Key Points
Responses to avapritinib in patients with previously treated AdvSM were rapid, deep, and sustained.
Efficacy was observed in all subtypes of AdvSM regardless of number/type of prior therapies or less favorable somatic mutations.
Abstract
Advanced systemic mastocytosis (AdvSM) is a rare myeloid neoplasm, driven by the KIT D816V mutation in >90% of patients. Avapritinib, a potent, highly selective D816V-mutant KIT inhibitor, is approved for treatment of adults with AdvSM by the US Food and Drug Administration, regardless of prior therapy, and the European Medicines Agency for patients with prior systemic therapy, based on EXPLORER (#NCT02561988; clinicaltrials.gov) and PATHFINDER (#NCT03580655; clinicaltrials.gov) clinical studies. We present latest pooled efficacy and safety analyses from patients who received ≥1 systemic therapy prior to avapritinib in EXPLORER/PATHFINDER. Overall response rate in response-evaluable patients (n = 31) was 71% (95% confidence interval: 52% to 86%; 22/31), including 19% (6/31) with complete remission (CR)/CR with partial recovery of peripheral blood counts (CRh). Median time to response was 2.3 months, median time to CR/CRh was 7.4 months, and median duration of response (DOR) was not reached. Reductions ≥50% in bone marrow mast cell infiltration (89%), KIT D816V variant allele fraction (66%), serum tryptase (89%), and reductions ≥35% in spleen size (70%) occurred in most patients. Median OS was not reached (median follow-up 17.7 months). Avapritinib was effective in all AdvSM subtypes, regardless of number/type of prior therapies or poor prognostic somatic mutations. Treatment-related adverse events (TRAEs) were observed in 94% of patients, most commonly grade 1/2; 57% had TRAEs of at least grade 3; 81% remained on treatment at 6 months. Avapritinib in adults with AdvSM who received prior systemic therapy was generally well tolerated, with high response rates regardless of prior systemic therapy.
Introduction
Systemic mastocytosis (SM) is a rare myeloid neoplasm, characterized by proliferation and accumulation of mast cells in the bone marrow, skin, and visceral organs.1-6 SM is associated with the KIT D816V mutation in >90% of cases.7-10 Advanced SM (AdvSM) includes 3 subtypes: aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).4 In SM-AHN, the AHN is most often myeloid, and the KIT D816V mutation as well as other myeloid-specific somatic mutations are often present in both the neoplastic mast cells and the AHN.11
Proliferation and infiltration of neoplastic mast cells can result in life-threatening organ damage due to infiltration by mast cells.4 Symptoms are often severe and debilitating and complicated by mast cell mediator release, leading to functional impairment and reduced quality of life.2,12
The median overall survival (OS) in patients with AdvSM is <3.5 years due to complications of high disease burden and mast cell–related organ damage or disease progression to secondary acute myeloid leukemia (AML) or MCL.3,4,13,14 In a study of patients with AdvSM treated with the multikinase inhibitor midostaurin, the median OS was 28.7 months, and the median progression-free survival was 14.1 months.14 Among AdvSM subtypes, MCL has a particularly poor prognosis, with a median OS ranging from 2 to 23 months.3,12,14-16 Reduction in KIT D816V expressed allele burden of at least 25% after 6 months was associated with longer OS.17,18
In addition to KIT D816V, patients with AdvSM often have other somatic mutations associated with myeloid neoplasms.19-22 Mutations in SRSF2, ASXL1, and RUNX1 (S/A/R) genes are associated with a significantly reduced treatment response, progression-free survival, and OS in patients with AdvSM, including those treated with midostaurin.11,17,23,24 The Mutation-Adjusted Risk Score (MARS) in AdvSM provides a validated risk estimate by combining clinical parameters (anemia, thrombocytopenia, and age >60 years) and the presence of S/A/R mutations.18,25
Several systemic therapies are used in the treatment of AdvSM. Midostaurin is licensed by the European Medicines Agency and the US Food and Drug Administration as monotherapy for the treatment of adults with AdvSM, regardless of prior systemic therapy.14,26,27 Cladribine and interferon-α (IFN-α) have also been used in the treatment of AdvSM.28,29 Allogeneic hematopoietic stem cell transplantation (alloHSCT) is another treatment option for some patients with AdvSM; to date, it has most often been used for patients with SM-AHN.30 In addition to SM, treatment of AHNs such as myelodysplastic syndrome (MDS) or overlap MDS/myeloproliferative neoplasms (MPN), including chronic myelomonocytic leukemia (CMML), may also require treatment of the AHN, for example with DNA methyltransferase inhibitors such as azacitidine or decitabine.
Avapritinib is a highly selective inhibitor of the KIT D816V mutation (half maximal inhibitory concentration, 0.27 nM).31 The clinical development of avapritinib in AdvSM comprises the phase 1 EXPLORER study (clinicaltrials.gov #NCT02561988) and the phase 2 PATHFINDER study (clinicaltrials.gov #NCT03580655), which tested efficacy and safety of avapritinib in both treatment-naïve and previously treated patients and demonstrated an overall response rate (ORR) of 75% in both studies.32-34 Based on these results, avapritinib at a starting dose of 200 mg once daily (QD) was approved by the US Food and Drug Administration in June 2021 for the treatment of adults with AdvSM (including ASM, SM-AHN, and MCL), regardless of prior therapy.35 This was followed by the approval of avapritinib at the 200 mg QD starting dose by the European Commission in March 2022 for patients with AdvSM (including ASM, SM-AHN, and MCL) who had received at least 1 prior systemic therapy.36 Here, we provide the first comprehensive report of the efficacy and safety of avapritinib in patients with AdvSM following 1 or more prior systemic therapies.
