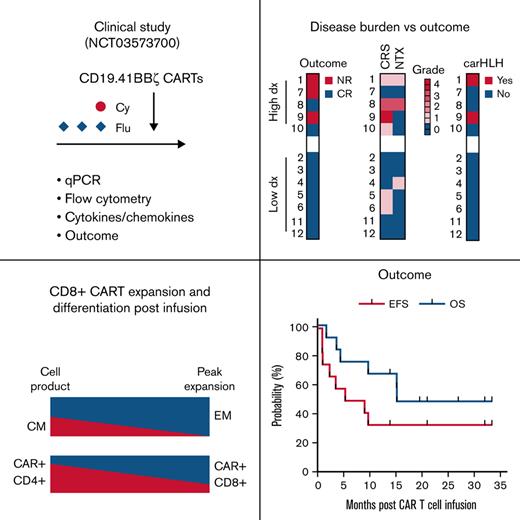
Key Points
CD8+ CD19-CAR T cells outcompete their CD4+ counterparts and undergo antigen-driven differentiation after infusion.
Our study highlights the role of disease burden on outcome after CD19-CAR T-cell therapy.
Abstract
T cells expressing CD19-specific chimeric antigen receptors (CD19-CARs) have potent antileukemia activity in pediatric and adult patients with relapsed and/or refractory B-cell acute lymphoblastic leukemia (B-ALL). However, not all patients achieve a complete response (CR), and a significant percentage relapse after CD19-CAR T-cell therapy due to T-cell intrinsic and/or extrinsic mechanisms. Thus, there is a need to evaluate new CD19-CAR T-cell products in patients to improve efficacy. We developed a phase 1/2 clinical study to evaluate an institutional autologous CD19-CAR T-cell product in pediatric patients with relapsed/refractory B-ALL. Here we report the outcome of the phase 1 study participants (n = 12). Treatment was well tolerated, with a low incidence of both cytokine release syndrome (any grade, n = 6) and neurotoxicity (any grade, n = 3). Nine out of 12 patients (75%) achieved a minimal residual disease-negative CR in the bone marrow (BM). High disease burden (≥40% morphologic blasts) before CAR T-cell infusion correlated with increased side effects and lower response rate, but not with CD19-CAR T-cell expansion. After infusion, CD8+ CAR T cells had a proliferative advantage over CD4+ CAR T cells and at peak expansion, had an effector memory phenotype with evidence of antigen-driven differentiation. Patients that proceeded to allogeneic hematopoietic cell transplantation (AlloHCT) had sustained, durable responses. In summary, the initial evaluation of our institutional CD19-CAR T-cell product demonstrates safety and efficacy while highlighting the impact of pre-infusion disease burden on outcomes. This trial was registered at www.clinicaltrials.gov as #NCT03573700.
Introduction
Adoptive immunotherapy with T cells expressing CD19-specific chimeric antigen receptors (CD19-CARs) has resulted in impressive clinical responses for relapsed and/or refractory B-cell acute lymphoblastic leukemia (B-ALL) or lymphoma, resulting in US Food and Drug Administration (FDA) approval of 4 CD19-CAR T-cell products.1 For pediatric, adolescent, and young adult (AYA) patients, several CD19-CAR T-cell products have been evaluated in large clinical studies and have demonstrated robust antitumor activity with similar toxicity profiles.2-5
Despite these successes, CD19-CAR T-cell therapy does not induce remission in all patients with B-ALL, and many who achieve an initial response eventually experience disease relapse.6-8 While the durability of responses has been correlated to the costimulatory signaling domain of the CAR, with CD19.41BBζ-CAR T cells better able to induce long-term remission as compared with CD19.CD28ζ-CAR T cells, CD19.41BBζ-CAR T cells do not induce durable remissions in all patients. Studies have also indicated that peak CAR T-cell expansion, T-cell subsets present in the CAR T-cell product, pretreatment leukemic burden, and/or absolute lymphocyte counts also influence outcome.3,6-14 Furthermore, at present, the optimal management for patients who achieve a complete response after CD19-CAR T-cell therapy is unknown, including which patients should proceed to consolidative allogeneic hematopoietic cell transplantation (AlloHCT).15,16
While a commercial CD19-CAR T-cell product is available for pediatric B-ALL, there is a continued need to study new CD19.41BBζ-CAR T-cell products, with the goal of identifying improvements in manufacturing techniques, modification of construct design, and optimization of administration conditions, which result in improvements in long-term response rates. We, therefore, developed an early phase clinical study (SJCAR19; NCT03573700) to evaluate the use of autologous T cells expressing CD19.41BBζ-CARs17 (CD19-CAR T cells) in patients ≤21 years of age with relapsed/refractory B-ALL. Here we report the safety and efficacy of this CAR T-cell product and a detailed immunophenotypic analysis of the first 12 consecutively enrolled patients in the phase 1 study.
