Log2 fold change of normalized counts of each sgRNA targeting CD58, CD81, ICAM1, or ITGA4 in the screening. (D) Efficacy of CD58, CD81, ICAM1, or ITGA4 KO in Nalm6 cells. (E-H) GFP-labeled indicated KO cells and mCherry-labeled WT cells were mixed at a ∼1:1 ratio and cocultured with control T or CAR T cells. The KO (GFP+)/WT (mCherry+) ratio was calculated over time. CD58KO/WT Nalm6 ratio (E), CD81KO/WT Nalm6 ratio (F), ICAM1KO/WT Nalm6 raito (G), and ITGA4KO/WT Nalm6 ratio (H) in growth competition assay. KO cell lines referred to sgRNA-1 targeting the indicated genes in panels E-H. A mixture of cells was cocultured with control T or CD19 CAR T cells at a 1:20 E:T ratio (n = 3). Statistical comparisons were performed using a 2-way ANOVA test with multiple comparisons. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. ns, not significant (P > .05); RRA, Robust Rank Aggregation.
Use of CRISPR/Cas9 screening to study intrinsic tumor resistance mechanisms to CAR T cells. (A) Schematic showing the CRISPR/Cas9 screening process. (B) The 10 genes with the most significant sgRNA enrichment. (C) Log2 fold change of normalized counts of each sgRNA targeting CD58, CD81, ICAM1, or ITGA4 in the screening. (D) Efficacy of CD58, CD81, ICAM1, or ITGA4 KO in Nalm6 cells. (E-H) GFP-labeled indicated KO cells and mCherry-labeled WT cells were mixed at a ∼1:1 ratio and cocultured with control T or CAR T cells. The KO (GFP+)/WT (mCherry+) ratio was calculated over time. CD58KO/WT Nalm6 ratio (E), CD81KO/WT Nalm6 ratio (F), ICAM1KO/WT Nalm6 raito (G), and ITGA4KO/WT Nalm6 ratio (H) in growth competition assay. KO cell lines referred to sgRNA-1 targeting the indicated genes in panels E-H. A mixture of cells was cocultured with control T or CD19 CAR T cells at a 1:20 E:T ratio (n = 3). Statistical comparisons were performed using a 2-way ANOVA test with multiple comparisons. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. ns, not significant (P > .05); RRA, Robust Rank Aggregation.
Loss of CD58 in tumor cells induces resistance to CAR T-cell therapy
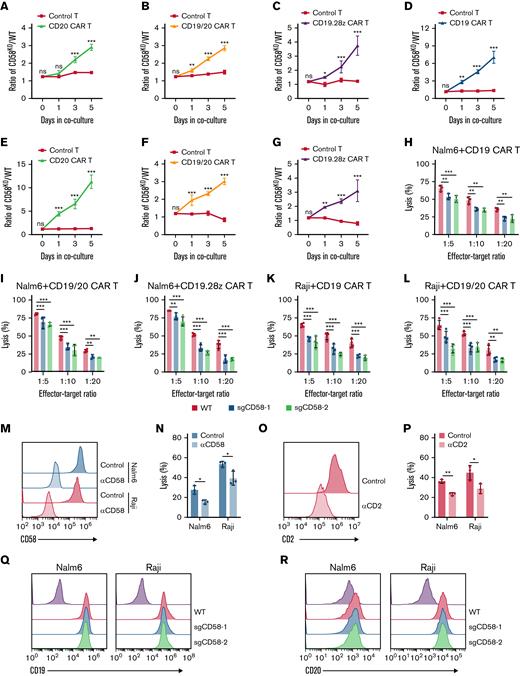
To explore whether CD58-deficient tumor cells were resistant to CAR T-cell therapy with different CAR design, we also generated distinct CAR T cells including CD20 CAR T, tandem CD19/CD20 CAR T cells,24 and CD19.28z CAR T cells from multiple healthy donors and observed that both CD58 loss in Nalm6 cells and Raji cells were relatively resistant to CAR T cells (Figure 2A-G; supplemental Figures 3 and 4A-J). We also found that tumor cells with disruption of CD58 exhibited low sensitivity to CAR T-cell–mediated killing in a cytotoxicity assay (Figure 2H-L; supplemental Figure 2K-M). This effect was recapitulated with an anti-CD58–blocking mAb (Figure 2M-N). Notably, blockage of CD2 on CAR T cells resulted in impaired CAR T-cell–mediated cytotoxicity (Figure 2O-P). As expected, resistance was not attributed to the downregulation of CD19 expression or CD20 expression, as determined by flow cytometry (Figure 2Q-R). Additionally, we observed that knocking out CD58 had no effect on the proliferation or apoptosis of tumor cells (supplemental Figure 5A-C). However, CD58 loss did not protect tumor cells from chemotherapy-mediated killing (supplemental Figure 5D), implying that CD58 loss in tumor cells may specifically confer resistance to CAR T-cell–mediated killing. Collectively, these findings imply that the CD58-CD2 axis is necessary in cytotoxic killing by CAR T cells and that lack of CD58 in lymphoid cancer cells could induce resistance to CAR T-cell therapy.