Methods
EXPLORER was a phase 1, open-label study of avapritinib in patients with AdvSM and relapsed or refractory myeloid malignancies, conducted in North America and Europe. This study comprised a dose escalation (part 1) and a dose expansion phase (part 2). The full protocol of EXPLORER was approved by the institutional review board or independent ethics committee of each participating center.
PATHFINDER is an ongoing, international, multicenter, open-label, single-arm, phase 2 registrational study of avapritinib administered at a starting dose of 200 mg QD in patients with a centrally confirmed diagnosis of AdvSM. The response to treatment for both studies was confirmed by central pathology review and adjudicated by the steering committee. The definition of response to treatment (presented as ORR) included CR, complete remission with partial recovery of peripheral blood counts (CRh; defined as CR but with residual cytopenias), partial remission (PR), and clinical improvement (CI) according to the modified International Working Group-Myeloproliferative Neoplasm Research and Treatment-European Competence Network on Mastocytosis response criteria (mIWG-MRT-ECNM).33,37 The full protocol of PATHFINDER was approved by the institutional review board or independent ethics committee of each participating center.
Both studies enrolled patients with AdvSM regardless of number of lines or type of prior systemic therapy.33,34 For SM-AHN disease subtype, the AHN was required to be myeloid. Patients with AML, very high- or high-risk MDS (as defined by the International Prognostic Scoring System for Myelodysplastic Syndromes),38 and Philadelphia chromosome–positive malignancies were excluded from both studies. In addition, patients with myeloid AHNs with ≥10% blasts in bone marrow or peripheral blood were excluded from the PATHFINDER study. Patients considered by the investigator to be candidates for alloHSCT for treatment of AdvSM were excluded from the PATHFINDER study; previous treatment with alloHSCT was permitted. Palliative and supportive care medications were allowed.
Following the observation of an increased risk of intracranial bleed (ICB) with thrombocytopenia, both study protocols were amended to exclude patients with platelets < 50 × 109/L at baseline. Other risk mitigation measures included increased platelet count monitoring, updated dose guidance for treatment interruption, support for severely low platelet counts, and treatment discontinuation following ICB of any grade according to common terminology criteria.
Full study design details, including statistical analysis methods for EXPLORER and PATHFINDER, have been previously described.33,34
In this analysis, data are included from both the EXPLORER and PATHFINDER studies, with a data cutoff date for all analyses of 20 April 2021. All pooled analyses are post hoc, and type 1 error control has not been applied to analysis of disease burden reductions.
Analysis populations
Post hoc efficacy and safety analyses were performed in the populations described as follows:
Efficacy analyses
Response rate and OS analyses were performed in patients who were centrally adjudicated as response-evaluable per mIWG-MRT-ECNM criteria. “C-findings” of weight loss due to malabsorption, osteolytic lesions, and platelets < 100 × 109/L but ≥ 75 × 109/L were not considered evaluable (patients needed “C-findings” other than these to be considered response evaluable). All patients had received ≥1 prior systemic therapy and initiated 200 mg QD avapritinib in the EXPLORER or PATHFINDER studies by 23 June 2020 (n = 31; prior systemic therapy pooled efficacy population). This enrollment cutoff was employed to ensure, based on previous experience,33,34 that patients had sufficient follow-up to achieve a response. Response rates were also analyzed in response-evaluable patients with ≥1 prior systemic therapy who initiated 200 mg avapritinib in PATHFINDER by 20 April 2021 (n = 47; prior systemic therapy PATHFINDER population); this analysis was done to capture responses in all pretreated patients who initiated the recommended dose of avapritinib in this pivotal phase 2 study.