Patients and methods
Detailed information regarding study design, eligibility criteria, disease response, toxicity evaluation, and correlative studies can be found in the supplemental Methods section.
Study design and participants
This is a single institution phase 1/2 clinical study evaluating the safety and efficacy of escalating doses of autologous CD19-CAR T cells in pediatric/AYA subjects ≤21 years old with relapsed/refractory CD19-positive B-ALL (SJCAR19; NCT03573700). The protocol was approved by the St. Jude Children’s Research Hospital institutional review board; written informed consent/assent was obtained from all participants/parents in accordance with institutional guidelines and the Declaration of Helsinki. Enrollment in the phase 1 study occurred between June 2018 and March 2020; enrollment in the phase 2 study is ongoing.
CD19-CAR T-cell products were manufactured at the Children’s Good Manufacturing Practice (GMP) facility of St. Jude from CD4/CD8-selected autologous leukapheresis products. CD19-CAR T-cell production is described in detail in the supplemental Methods section. Briefly, activated T cells were transduced with a self-inactivating lentiviral vector18 that encoded a CAR consisting of the CD19-specific single-chain variable fragment FMC63, the hinge/transmembrane domain of CD8α, and 41BB and CD3ζ signaling domains.17 After transduction, CD19-CAR T cells were expanded with interleukin-7 (IL-7) and IL-15 for 7 to 8 days before cryopreservation. Protocol treatment included lymphodepletion (fludarabine [25 mg/m2, days −4 to −2] and cyclophosphamide [900 mg/m2, day −2]) followed by CAR T-cell infusion (day 0; infusion occurred after thawing of a cryopreserved product). The phase 1 study used a 3 + 3 design to evaluate 2 dose levels (DLs): 1 × 106 and 3 × 106 CAR-positive T cells/kg, with a maximum cell dose of 2.5 × 108 CAR-positive T cells. Pretherapy disease evaluation (bone marrow [BM] and cerebrospinal fluid [CSF]) occurred after completion of any bridging therapy and within 2 weeks before protocol treatment. Bridging chemotherapy was per the treating physician’s discretion. After infusion, routine supportive care included anticonvulsant, antiviral, and antifungal prophylaxis, and IV immunoglobulin supplementation, as per institutional guidelines. After infusion, leukemia-directed therapy was not protocol defined, including the use of consolidative AlloHCT. Three patients received additional CD19-CAR T-cell infusions for recurrent leukemia on individualized, FDA-reviewed single-patient investigational plans.
Toxicity and outcome evaluations
Adverse events were captured using NCI Common Terminology Criteria (Version 5.0), except for cytokine release syndrome (CRS) and neurotoxicity (NTX). CRS was graded according to the consensus grading of the American Society of Transplant and Cellular Therapy (ASTCT).19 Before the adoption of the ASTCT consensus grading, NTX was graded using the common terminology adverse event criteria, in conjunction with a protocol-defined grading scheme to determine an overall NTX grade. With institutional adoption of the ASTCT consensus grading, NTX was then graded using immune effector cell-associated syndrome (ICANs).19 Disease response was determined using pre and 4 weeks post infusion leukemic burden in the BM. The response was categorized as complete response (CR), either minimal residual disease (MRD)-negative, MRD-positive, or no response (NR). MRD testing included flow cytometry (cut off 0.01%) and, when available for a given patient, reverse transcription-polymerase chain reaction (RT-PCR) and/or next-generation sequencing (NGS; Adaptive Biotechnologies) techniques. Extramedullary (EM) disease response was determined independently of marrow response. Relapse was defined as the development of any recurrent detectable disease (including MRD) after initial CR.