Role of CD58 in resistance to CAR T-cell–mediated killing. (A-C) CD58KO/WT Nalm6 cell ratio in growth competition assays. CD58KO Nalm6 cells referred to sgCD58-2 Nalm6 cells. (A) A mixture of Nalm6 cells were cocultured with control T cells or CD20 CAR T cells at a 1:1 E:T ratio (n = 3). (B) A mixture of Nalm6 cells were cocultured with control T cells or tandem CD19/20 CAR T cells at a 1:20 E:T ratio (n = 3). (C) A mixture of Nalm6 cells were cocultured with control T cells or CD19.28z CAR T cells at a 1:20 E:T ratio (n = 4). (D-G) CD58KO/WT Raji cell ratio in a growth competition assay. CD58KO Raji cells referred to sgCD58-2 Raji cells. (D) A mixture of Raji cells were cocultured with control T cells or CD19 CAR T cells at a 1:20 E:T ratio (n = 3). (E) A mixture of Raji cells were cocultured with control T cells or CD20 CAR T cells at a 1:20 E:T ratio (n = 3). (F) A mixture of Raji cells were cocultured with control T cells or CD19/20 CAR T cells at a 1:20 E:T ratio (n = 4). (G) A mixture of Raji cells were cocultured with control T cells or CD19.28z CAR T cells at a 1:20 E:T ratio (n = 4). (H) Cytotoxic analysis of WT and CD58KO Nalm6 cells cocultured with CD19 CAR T cells at indicated E:T ratios for 24 hours (n = 3). (I) Cytotoxic analysis of WT and CD58KO Nalm6 cells cocultured with CD19/20 CAR T cells at indicated E:T ratios for 24 hours (n = 4). (J) Cytotoxic analysis of WT and CD58KO Nalm6 cells cocultured with CD19.28z CAR T cells at indicated E:T ratios for 24 hours (n = 4). (K) Cytotoxic analysis of WT and CD58KO Raji cells cocultured with CD19 CAR T cells at indicated E:T ratios for 24 hours (n = 4). (L) Cytotoxic analysis of WT and CD58KO Raji cells cocultured with CD19/20 CAR T cells at indicated E:T ratios for 24 hours (n = 4). (M) FACS histogram showing the CD58 level in Nalm6 or Raji cell lines pretreated with control or blocking anti-CD58 mAbs for 30 minutes. (N) Cytotoxic analysis of Nalm6 or Raji cells pretreated with control or anti-CD58–blocking mAbs and cocultured with CD19 CAR T cells for 24 hours at a 1:20 E:T ratio (n = 3). (O) Representative FACS histogram showing the CD2 level in CD19 CAR T cells pretreated with control or anti-CD2–blocking mAbs for 30 minutes. (P) Cytotoxicity analysis of Nalm6 or Raji cells cocultured with control or anti-CD2 mAb-blocked CD19 CAR T cells at a 1:20 E:T ratio for 24 hours (n = 3). (Q) Representative FACS plot showing the level of CD19 expression in WT and CD58KO cell lines. (R) Representative FACS plot showing the level of CD20 expression in WT and CD58KO cell lines. Significance was assessed using a 2-way ANOVA test with multiple comparisons in panels A-L. Statistical comparisons were performed using a 2-tailed unpaired t test in panels N and P. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. FACS, fluorescence-activated cell sorter; ns, not significant (P > .05).
Role of CD58 in resistance to CAR T-cell–mediated killing. (A-C) CD58KO/WT Nalm6 cell ratio in growth competition assays. CD58KO Nalm6 cells referred to sgCD58-2 Nalm6 cells. (A) A mixture of Nalm6 cells were cocultured with control T cells or CD20 CAR T cells at a 1:1 E:T ratio (n = 3). (B) A mixture of Nalm6 cells were cocultured with control T cells or tandem CD19/20 CAR T cells at a 1:20 E:T ratio (n = 3). (C) A mixture of Nalm6 cells were cocultured with control T cells or CD19.28z CAR T cells at a 1:20 E:T ratio (n = 4). (D-G) CD58KO/WT Raji cell ratio in a growth competition assay. CD58KO Raji cells referred to sgCD58-2 Raji cells. (D) A mixture of Raji cells were cocultured with control T cells or CD19 CAR T cells at a 1:20 E:T ratio (n = 3). (E) A mixture of Raji cells were cocultured with control T cells or CD20 CAR T cells at a 1:20 E:T ratio (n = 3). (F) A mixture of Raji cells were cocultured with control T cells or CD19/20 CAR T cells at a 1:20 E:T ratio (n = 4). (G) A mixture of Raji cells were cocultured with control T cells or CD19.28z CAR T cells at a 1:20 E:T ratio (n = 4). (H) Cytotoxic analysis of WT and CD58KO Nalm6 cells cocultured with CD19 CAR T cells at indicated E:T ratios for 24 hours (n = 3). (I) Cytotoxic analysis of WT and CD58KO Nalm6 cells cocultured with CD19/20 CAR T cells at indicated E:T ratios for 24 hours (n = 4). (J) Cytotoxic analysis of WT and CD58KO Nalm6 cells cocultured with CD19.28z CAR T