Disease burden analyses, including changes in clinicopathological measures of response (bone marrow mast cell infiltration, serum tryptase, KIT D816V variant allele fraction [VAF], and spleen volume), are presented for all patients from the EXPLORER and PATHFINDER study populations who were treated with avapritinib 200 mg QD starting dose, had ≥1 prior systemic therapy, and were enrolled by 23 June 2020 (n = 53; prior systemic therapy pooled safety population) and had available baseline and on-treatment evaluations. KIT D816V VAF was quantified by digital droplet polymerase chain reaction, with a limit of detection of 0.17%. Analyses of maximal change in absolute blood eosinophil counts are presented for the subgroup of patients in the prior systemic therapy pooled safety population with baseline eosinophil counts ≥0.5/mL, and analyses of maximal change in absolute blood monocyte count are shown for patients in the subgroup with an AHN of CMML. Survival analyses are included for the prior systemic therapy pooled efficacy and prior systemic therapy pooled safety populations. Within the latter, we also analyzed the subset of patients for whom KIT D816V VAF evaluations at baseline and at week 8 were available (n = 37). Consistent with previous studies, we grouped patients according to ≥25% or <25% reductions in KIT D816V VAF levels.17,18 MARS risk groups were defined as low (0-1), intermediate (2), and high (3-5) according to published criteria.25
Safety analyses
Safety analyses are presented for patients in the prior systemic therapy pooled safety population, and also for all patients in EXPLORER or PATHFINDER who were treated with avapritinib 200 mg QD starting dose, regardless of whether prior systemic treatment was received (n = 126; full pooled safety population); analyses include a summary of treatment-related adverse events (AEs) (TRAEs) and TRAEs leading to dose interruptions, dose modifications, and treatment discontinuations, as well as cognitive effects and ICBs, which have previously been observed in clinical trials of avapritinib.33,34
Results
Patients and treatment
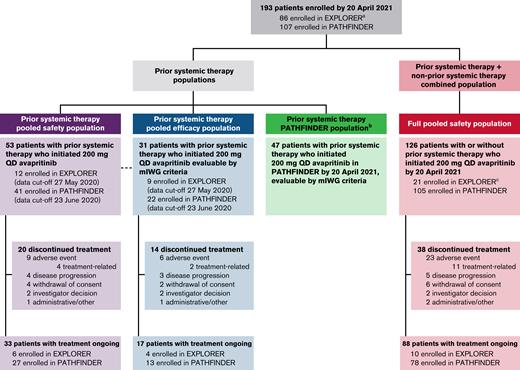
Demographic and baseline characteristics for the prior systemic therapy pooled efficacy population (n = 31), which only included patients who were evaluable per the mIWG-MRT-ECNM criteria, and the prior systemic therapy pooled safety population (n = 53), which included all patients with prior systemic therapy treated at a dose of 200 mg, are presented in Table 1, and those for the full pooled safety population (n = 126) and prior systemic therapy PATHFINDER population (n = 47) are shown in supplemental Table 1. Patient disposition and analysis populations for EXPLORER and PATHFINDER studies are shown in Figure 1. A large proportion of patients in the prior systemic therapy pooled efficacy population were treated with 2 or more prior systemic therapies (42%). Most had previously received midostaurin (n = 23; 74%); other treatments included azacitidine (n = 2), cladribine (n = 4), hydroxyurea (n = 3), and IFN-α (n= 4), along with small numbers of patients who received other treatments including other tyrosine kinase inhibitors (imatinib or dasatinib), alloHSCT, or investigational drugs. The most common reasons for discontinuation of prior systemic therapy were disease progression (18 patients; 58%) and toxicity (7 patients; 23%) (Table 1).
Patient disposition from EXPLORER and PATHFINDER studies.aEighty-six patients in EXPLORER include 16 non-AdvSM and 1 with CMML by central diagnosis. bSmPC population included in supplemental Appendix. cOne patient enrolled who initiated 200 mg QD in EXPLORER with a local diagnosis of AdvSM was centrally adjudicated to have indolent SM. SmPC, summary of medicinal product characteristics.
Patient disposition from EXPLORER and PATHFINDER studies.aEighty-six patients in EXPLORER include 16 non-AdvSM and 1 with CMML by central diagnosis. bSmPC population included in supplemental Appendix. cOne patient enrolled who initiated 200 mg QD in EXPLORER with a local diagnosis of AdvSM was centrally adjudicated to have indolent SM. SmPC, summary of medicinal product characteristics.
Of the 53 patients in the prior systemic therapy pooled safety population, 33 (62%) remained on treatment at the data cutoff; the most frequent reasons for discontinuation were AEs (n = 9 [17%]), withdrawal of consent (n = 4 [8%]), and disease progression (n = 4 [8%], of which 1 [2%] was due to progression to secondary AML).
Median baseline platelet counts were similar in the prior systemic therapy pooled safety population (n = 53) and in treatment-naïve patients who initiated 200 mg QD avapritinib in EXPLORER or PATHFINDER (n = 28; 148.0 × 109/L and 173.5 × 109/L, respectively; supplemental Table 2).
Efficacy
Response rates
The ORR in the prior systemic therapy pooled efficacy population (n = 31) was 71% (22/31; 95% confidence interval [95% CI], 52% to 86%). The median time to response across all AdvSM subtypes was 2.3 months (range, 0.5-26.7 months). Median time to CR/CRh was 7.4 months (range, 1.8-32.2 months). Median duration of response was not reached. At 12 and 24 months, respectively, 95% and 84% of initial responders continued to respond to treatment.
Response to avapritinib occurred across all AdvSM subtypes, including SM-AHN (77%; 17/22 patients) and MCL (50%; 4/8 patients) (Table 2). Responses in the SM component were observed across all SM-AHN subtypes. ORRs were similar in patients without (78%) vs with (62%) 1 or more S/A/R mutations (Table 3). Treatment discontinuation due to progressive disease (PD) was similar in patients with (1/20; 5%) or without (3/33; 9%) baseline S/A/R mutations in the prior systemic therapy pooled safety population.
Among patients with a recorded response to prior therapy, ORR to avapritinib was similar across response categories, with the highest response rate to avapritinib found in patients who had previously achieved PR (83%; 5/6). In patients who previously had stable disease, the largest group in this population, 75% (6/8) patients had a response to avapritinib (supplemental Table 3).
Responses were observed in patients regardless of prior therapy, including midostaurin (17/23; 74%), cladribine (2/4; 50%), IFN-α (4/4; 100%), hydroxyurea (3/3; 100%), and azacitidine (1/2; 50%) (supplemental Table 4).
ORR was 80% (95% CI, 52% to 96%) in 15 of 31 patients who discontinued their most recent prior therapy due to PD or relapse. Detailed response data in these patients are provided in supplemental Table 5.
The ORR for patients in the prior systemic therapy PATHFINDER population was 60% (95% CI, 44% to 74%) but with a shorter median follow-up of 14.6 months compared with 17.7 months (supplemental Table 6).