Correlative studies
Peripheral blood samples were collected before lymphodepleting chemotherapy on the day of CAR T-cell infusion and at weeks 1, 2, 3, and 4 after infusion; BM and CSF samples were obtained at 4 weeks after infusion. Quantitative polymerase chain reaction (qPCR) assays and flow cytometric and multiplex analyses were performed.
Statistical analysis
Overall survival (OS) and event-free survival (EFS) rates were estimated by the Kaplan-Meier method. Pairwise comparisons were made using t tests. Adjustment for multiple testing was not performed due to the small sample size and the exploratory nature of the analysis. OS was defined by time from CAR T-cell infusion to death, censoring at the time of the last follow-up. EFS was defined as the time from CAR T-cell infusion to NR at the week 4 evaluation, relapse, or death, with censoring at the time of the last follow-up. Statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
Thirteen patients enrolled in the phase 1 study. Twelve patients received protocol therapy. The first enrolled patient did not proceed due to poor clinical status in the setting of rapidly progressive B-ALL and is not included in this analysis. The median age at the time of B-ALL diagnosis and CD19-CAR T-cell infusion was 8.2 (range: 0.01-19.3) and 13.9 (range: 1.8-21.7) years, respectively (Table 1). Most patients identified as White (n = 10), of whom 4 were non-Hispanic. Participants were heavily pretreated, including prior AlloHCT (n = 4; median days from HCT infusion: 196.5; range: 132-254) and/or treatment with CD19- and/or CD22-directed non-CAR therapies (n = 5). No patient had previously received a CAR T-cell product, though this was not an exclusion criterion for the clinical study. Most participants harbored high-risk leukemic genetic and/or cytogenetic abnormalities.20 Pretherapy leukemic burden in the BM included a median morphologic blast count and MRD of 11% (range: 0-98; n = 12) and 20.6% (flow cytometry; range: 0-82.9; n = 11), respectively, with a median CD19 positivity of 99.1% (range: 67.2-100; n = 11). Pretherapy EM disease included 2 patients with disease in the CSF (central nervous system [CNS]-2) and 5 patients with areas of increased metabolic uptake on positron emission tomography (PET) imaging concerning possible leukemic involvement (Table 1). The median time between apheresis and protocol treatment was 20.5 (range: 17-45) days; most patients received bridging therapies (n = 10) during this time. Bridging therapy included systemic chemotherapy (n = 8) or focal radiation therapy to non-CNS EM sites (n = 2; 24 Gy to multiple sites of disease).
Patient characteristics
| Patient no./ dose level . | Age at infusion/ sex . | Prior HCT . | Prior antigen-directed therapy . | Genetic alterations . | Cytogenetics . | Disease indication . | Before infusion∗ . | Max CRS/ NTX grade . | carHLH . | Response (BM; 4 wk after infusion) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNS status . | PET† . | Marrow blast/ MRD (%) . | ||||||||||
| 1/1 | 20.4/M | N | B | KMT2A-AFF1 | pseudodiploid | Relapse 3 | 2 | Pos | 98 (ND) | 1/1 | Y | NR |