cells at indicated E:T ratios for 24 hours (n = 4). (K) Cytotoxic analysis of WT and CD58KO Raji cells cocultured with CD19 CAR T cells at indicated E:T ratios for 24 hours (n = 4). (L) Cytotoxic analysis of WT and CD58KO Raji cells cocultured with CD19/20 CAR T cells at indicated E:T ratios for 24 hours (n = 4). (M) FACS histogram showing the CD58 level in Nalm6 or Raji cell lines pretreated with control or blocking anti-CD58 mAbs for 30 minutes. (N) Cytotoxic analysis of Nalm6 or Raji cells pretreated with control or anti-CD58–blocking mAbs and cocultured with CD19 CAR T cells for 24 hours at a 1:20 E:T ratio (n = 3). (O) Representative FACS histogram showing the CD2 level in CD19 CAR T cells pretreated with control or anti-CD2–blocking mAbs for 30 minutes. (P) Cytotoxicity analysis of Nalm6 or Raji cells cocultured with control or anti-CD2 mAb-blocked CD19 CAR T cells at a 1:20 E:T ratio for 24 hours (n = 3). (Q) Representative FACS plot showing the level of CD19 expression in WT and CD58KO cell lines. (R) Representative FACS plot showing the level of CD20 expression in WT and CD58KO cell lines. Significance was assessed using a 2-way ANOVA test with multiple comparisons in panels A-L. Statistical comparisons were performed using a 2-tailed unpaired t test in panels N and P. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. FACS, fluorescence-activated cell sorter; ns, not significant (P > .05).
CD58 disruption in tumor cells impairs CAR T cells
The CD58-CD2 interaction has been reported to be a crucial costimulatory signal for T-cell activation in response to target cells.25 Using TCGA RNA sequencing data and Tumor Immune Estimation Resource 2.0, (TIMER),26 we found that messenger RNA levels of CD58 were positively correlated with CD8 T-cell infiltration in many human cancer types (supplemental Figure 6A). Besides, we found that the low expression of CD58 was associated with the low expression of interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) (supplemental Figure 6B-C), which suggested that the downregulated expression of CD58 in tumor cells may be related to the dysfunction of T cells. Hence, we wondered whether resistance to CAR T-cell therapy caused by CD58 disruption in cancer cells is due to attenuated CAR T-cell function. We found decreased expansion of CD19 CAR T cells cocultured with CD58KO Nalm6 cells or CD58KO Raji cells (Figure 3A-B). We also observed that lack of CD58 in tumor cells initiated dysfunctional degranulation, as measured by CD107a level (Figure 3B; supplemental Figure 7A). In parallel, adding an anti-CD58–blocking mAb to Nalm6 cell cultures and an anti-CD2–blocking mAb to CD19 CAR T-cell cultures significantly inhibited degranulation (Figure 3C-D). Moreover, we found that loss of CD58 in tumor cells led to the reduced secretion of cytokines, such as interleukin-2 (IL-2), TNF-α, and IFN-γ (Figure 3E; supplemental Figure 7B). To further explore the effect of tumor cells with disrupted CD58 on CAR T cells, we sorted CAR T cells cultured with WT or CD58KO tumor cells and then tested their kinetic responses when cocultured with WT tumor cells (Figure 3F). Remarkably, we found that CD19 CAR T cells initially cultured with CD58KO tumor cells exhibited low expansion capacity, a reduced degranulation, and impaired ability to kill WT tumor cells again (Figure 3G-J; supplemental Figure 8A-D). We also noted increased apoptosis in CD19 CAR T cells initially cultured with CD58KO tumor cells, and this increased apoptosis was not driven by FAS or TNFR2 upregulation (Figure 3K; supplemental Figure 8E-F). To investigate the effect of chronic CD58KO tumor cell stimulation on the function of CAR T cells, we established a coculture system in which WT or CD58KO tumor cells were added to a CAR T-cell culture every 72 hours (Figure 3L). Repetitive CD58KO tumor cell stimulation attenuated CAR T-cell expansion and reduced CAR T-cell activation as measured by Ki67, CD25, and CD69 level (Figure 3M-O; supplemental Figure 8G-K). Overall, these results suggest that CD58 disruption in cancer cells conferred functional impairment of CAR T cells, including reduced CAR T-cell expansion, survival, activation, degranulation, cytotoxicity, and cytokine secretion and increased CAR T-cell death, which might be responsible for resistance to CAR T-cell therapy.