Disease burden measures
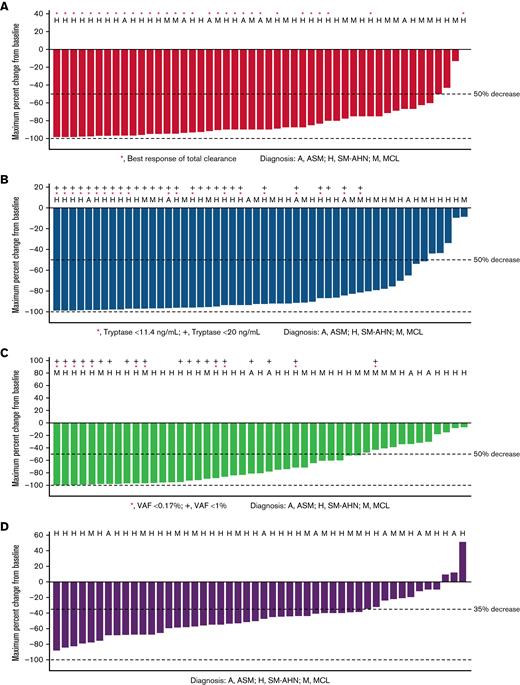
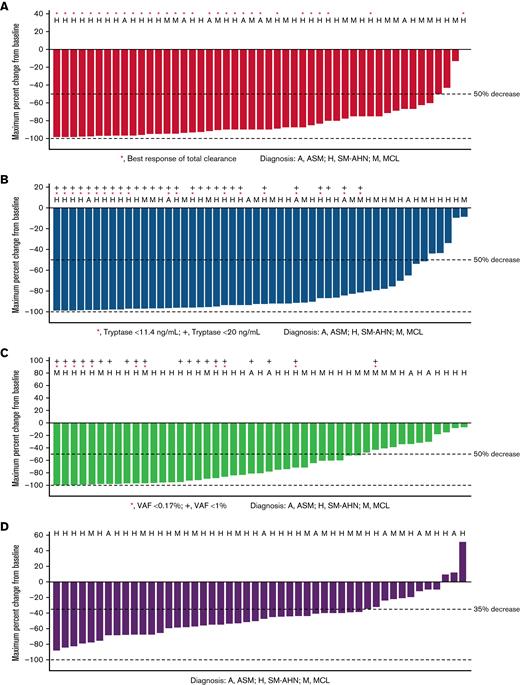
Waterfall plots for reductions from baseline of key clinicopathologic disease burden parameters in AdvSM in the prior systemic therapy pooled safety population, including bone marrow mast cell infiltrates, serum tryptase levels, KIT D816V VAF in peripheral blood, and spleen volume, are shown in Figure 2. Reductions were observed across all disease burden parameters. Neoplastic mast cells were measurable at baseline in 52 patients, and 89% (46 patients) achieved at least 50% reduction from baseline in bone marrow mast cell infiltrates, with 60% (31 patients) achieving total clearance of mast cell aggregates. All patients had serum tryptase assessment at baseline; reduction of serum tryptase ≥50% from baseline was achieved in 89% (47 patients), and 55% (29 patients) achieved serum tryptase levels <20 ng/mL. Baseline assessments of KIT D816V VAF in peripheral blood were available for all patients. Of these, 66% (35 patients) had a reduction of ≥50% from baseline, and 21% (11 patients) had VAF below the limit of detection. Baseline spleen volume assessment was available in all patients, of whom 70% (37 patients) had a reduction in spleen volume of at least 35% from baseline.
Percentage reduction from baseline in clinicopathological measures of response (prior systemic therapy pooled safety population, patients with baseline assessment data available). (A) Bone marrow mast cell infiltrates (median percent). (B) Serum tryptase (ng/mL). (C) KIT D816V VAF (median percent). (D) Spleen volume (mL).
Percentage reduction from baseline in clinicopathological measures of response (prior systemic therapy pooled safety population, patients with baseline assessment data available). (A) Bone marrow mast cell infiltrates (median percent). (B) Serum tryptase (ng/mL). (C) KIT D816V VAF (median percent). (D) Spleen volume (mL).
Of 16 patients with CMML as AHN, 15 (94%) showed reductions in monocytes of >50% and 7 (44%) of at least 80% during avapritinib treatment; only 2/16 patients still had raised levels at the latest assessment point (supplemental Figure 1A). All 10 patients with baseline eosinophil counts > 0.5x 109/L, 2 of whom had chronic eosinophilic leukemia (CEL), had reductions in eosinophils with avapritinib; 9/10 had reductions of >50%, and 8/10 had normal levels at the latest assessment point (supplemental Figure 1B).