Loss of CD58 in tumor cells gives rise to CAR T-cell dysfunction. (A) Expansion of CD19 CAR T cells after coculturing with WT or CD58KO Nalm6 or Raji cells at a 1:1 E:T ratio (n = 3). (B) FACS-based measurement of CD107a expression in CD19 CAR T cells stimulated by WT or CD58KO Nalm6 or Raji cells (n = 4). (C) FACS-based measurement of CD107a expression in CD19 CAR T cells stimulated with Nalm6 or Raji cells pretreated with control or anti-CD58–blocking mAbs (n = 4). (D) FACS-based measurement of CD107a expression in CD19 CAR T cells pretreated with control or anti-CD2–blocking mAbs after stimulation with Nalm6 cells or Raji cells (n = 4). (E) FACS-based quantification of intracellular interleukin-2, IFN-γ, and TNF-α expression in CD19 CAR T cells stimulated with WT or CD58KO Nalm6 cells (n = 4) or Raji cells (n = 5) at a 1:1 E:T ratio for 8 hours. (F) Schematic showing the functional assessment study. CD19 CAR T cells and WT or CD58KO Nalm6 cells were initially cocultured at an E:T ratio of 1:1 for 3 days (first coculture). CD19 CAR T cells were sorted by the phycoerythrin magnetic beads method based on CAR staining in first coculture and then cocultured again with WT Nalm6 cells at an E:T ratio of 1:1 for 24 hours and 48 hours (secondary coculture). Expansion (G) and Ki67 expression (H) of CD19 CAR T cells after 24 hours and 48 hours in secondary coculture (n = 3). (I) CD19 CAR T cells were sorted in first coculture and then cocultured again with WT Nalm6 cells at an E:T ratio of 1:1 to test the degranulation (CD107a expression) of CAR T cells for 0.5 hours or 1 hour (n = 4). (J) Survival of WT Nalm6 cells after 24 hours and 48 hours in secondary coculture (n = 3). (K) Representative FACS plots and quantification of annexin V expression of CD19 CAR T cells after 24 hours and 48 hours in secondary coculture (n = 3). (L) Pattern of repeated antigen stimulation in vitro. (M) CD19 CAR T-cell expansion after repeated stimulation with WT or CD58KO Nalm6 cells. CD25 (N) and CD69 expression (O) in CD19 CAR T cells after repeated stimulation with WT or CD58KO Nalm6 cells (n = 3). Statistical comparisons were performed using a 2-tailed unpaired t test in panels C-D. Statistical comparisons were performed using a 1-way ANOVA test in panels B and E. Significance was assessed using a 2-way ANOVA test with multiple comparisons in panels A, G-K, and M-O. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ↓, the time point when WT or CD58KO Nalm6 or Raji cells were added; 7-AAD-Percp, 7-Aminoactinomycin D (Peridinin-Chlorophyll-Protein Complex); FACS, fluorescence-activated cell sorter; ns, not significant (P > .05).
Loss of CD58 in tumor cells gives rise to CAR T-cell dysfunction. (A) Expansion of CD19 CAR T cells after coculturing with WT or CD58KO Nalm6 or Raji cells at a 1:1 E:T ratio (n = 3). (B) FACS-based measurement of CD107a expression in CD19 CAR T cells stimulated by WT or CD58KO Nalm6 or Raji cells (n = 4). (C) FACS-based measurement of CD107a expression in CD19 CAR T cells stimulated with Nalm6 or Raji cells pretreated with control or anti-CD58–blocking mAbs (n = 4). (D) FACS-based measurement of CD107a expression in CD19 CAR T cells pretreated with control or anti-CD2–blocking mAbs after stimulation with Nalm6 cells or Raji cells (n = 4). (E) FACS-based quantification of intracellular interleukin-2, IFN-γ, and TNF-α expression in CD19 CAR T cells stimulated with WT or CD58KO Nalm6 cells (n = 4) or Raji cells (n = 5) at a 1:1 E:T ratio for 8 hours. (F) Schematic showing the functional assessment study. CD19 CAR T cells and WT or CD58KO Nalm6 cells were initially cocultured at an E:T ratio of 1:1 for 3 days (first coculture). CD19 CAR T cells were sorted by the phycoerythrin magnetic beads method based on CAR staining in first coculture and then cocultured again with WT Nalm6 cells at an E:T ratio of 1:1 for 24 hours and 48 hours (secondary coculture). Expansion (G) and Ki67 expression (H) of CD19 CAR T cells after 24 hours and 48 hours in secondary coculture (n = 3). (I) CD19 CAR T cells were sorted in first coculture and then cocultured again with WT Nalm6 cells at an E:T ratio of 1:1 to test the degranulation (CD107a expression) of CAR T cells for 0.5 hours or 1 hour (n = 4). (J) Survival of WT Nalm6 cells after 24 hours and 48 hours in secondary coculture (n = 3). (K) Representative FACS plots and quantification of annexin V expression of CD19 CAR T cells after 24 hours and 48 hours in secondary coculture (n = 3). (L) Pattern of repeated antigen stimulation in vitro. (M) CD19 CAR T-cell expansion after repeated stimulation with WT or CD58KO Nalm6 cells. CD25 (N) and CD69 expression (O) in CD19 CAR T cells after repeated stimulation with WT or CD58KO Nalm6 cells (n = 3). Statistical comparisons were performed using a 2-tailed unpaired t test in panels C-D. Statistical comparisons were performed using a 1-way ANOVA test in panels B and E. Significance was assessed using a 2-way ANOVA test with multiple comparisons in panels A, G-K, and M-O. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. ↓, the time point when WT or CD58KO Nalm6 or Raji cells were added; 7-AAD-Percp, 7-Aminoactinomycin D (Peridinin-Chlorophyll-Protein Complex); FACS, fluorescence-activated cell sorter; ns, not significant (P > .05).
CD58-deficient tumor cells prevent effective IS formation with CAR T cells and attenuate CAR signaling transduction
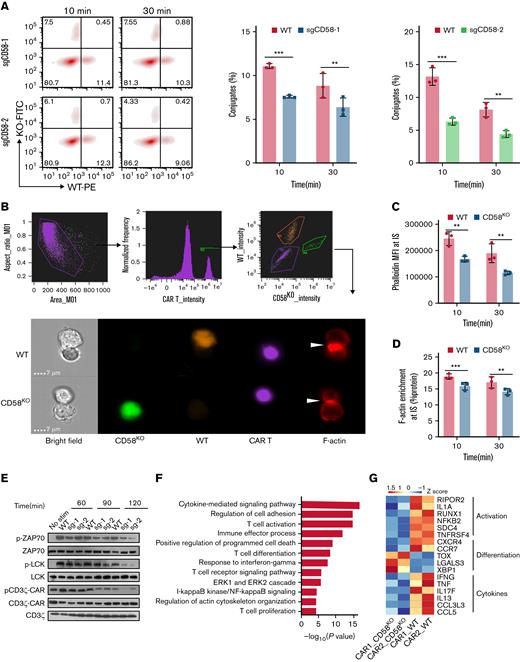
The CD2-CD58 interaction is essential for the formation of effective IS, to sustaining the activation and proliferation of T cells and trigger a series of intracellular signaling pathways in T cells.25 Recent studies have revealed that nonclassical IS formed by CAR T cells and tumor cells has been regarded as an important indicator for predicting the effectiveness of CAR T-cell therapy.27-29 Therefore, we hypothesized that the inhibition of CAR T-cell function induced by CD58-deficient tumor cells is caused by the ineffective formation of IS and weakened CAR signaling strength. To test this hypothesis, we performed an in vitro conjugation assay.30 Compared with WT cells, CD58KO Nalm6 cells formed significantly fewer conjugates with CAR-expressing Jurkat cells or CAR T cells (Figure 4A; supplemental Figure 9).
KO of CD58 in tumor cells forms ineffective IS with CAR T cells and attenuates CAR signaling. (A) CD19 CAR T cells were stained with CellTrace Violet dye and incubated with WT Nalm6 (mCherry+) and CD58KO (GFP+) Nalm6 cells for 10 minutes or 30 minutes. Representative FACS and bar plots (gating from Violet+ cells) representing the quantification of conjugates formed by WT or CD58KO with CAR T cells (n = 3). (B) CD19 CAR T cells prestained with CellTrace Violet dye were cocultured with WT (mCherry+) or CD58KO (GFP+) Nalm6 cells for 10 minutes or 30 minutes. After incubation, the cells were analyzed for the expression of CellTrace Violet, GFP, mCherry, and phalloidin (AF647) using ImageStream. Phalloidin was used to stain F-actin. The gating strategy used for the identification of IS and a representative image of an IS are shown. The white arrow points to an IS. (C) Median fluorescence intensity (MFI) of phalloidin at IS (n = 3). (D) F-actin enrichment at IS with WT or CD58KO is reported as percent protein (n = 3). (E) Proximal signaling events of CAR-expressing Jurkat cells upon stimulation with WT or CD58KO Nalm6 cells. Similar results were obtained from 3 independent biological experiments. (F) Selected pathways of gene ontology enrichment analysis in the biological process category of differentially expressed genes in CD19 CAR T cells sorted by flow cytometry 3 days after stimulation with WT or CD58KO Nalm6 cells (n = 2 different peripheral blood mononuclear cell donors). (G) Heatmap showing select differentially expressed genes related to T-cell activation, T-cell differentiation, and cytokines in CD19 CAR T cells after 3 days of stimulation with WT or CD58KO Nalm6 cells (n = 2 different PBMC donors). Statistical comparisons were performed using a 2-way ANOVA test with multiple comparisons. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. FACS, fluorescence-activated cell sorter; KO-FITC, CD58KO cells (Fluorescein Isothiocyanate); ns, not significant (P > .05); WT-PE, WT cells (phycoerythrin).