Overall Survival
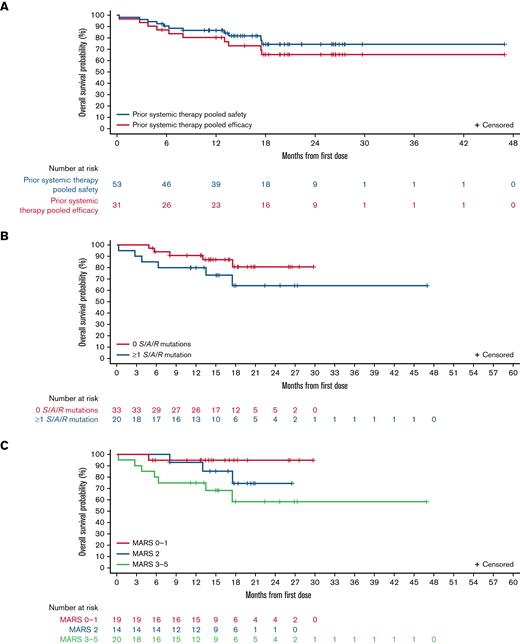
Median OS was not reached in the prior systemic therapy pooled efficacy population (Figure 3A), with estimated OS rates of 80% (95% CI, 66% to 95%) at 12 months and 65% (95% CI, 48% to 83%) at 24 months. In the prior systemic therapy pooled safety population, median OS was also not reached after a median follow-up of 17.7 months (95% CI, 15.0-19.7; Figure 3A), with estimated survival rates of 87% (95% CI, 77% to 96%) and 74% (95% CI, 60% to 88%) at 12 and 24 months, respectively. OS in these populations by AdvSM subtype is shown in supplemental Figure 2A-B. Median OS was not reached in any S/A/R status or MARS risk category subgroup, and there were no statistically significant differences between OS Kaplan-Meier curves (by log-rank test) between S/A/R status (Figure 3B; P = .19) or MARS risk category subgroups (Figure 3C; P = .08). At 12 months, OS in S/A/R− patients (n = 33) was 91% (95% CI, 81% to 100%) and in S/A/R+ patients (n = 20) was 80% (95% CI, 63% to 98%), whereas at 24 months, OS was 81% (95% CI, 64% to 97%) in S/A/R− and 64% (95% CI, 40% to 89%) in S/A/R+ patients. OS at 12 months was 95% in low- (n = 19; 95% CI, 85% to 100%), 93% in intermediate- (n = 14; 95% CI, 79% to 100%), and 75% in high-risk (n = 20; 95% CI, 56% to 94%) MARS categories, whereas at 24 months, OS was 95% in low- (95% CI, 85% to 100%), 75% in intermediate- (95% CI, 49% to 100%), and 58% in high-risk (95% CI, 33% to 84%) MARS categories. The difference in OS between patients with <25% vs ≥25% reduction in levels of KIT D816V VAF at week 8 as compared with baseline was not significant (P = .16) (supplemental Figure 2C).
OS in different populations and according to baseline mutations and risk scores. OS shown in (A) all patients in the prior systemic therapy pooled efficacy population and prior systemic therapy pooled safety population; (B) S/A/R− (0 mutations at baseline) and S/A/R+ (≥1 mutation at baseline) patients in the prior systemic therapy pooled safety population; and (C) low, intermediate, and high MARS categories in prior systemic therapy pooled safety population.
OS in different populations and according to baseline mutations and risk scores. OS shown in (A) all patients in the prior systemic therapy pooled efficacy population and prior systemic therapy pooled safety population; (B) S/A/R− (0 mutations at baseline) and S/A/R+ (≥1 mutation at baseline) patients in the prior systemic therapy pooled safety population; and (C) low, intermediate, and high MARS categories in prior systemic therapy pooled safety population.
Safety
Median treatment duration (range) in the prior systemic therapy pooled safety population (n = 53) was 14.6 months (0.2-43.3), and the median daily dose (range) was 123 mg (32-289), with a median relative dose intensity (range) of 56% (20-140). Median time to dose reduction was 2.9 months (95% CI, 1.7-3.2). At 6 months, 15 (35%) patients were being treated with avapritinib at 200 mg QD. The dose distribution at 6 months is provided in supplemental Table 7.
TRAEs by preferred term occurring in ≥10% of patients in the prior systemic therapy pooled safety population are shown in Table 4. The most common TRAEs (occurring in ≥20% of patients) of any grade were peripheral edema, thrombocytopenia, periorbital edema, and diarrhea. The most common grade 3 or above TRAEs according to common terminology criteria occurring in ≥10% of patients were neutropenia, thrombocytopenia, and anemia. TRAEs in the full pooled safety population are shown in supplemental Table 8.
Dose interruptions due to TRAEs were required in 30 patients (57%). The most common TRAEs leading to dose interruption were neutropenia and thrombocytopenia (each n = 6; 11%), cognitive disorder (n = 4; 8%), decreased white blood cell count, and decreased platelet count (each n = 3; 6%). Of the 53 patients in this population, 38 (72%) had at least 1 TRAE leading to dose reduction. The most common TRAEs leading to dose reduction were thrombocytopenia (n = 8; 15%), neutropenia (n = 5; 9%), periorbital edema (n = 4; 8%), peripheral edema, asthenia, decreased platelet count, and cognitive disorder (each n = 3; 6%). In this population, 2 patients (4%) discontinued primarily due to a TRAE, including 1 (2%) patient due to subdural hematoma and 1 (2%) due to decreased hemoglobin. There were no deaths due to AEs.