KO of CD58 in tumor cells forms ineffective IS with CAR T cells and attenuates CAR signaling. (A) CD19 CAR T cells were stained with CellTrace Violet dye and incubated with WT Nalm6 (mCherry+) and CD58KO (GFP+) Nalm6 cells for 10 minutes or 30 minutes. Representative FACS and bar plots (gating from Violet+ cells) representing the quantification of conjugates formed by WT or CD58KO with CAR T cells (n = 3). (B) CD19 CAR T cells prestained with CellTrace Violet dye were cocultured with WT (mCherry+) or CD58KO (GFP+) Nalm6 cells for 10 minutes or 30 minutes. After incubation, the cells were analyzed for the expression of CellTrace Violet, GFP, mCherry, and phalloidin (AF647) using ImageStream. Phalloidin was used to stain F-actin. The gating strategy used for the identification of IS and a representative image of an IS are shown. The white arrow points to an IS. (C) Median fluorescence intensity (MFI) of phalloidin at IS (n = 3). (D) F-actin enrichment at IS with WT or CD58KO is reported as percent protein (n = 3). (E) Proximal signaling events of CAR-expressing Jurkat cells upon stimulation with WT or CD58KO Nalm6 cells. Similar results were obtained from 3 independent biological experiments. (F) Selected pathways of gene ontology enrichment analysis in the biological process category of differentially expressed genes in CD19 CAR T cells sorted by flow cytometry 3 days after stimulation with WT or CD58KO Nalm6 cells (n = 2 different peripheral blood mononuclear cell donors). (G) Heatmap showing select differentially expressed genes related to T-cell activation, T-cell differentiation, and cytokines in CD19 CAR T cells after 3 days of stimulation with WT or CD58KO Nalm6 cells (n = 2 different PBMC donors). Statistical comparisons were performed using a 2-way ANOVA test with multiple comparisons. The values are shown as the means plus or minus SD. ∗P < .05; ∗∗P < .01; ∗∗∗ P < .001. FACS, fluorescence-activated cell sorter; KO-FITC, CD58KO cells (Fluorescein Isothiocyanate); ns, not significant (P > .05); WT-PE, WT cells (phycoerythrin).
Next, we used high-throughput imaging flow cytometry (ImageStream) to evaluate the stability of IS (Figure 4B). CD58KO Nalm6 cells formed IS structures with potentially disadvantageous cytoskeletal properties, as measured by F-actin intensity and enrichment at the IS (Figure 4C-D), implying that CD58KO Nalm6 cells prevented effective IS formation with CAR T cells.
Considering that the production of CAR T cells requires the activation of anti-CD3 antibody, the addition of anti-CD3 antibody caused high background phosphorylation level and we could not detect differences between groups. Therefore, we observed that the phosphorylation of CD3ζ-CAR, LCK, or ZAP70 in CAR-expressing Jurkat cells stimulated with CD58KO Nalm6 cells was inhibited compared with that stimulated with WT Nalm6 cells (Figure 4E). Of note, an anti-CD2 antibody can activate CAR T cells by detecting the expression of CD25 in CAR T cells (supplemental Figure 10A). However, CD58 loss in tumor cells did not show increased sensitivity to CAR T cells after the addition of an anti-CD2 antibody (supplemental Figure 10B), indicating the stable IS formed by CD58 on tumor cells is necessary for CAR T cells to successfully kill tumor cells.
To decipher the underlying molecular programs accounting for CD58-deficient tumor-mediated CAR T-cell dysfunction, we leveraged the transcriptional profiles of CD19 CAR T cells cocultured with WT Nalm6 cells or CD58KO Nalm6 cells. We observed differentially expressed genes in CD19 CAR T cells stimulated with WT Nalm6 cells or CD58KO Nalm6 cells (supplemental Figure 11A). A gene enrichment analysis of these differentially expressed genes revealed that these genes were significantly enriched in regulation of cell adhesion, T-cell activation, cytokine-related signaling, and cell proliferation (Figure 4F; supplemental Figure 11B-C; supplemental Table 2). More specifically, CD19 CAR T cells cocultured with CD58KO Nalm6 cells showed marked downregulation of genes associated with activation (RIPOR2, IL1A, RUNX1, NFKB2, SDC4, and TNFRSF4)31-35 and significantly differentially expressed genes associated with T-cell differentiation (CXCR4, CCR7, TOX, LGALS3, and XBP1).36-38 In addition, we found that the expression of a cluster of cytokine genes (IFNG, TNF, IL17F, IL13, CCL3L3, and CCL5) was decreased in CAR T cells cocultured with CD58KO Nalm6 cells (Figure 4G). Taken together, these findings indicate that CD58KO tumor cells and CAR T cells form inefficient IS, which drives a reduction in CAR T-cell activation, resulting in CAR T-cell dysfunction.
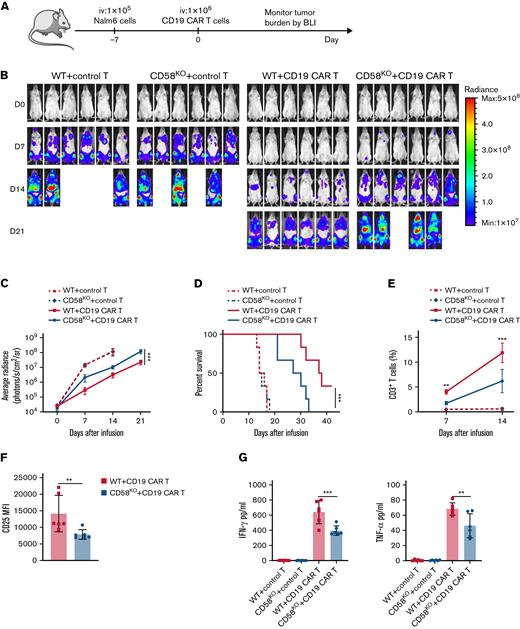
Loss of CD58 in tumor cells exhibits resistance to CAR T-cell therapy in vivo
To evaluate the effect of CD58KO tumor cells on the anti-tumor ability of CAR T-cell therapy in mouse xenograft models, we examined the tumor-suppressive ability of equal amounts of CAR T cells in mice transplanted with WT tumor cells or CD58KO tumor cells (Figure 5A). Consistent with our in vitro observations, the CD19 CAR T cells in CD58KO Nalm6 cell-bearing mice showed low tumor clearance capacity (Figure 5B-C). Moreover, xenograft mice with CD58KO Nalm6 cell transplants exhibited a survival disadvantage compared with xenograft mice with WT Nalm6 cell transplants (Figure 5D). Consistently, the expansion of CAR T cells in peripheral blood in CD58KO tumor-bearing mice was significantly lower than that in WT tumor-bearing mice (Figure 5E). Besides, we found that loss of CD58 in tumor cells suppressed activation of CAR T cells, as measured by CD25 level (Figure 5F). CAR T cells in the CD58KO tumor group secreted fewer cytokines than those in the WT tumor group (Figure 5G). These results indicate that loss of CD58 in tumor cells results in reduced sensitivity to CD19 CAR T-cell therapy in vivo.
CD58-deficient tumors show decreased sensitivity to CAR T-cell therapy in mouse xenograft model. (A) Schematic showing the generation of xenograft mouse models. NPG mice (n = 6 per group) were injected IV with 1 × 105 WT or CD58KO Nalm6-luk cells 7 days after a single IV injection of 1 × 106 control T cells or CD19 CAR T cells. Tumor burden was monitored every 7 days with BLI with an IVIS imaging system. (B) IVIS-obtained images showing tumor burden; BLI was performed at the indicated time points. (C) Average radiance measurement at the indicated time points (n = 6). (D) Mouse survival was monitored and recorded (n = 6 per group). (E) T-cell persistence in peripheral blood on days 7 and 14 (n = 6). (F) FACS-based measurement of CD25 expression in CD19 CAR T cells in peripheral blood on days 7 (n = 6). (G) Cytokines in peripheral blood at 7 days after CAR T-cell infusion were measured by enzyme-linked immunosorbent assays (n = 6). Significance was assessed using a 2-way ANOVA with multiple comparisons in panels C and E. Log-rank tests were performed to assess the significance of difference in panel D. Statistical comparisons were performed using a 2-tailed unpaired t test in panel F. Statistical comparisons were performed using a 1-way ANOVA test in panel G. The values are shown as the means plus or minus SD of 6 mice per group. For panels B-G, the results were from 1 of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. FACS, fluorescence-activated cell sorter; MFI, median fluorescence intensity; ns, not significant (P > .05).
CD58-deficient tumors show decreased sensitivity to CAR T-cell therapy in mouse xenograft model. (A) Schematic showing the generation of xenograft mouse models. NPG mice (n = 6 per group) were injected IV with 1 × 105 WT or CD58KO Nalm6-luk cells 7 days after a single IV injection of 1 × 106 control T cells or CD19 CAR T cells. Tumor burden was monitored every 7 days with BLI with an IVIS imaging system. (B) IVIS-obtained images showing tumor burden; BLI was performed at the indicated time points. (C) Average radiance measurement at the indicated time points (n = 6). (D) Mouse survival was monitored and recorded (n = 6 per group). (E) T-cell persistence in peripheral blood on days 7 and 14 (n = 6). (F) FACS-based measurement of CD25 expression in CD19 CAR T cells in peripheral blood on days 7 (n = 6). (G) Cytokines in peripheral blood at 7 days after CAR T-cell infusion were measured by enzyme-linked immunosorbent assays (n = 6). Significance was assessed using a 2-way ANOVA with multiple comparisons in panels C and E. Log-rank tests were performed to assess the significance of difference in panel D. Statistical comparisons were performed using a 2-tailed unpaired t test in panel F. Statistical comparisons were performed using a 1-way ANOVA test in panel G. The values are shown as the means plus or minus SD of 6 mice per group. For panels B-G, the results were from 1 of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. FACS, fluorescence-activated cell sorter; MFI, median fluorescence intensity; ns, not significant (P > .05).
Discussion
Resistance to CAR T-cell therapy is a primary obstacle to its broader therapeutic use.39 Performing unbiased CRISPR/Cas9 screening with the Nalm6 cells, CD19+ human ALL cell line, we revealed several genetic perturbations potentially capable of mediating resistance to CAR T-cell therapy. In addition to antigen loss and T-cell dysfunction, tumor-intrinsic resistance mechanisms, such as impaired death receptor signaling and NOXA, have been recently reported.18,22,40 In the present study, we identified a potential mechanism of tumor-intrinsic resistance to CAR T-cell therapy mediated by the loss of CD58 in tumor cells.