ICB and cognitive disorder TRAEs in the prior systemic therapy pooled safety population and the full pooled safety population are shown in supplemental Table 9. Two patients (4%) experienced an ICB, both subdural hematomas (1 grade 1 and 1 grade 4) (Table 4; supplemental Tables 8 and 9). One ICB event (subdural hematoma, grade 4) was reported in a patient with a platelet count < 50 × 109/L at baseline, who was enrolled prior to protocol amendments requiring platelet counts ≥ 50 × 109/L at screening. The other ICB event (subdural hematoma, grade 1) occurred in a patient with a baseline platelet count of 76 × 109/L. This patient had a platelet count of 60 × 109/L at the time of the ICB but previously had platelet counts < 50 × 109/L during treatment with avapritinib. The patient was also being treated with acetylsalicylic acid concurrently. Thirteen patients (24.5%) experienced cognitive TRAEs, of which only 2 (4%) were grade 3 (cognitive disorder). The most common cognitive TRAEs of any grade in this population were cognitive disorder (n = 8; 15%) and memory impairment (n = 3, 6%).
Discussion
Patients with AdvSM treated with avapritinib at a starting dose of 200 mg QD following at least 1 line of prior systemic therapy achieved rapid (median time to response, 2.3 months), deep (71% ORR, with reductions in all measures of disease burden), and sustained (median DOR not reached) response to treatment, including sustained CR/CRh in almost 20% of patients. Similar response rates were observed across all disease subtypes regardless of the number or type of prior therapies.
Importantly, we demonstrate that the ORR to avapritinib therapy in AdvSM patients following at least 1 line of prior systemic therapy was comparable to previously reported data from the EXPLORER and PATHFINDER studies for all patients, regardless of prior therapy.34 Responses were durable (with median DOR not reached) and were maintained despite dose reductions.34
Even patients with PD as their best overall response to their most recent prior systemic therapy had an ORR of 60% to avapritinib, and patients who discontinued their most recent prior therapy due to either PD or relapse, following an initial response, had an ORR of 80%.
Avapritinib reduced bone marrow mast cell infiltration, serum tryptase, KIT D816V VAF in peripheral blood, and spleen volume in the vast majority of patients. Two-thirds of patients treated with avapritinib had a reduction of ≥50% in KIT D816V VAF in blood, with almost one-fourth achieving levels below the limit of detection (0.17%). There was also a trend (P = .16) toward longer OS in patients who achieved ≥25% reduction in KIT D816V VAF by week 8 compared with those who did not. However, disease burden data analyses were limited by small patient numbers and short follow-up and were not controlled for type 1 errors.
In patients with AdvSM treated with prior systemic therapies, avapritinib demonstrated durable efficacy, with median OS not reached after median 17.7 months of follow-up and a 74% estimated OS rate at 24 months. Although direct comparisons cannot be made in the absence of head-to-head trials, in a registry-based study of patients with AdvSM, median OS was only 1.2 years with cladribine and 1.5 years with midostaurin in the second-line setting.18
Additionally, responses to avapritinib and OS in this population of patients with AdvSM were similar in patients with or without S/A/R mutation(s) (log-rank test P = .19), in agreement with previously reported results from the EXPLORER and PATHFINDER studies.33,34 OS rates at 12 months for avapritinib were similar across MARS risk categories (log-rank test P = .08).25 Median OS was not reached in any S/A/R status or MARS risk category subgroup. S/A/R mutations have previously been associated with shorter survival in AdvSM and lower response rates to midostaurin treatment.17,23,24 Higher MARS scores have also been associated with shorter median OS in patients treated with midostaurin.18 In contrast, the response and survival data in AdvSM patients suggest avapritinib treatment beyond the first-line setting can be effective and may prolong survival in many patients, including those with historically poorer prognoses.
The impact of avapritinib was also observed within the AHN component of disease. Eosinophils were reduced in all patients with elevated baseline eosinophils, and levels of peripheral monocytes were also reduced in almost all patients with baseline monocytosis, suggesting an effect of avapritinib across multiple disease components. These data may reflect the fact that cells derived from the AHN component in AdvSM often carry the KIT D816V mutation.11
These observations demonstrate high selectivity and potency of avapritinib in targeting and inhibiting the primary oncogenic driver in AdvSM regardless of prior therapy. Because SM-AHN represented >70% of the population in this analysis, the high response rate and low rate of progression (only 1 response-evaluable patient with PD) suggest a clinically important effect of avapritinib across disease subtypes, indicating that evaluation of avapritinib combined with AHN-directed therapy may be worth exploring. It should be noted that although patients in the study with SM-AHN responded well to avapritinib, patients with certain AHN subtypes, including AML, high-risk/very high-risk MDS as well as myeloid AHN with ≥10% blasts in bone marrow or peripheral blood were excluded from enrollment. These data, therefore, do not speak to the efficacy of avapritinib or its role as a treatment in patients with more advanced types of AHN.
The safety profile of avapritinib 200 mg QD starting dose was generally well tolerated in this heavily pretreated patient population, with few patients discontinuing treatment due to AEs. Dose reduction was an important component in managing the tolerability of avapritinib, along with platelet monitoring and other related safety precautions. Comparable to EXPLORER (72%) and PATHFINDER (68%) results,33,34 more than two-thirds of patients (72%) required dose reduction due to TRAEs, with the most common being myelosuppression, including thrombocytopenia. The rate of ICBs (2%) in patients with platelet counts > 50 × 109/L was low. Although cognitive disorder was among the common TRAEs (15%), <5% were grade 3 or above and <10% of patients required dose reduction due to cognitive disorders, and importantly, no cognitive disorders resulted in treatment discontinuation. Peripheral edema and edema in facial areas (including periorbital, eyelid, and face edema) were observed as common nonhematologic TRAEs, with only 1 edema event above grade 2 and no patients requiring treatment discontinuation due to any type of edema.