CD58 is a member of the immunoglobulin superfamily and is a ligand for the costimulatory molecule CD2 expressed in T cells.41 Disruption of the CD58-CD2 axis by blocking antibodies results in decreased T-cell activation, reduced IFN-γ secretion, and reduced cytotoxicity.42,43 Several reports have shown that loss of CD58 in tumor cells is an unfavorable prognostic factor and a frequent genetic abnormality in patients with hematologic malignancies.44,45 On the basis of CRISPR/Cas9 screening, a recent study has revealed that CD58 loss can confer immune evasion in tumor-infiltrating lymphocyte-mediated killing and that CD58 expression is downregulated in tumors of melanoma patients with resistance to immune checkpoint inhibitors.46 Likewise, CRISPR/Cas9 screening was implemented showed that CD58 deletion caused Nalm6 cell escape from natural killer cell–mediated killing in another study.47 These data suggest that abnormal CD58 expression in tumor cells may confer general resistance to various immunotherapies and that further investigation is needed in future studies.
CD58 is essential for the formation of stable IS, maintenance of T-cell activation, T-cell survival, and T-cell–mediated killing.25,48 However, the effect of CD58 expression in tumor cells on CAR T-cell therapy remains unknown. In this study, we observed that CD58KO in 2 B-lymphoid cell lines showed significantly less sensitive to a series of CAR T cells, including CD19 CAR T, CD20 CAR T, and tandem CD19/20 CAR T cells. In addition, we found that the loss of CD58 in tumor cells triggered impaired CAR T cells, which resulted in decreased CAR T-cell proliferation, degranulation, cytokine secretion, and cytotoxic effects and increased cell death. Even if the structure of IS formed by CAR T cells and target cells is saliently distinct from that the structure of classical IS, but an increasing number of studies demonstrated that the IS formed by CAR T cells plays an important role in driving the cytotoxic function of CAR T cells.27-29,49,50 In this study, we observed that CD58 loss in tumor cells prevented effective IS formation with CAR T cells, as measured by the intensity and enrichment of F-actin, a key component in IS.16,51 Furthermore, we found that CAR T cells saliently attenuated CAR signaling and CAR T-cell activation stimulation by CD58-deficient tumor cells. These results may shed light on the reasons that loss of CD58 in tumor cells induces resistance to CAR T-cell therapy.
Unfortunately, we were unable to provide clinical relevance of CD58 protein levels to CAR T-cell therapy in hematologic malignancies. Factually, we detected CD58 protein levels in tumor specimens from 34 patients with B-cell lymphoma before infusions of tandem CD19/20 CAR T cells by immunohistochemistry.24,52 Unexpectedly, we found only 2 patients with low CD58 expression (data not shown), which is totally distinct from the 60% DLBCL patients of CD58 protein downregulation reported by others53,54 in the Western population. In addition, we noted a large difference of CD58 mutation frequency in patients with DLBCL between the Chinese population (∼5% to 10% mutation rate)44,55 and Western population (>20% mutation rate).53,54 Although the 2 patients with CD58 low expression had poor response in our tandem CD19/20 CAR T-cell clinical trial, few sample number limited further comparative analysis. We preliminarily speculate that the expression of CD58 may be affected by racial disparities; CD58 loss or downregulation was not a main contributing factor for resistance to CAR T-cell therapy, just a low-probability event in Chinese patients with DLBCL.
Strategies to overcome tumor resistance to CAR T-cell therapy caused by CD58 loss remains to be further explored. A recent study illuminated that bypassing CD58 loss in tumor cells using a novel CAR T-cell construct integrating CD2 costimulatory domains with CAR molecules may be a potential therapeutic strategy.56 CD58 is regulated by both genetic and nongenetic factors.57 A previous study suggested that EZH2 inhibitors can restore epigenetically silenced CD58 expression on the surface of lymphoma cells, which in turn enhance IFN-γ secretion by T/natural killer cells.43 Reversing the functional downregulation of CD58 in tumor cells using drugs such as epigenetic modulators may contribute to novel combinatorial treatment strategies that can improve clinical responses to CAR T-cell therapy.
Overall, our findings emphasize a potential molecular mechanism determining the resistance of B-cell malignancies to CAR T-cell therapy. Our observations suggest that CD58 may be a clinically predictive biomarker for evaluating response to CAR T-cell therapy in hematologic malignancies, and therefore, targeting CD58 may be a novel therapeutic avenue to enhance the sensitivity or overcome resistance to CAR T-cell therapy.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (Nos. 81830002, 82150108, and 31991171) and Translational Research Grant of National Clinical Research Center for Hematologic Diseases (2021WWC04).
Authorship
Contribution: X.Y., D.C., Y.W., Z.W., and W.H. designed the study; X.Y., D.C., X.M., and Y.W. performed the experiments; Y.W., Y.G., J.W, Q.Z., Y.L., Y.Y., and C.T. assisted with the experiments; X.Y., D.C., Z.W., and W.H. analyzed the data and wrote the original manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhiqiang Wu, Department of Bio-therapeutic, the First Medical Center, Chinese PLA General Hospital, Beijing, China; e-mail: wuzhiqiang1006@163.com; and Weidong Han, Department of Bio-therapeutic, the First Medical Center, Chinese PLA General Hospital, Beijing, China; e-mail: hanwdrsw@163.com.
References
Author notes
The RNA sequencing data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE201970).
All data that support the findings of this study are available to the researchers on reasonable request.
The full-text version of this article contains a data supplement.
X.Y., D.C., X.M., and Y.W. contributed equally to this study.