Limitations of the study, in the context of a rare disease, include the lack of a comparator in a randomized controlled trial setting and the retrospective nature of the analysis.
Summary
Data presented here support avapritinib 200 mg QD as a safe and efficacious treatment for adult patients with all disease subtypes of AdvSM who were previously treated with ≥1 prior systemic therapy. Treatment with avapritinib was associated with rapid, deep, and durable responses, including the major reduction of disease burden in SM as well as AHN disease components. The current analysis demonstrates that prior systemic therapy does not affect the efficacy or safety of treatment with avapritinib.
Acknowledgments
The authors would like to thank the patients, their families, all investigators involved in this study, and Cheryl Langford for assistance with data management. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Eloise Aston, and editorial support was provided by Travis Taylor, all of Paragon, Knutsford, United Kingdom, supported by Blueprint Medicines Corporation according to Good Publication Practice guidelines (https://www.ismpp.org/gpp3). The Sponsor was involved in the study design, collection, analysis, and interpretation of data. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
These studies were funded by Blueprint Medicines Corporation.
Authorship
Contribution: All authors contributed to the design of the study and/or interpretation of data for this article, contributed to drafts of the article, and approved the final version to be published. All authors had access to the data contained within this manuscript.
Conflict-of-interest disclosure: A.R. has received advisory board fees, speaking fees, and travel support from AbbVie, AOP Orphan Pharmaceuticals, Blueprint Medicines Corporation, BMS-Celgene, Deciphera, GSK, Incyte Corporation, and Novartis and received research support from Blueprint Medicines Corporation and Novartis. J.S. has received advisory board fees from Blueprint and Novartis. D.J.D. has served as a consultant for Amgen, Agios, Autolus, Blueprint Medicines Corporation, Forty-Seven, Incyte Corporation, Jazz, Novartis, Pfizer, Shire, and Takeda and has received research funding from AbbVie, GlycoMimetics, and Novartis. J.G. is Chair of the Response Adjudication Committee for the EXPLORER study and cochair of the Study Steering Committee for the PATHFINDER study and has received research funding and honoraria for these roles; has received research funding, served on advisory boards, and received honoraria and funding to cover travel expenses from Blueprint Medicines Corporation; has received research funding and serves on advisory boards for Deciphera; serves as the Chair for the Eligibility and Central Response Review Committee for Cogent Biosciences; and receives funding and honoraria for a study of bezuclastinib in patients with advanced SM. M.W.D. has received honoraria fees from Blueprint Medicines Corporation, Incyte Corporation, Medscape, Sangamo, and Takeda; has received consultancy fees from Blueprint Medicines Corporation, DisperSol, Fusion Pharma, Novartis, and Sangamo; has received research funding from Blueprint Medicines Corporation, Incyte Corporation, Leukemia & Lymphoma Society, Novartis, Pfizer, SPARC, and Takeda; is part of a study management committee for Blueprint Medicines Corporation and Takeda; and is a case author for Medscape. K.M.P. has participated in advisory boards for AbbVie, CTI Biopharma, PharmaEssentia, and Kura Oncology. I.A.-T. has served on advisory boards for and received honoraria from Blueprint Medicines Corporation and has participated in educational events for Novartis. A.M.V. has participated in speakers’ bureau for AOP Orphan Pharmaceuticals, BMS-Celgene, Novartis, and Shire and in advisory boards for Abbvie, BMS-Celgene, CTI, Incyte, and Novartis. J.P. has received honorarium fees from Alexion, Apellis, Blueprint Medicines Corporation, BMS, MSD, Novartis, and F. Hoffmann-La Roche and served on the speaker’s bureau of Alexion, Boehringer Ingelheim, Neopharm Israel, and Sobi. U.P. has received research funding from BMS, Amgen, Novartis, Curis, and BerGenBio. O.H. has received research funding from AB Science, BMS-Celgene, Alexion, Novartis, and Inatherys; consulted for AB Science; and is a shareholder for AB Science. I.D. has received advisory board fees from Novartis. H.-M.L., S.N.R., K.E., and S.D. are employees of and equity holders in Blueprint Medicines Corporation. D.H.R. has been a clinical advisory board/study steering committee member (EXPLORER and PATHFINDER studies) for Blueprint Medicines Corporation, was a Cogent Biosciences steering committee member for the APEX trial, and was involved with educational events and advisory boards for Novartis.
A full list of EXPLORER and PATHFINDER study investigators appears in “Appendix.”
Correspondence: Prof Andreas Reiter, University Hospital Mannheim, Theodor-Kutzer-Ufer 1-3, Mannheim 68167, Germany; e-mail: andreas.reiter@medma.uni-heidelberg.de.
Appendix: EXPLORER and PATHFINDER study investigators
EXPLORER
| Investigator . | Center . |
|---|---|
| Deepti Radia | Guy's and St Thomas' NHS Foundation Trust, Guy's Hospital, London, UK |
| Mark Drummond | Beatson West of Scotland Cancer Centre, Glasgow, UK |
| Elizabeth Hexner | Hospital of the University of Pennsylvania, Pennsylvania, PA, USA |
| Daniel J. DeAngelo | Dana-Farber Cancer Institute, Boston, MA, USA |
| Michael W. Deininger (Tsewang Tashi from 2021) | Huntsman Cancer Institute, Salt Lake City, UT, USA |
| Kristin Pettit | University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA |
| Prithviraj Bose | MD Anderson Cancer Center, Houston, TX, USA |
| Jason Gotlib | Stanford Cancer Institute, Stanford, CA, USA |
| William Robinson | University of Colorado Comprehensive Cancer Center, Aurora, CO, USA |
| Anthony Hunter | Winship Cancer Institute of Emory University, Atlanta, GA, USA |
| Investigator . | Center . |
|---|---|
| Deepti Radia | Guy's and St Thomas' NHS Foundation Trust, Guy's Hospital, London, UK |
| Mark Drummond | Beatson West of Scotland Cancer Centre, Glasgow, UK |
| Elizabeth Hexner | Hospital of the University of Pennsylvania, Pennsylvania, PA, USA |
| Daniel J. DeAngelo | Dana-Farber Cancer Institute, Boston, MA, USA |
| Michael W. Deininger (Tsewang Tashi from 2021) | Huntsman Cancer Institute, Salt Lake City, UT, USA |
| Kristin Pettit | University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA |
| Prithviraj Bose | MD Anderson Cancer Center, Houston, TX, USA |
| Jason Gotlib | Stanford Cancer Institute, Stanford, CA, USA |
| William Robinson | University of Colorado Comprehensive Cancer Center, Aurora, CO, USA |
| Anthony Hunter | Winship Cancer Institute of Emory University, Atlanta, GA, USA |
PATHFINDER
| Investigator . | Center . |
|---|---|
| Deepti Radia | Guy's and St Thomas' NHS Foundation Trust, Guy's Hospital, London, UK |
| Daniel J. DeAngelo | Dana-Farber Cancer Institute, Boston, MA, USA |
| Tsewang Tashi | Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA |
| Kristin Pettit | University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA |
| Jason Gotlib | Stanford Cancer Institute, Stanford, CA, USA |
| Mark Heaney | Herbert Irving Comprehensive Cancer Center, New York City, NY, USA |
| Ruben Mesa | Mays Cancer Center, San Antonio, TX, USA |
| Celalettin Ustun | Rush University Medical Center, Chicago, IL, USA |
| Stephen Oh | Washington University School of Medicine, St Louis, MO, USA |
| Elizabeth Griffiths | Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA |
| Wolfgang Sperr | Medical University of Vienna, Vienna, Austria |
| Olivier Hermine | Hôpital Necker-Enfants Malades, Paris, France |
| Cristina Livideanu | CHU Toulouse – Hôpital Larrey, Toulouse, France |
| Andreas Reiter | University Hospital Mannheim, Mannheim, Germany |
| Jens Panse | University Hospital RWTH Aachen, Aachen, Germany |
| Uwe Platzbecker | Leipzig University, Leipzig, Germany |
| Massimo Triggiani | Ospedale San Giovanni di Dio e Ruggi d'Aragona, University of Salerno, Salerno, Italy |
| Roberta Zanotti | Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy |
| Alessandro Vannucchi | Azienda Ospedaliero-Universitaria Careggi, CRIMM, Florence, Italy |
| Ivan Alvarez-Twose | Hospital Virgen del Valle – Complejo Hospitalario de Toledo, Toledo, Spain |
| Ingunn Dybedal | Oslo University Hospital-Rikshospitalet, Oslo, Norway |
| Investigator . | Center . |
|---|---|
| Deepti Radia | Guy's and St Thomas' NHS Foundation Trust, Guy's Hospital, London, UK |
| Daniel J. DeAngelo | Dana-Farber Cancer Institute, Boston, MA, USA |
| Tsewang Tashi | Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA |
| Kristin Pettit | University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA |
| Jason Gotlib | Stanford Cancer Institute, Stanford, CA, USA |
| Mark Heaney | Herbert Irving Comprehensive Cancer Center, New York City, NY, USA |
| Ruben Mesa | Mays Cancer Center, San Antonio, TX, USA |
| Celalettin Ustun | Rush University Medical Center, Chicago, IL, USA |
| Stephen Oh | Washington University School of Medicine, St Louis, MO, USA |
| Elizabeth Griffiths | Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA |
| Wolfgang Sperr | Medical University of Vienna, Vienna, Austria |
| Olivier Hermine | Hôpital Necker-Enfants Malades, Paris, France |
| Cristina Livideanu | CHU Toulouse – Hôpital Larrey, Toulouse, France |
| Andreas Reiter | University Hospital Mannheim, Mannheim, Germany |
| Jens Panse | University Hospital RWTH Aachen, Aachen, Germany |
| Uwe Platzbecker | Leipzig University, Leipzig, Germany |
| Massimo Triggiani | Ospedale San Giovanni di Dio e Ruggi d'Aragona, University of Salerno, Salerno, Italy |
| Roberta Zanotti | Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy |
| Alessandro Vannucchi | Azienda Ospedaliero-Universitaria Careggi, CRIMM, Florence, Italy |
| Ivan Alvarez-Twose | Hospital Virgen del Valle – Complejo Hospitalario de Toledo, Toledo, Spain |
| Ingunn Dybedal | Oslo University Hospital-Rikshospitalet, Oslo, Norway |
References
Author notes
The anonymized derived data from the EXPLORER and PATHFINDER studies that underlie the results reported in this article will be made available, beginning 12 months and ending 5 years after this article’s publication, to any investigators who sign a data access agreement and provide a methodologically sound proposal to medinfo@blueprintmedicines.com. The trial protocol will also be made available, as will a data fields dictionary.
The full-text version of this article contains a data supplement.
A.R. and J.S. contributed equally to this study.