| 2/1 | 18.5/F | N | N | SMU1-JAK2‡ | normal | Relapse 2 | 1 | ND | 10 (20.6) | 0/0 | N | MRDneg CR§ |
| 3/1 | 16.3/M | N | N | none identified | normal | Primary Refractory | 1 | ND | 1 (6.1) | 0/0 | N | MRDneg CR§ |
| 4/1 | 10.4/M | Y | B | IKZF1 truncation | hyperdiploid | Relapse 2 | 1 | Pos | 0 (0.058) | 0/1 | N | MRDneg CR§ |
| 5/1 | 15.4/F | Y | N | none identified | normal | Relapse 3 | 1 | Neg | 12 (56) | 1/0 | N | MRDneg CR§ |
| 6/1 | 1.8/F | Y | B/I | KMT2A-RELA | pseudodiploid | Relapse 2 | 1 | Pos | 0 (NGS 70) | 1/0 | N | MRDneg CR§ |
| 7/2 | 12.4/F | Y | B | JAK/STAT iAMP21‡ | hypodiploid/ pseudodiploid | Relapse 2 | 1 | Pos | 80 (43) | 0/0 | N | NR |
| 8/2 | 15.4/F | N | B | CRLF2-r‡ | hyperdiploid | Relapse 2 | 1 | ND | 72 (95.2) | 3/3 | N | MRDneg CR |
| 9/2 | 6.2/F | N | N | none identified | hyperdiploid | Relapse 2 | 2 | Neg | 78 (83) | 4/0 | Y | NR |
| 10/2 | 5.6/F | N | N | NUMA1-CSF1R‡ | ND | Relapse 2 | 1 | ND | 84 (59.2) | 1/0 | N | MRDneg CR§ |
| 11/2 | 21.8/M | N | N | none identified | normal | Relapse 2 | 1 | Pos | Aplastic (6.8) | 0/0 | N | MRDneg CR§ |
| 12/2 | 12.3/F | N | N | none identified | hyperdiploid | Relapse 1 (refractory) | 1 | Neg | 2 (0.07) | 0/0 | N | MRDneg CR |
| Patient no./ dose level . | Age at infusion/ sex . | Prior HCT . | Prior antigen-directed therapy . | Genetic alterations . | Cytogenetics . | Disease indication . | Before infusion∗ . | Max CRS/ NTX grade . | carHLH . | Response (BM; 4 wk after infusion) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNS status . | PET† . | Marrow blast/ MRD (%) . | ||||||||||
| 1/1 | 20.4/M | N | B | KMT2A-AFF1 | pseudodiploid | Relapse 3 | 2 | Pos | 98 (ND) | 1/1 | Y | NR |
| 2/1 | 18.5/F | N | N | SMU1-JAK2‡ | normal | Relapse 2 | 1 | ND | 10 (20.6) | 0/0 | N | MRDneg CR§ |
| 3/1 | 16.3/M | N | N | none identified | normal | Primary Refractory | 1 | ND | 1 (6.1) | 0/0 | N | MRDneg CR§ |
| 4/1 | 10.4/M | Y | B | IKZF1 truncation | hyperdiploid | Relapse 2 | 1 | Pos | 0 (0.058) | 0/1 | N | MRDneg CR§ |
| 5/1 | 15.4/F | Y | N | none identified | normal | Relapse 3 | 1 | Neg | 12 (56) | 1/0 | N | MRDneg CR§ |
| 6/1 | 1.8/F | Y | B/I | KMT2A-RELA | pseudodiploid | Relapse 2 | 1 | Pos | 0 (NGS 70) | 1/0 | N | MRDneg CR§ |
| 7/2 | 12.4/F | Y | B | JAK/STAT iAMP21‡ | hypodiploid/ pseudodiploid | Relapse 2 | 1 | Pos | 80 (43) | 0/0 | N | NR |
| 8/2 | 15.4/F | N | B | CRLF2-r‡ | hyperdiploid | Relapse 2 | 1 | ND | 72 (95.2) | 3/3 | N | MRDneg CR |
| 9/2 | 6.2/F | N | N | none identified | hyperdiploid | Relapse 2 | 2 | Neg | 78 (83) | 4/0 | Y | NR |
| 10/2 | 5.6/F | N | N | NUMA1-CSF1R‡ | ND | Relapse 2 | 1 | ND | 84 (59.2) | 1/0 | N | MRDneg CR§ |
| 11/2 | 21.8/M | N | N | none identified | normal | Relapse 2 | 1 | Pos | Aplastic (6.8) | 0/0 | N | MRDneg CR§ |
| 12/2 | 12.3/F | N | N | none identified | hyperdiploid | Relapse 1 (refractory) | 1 | Neg | 2 (0.07) | 0/0 | N | MRDneg CR |
B, blinatumomab; carHLH, CAR-associated hemophagocytic lymphohistiocytosis; CNS, central nervous system; F, female; HCT, hematopoietic cell transplant; I, inotuzumab; M, male; MRD, minimal residual disease (flow-based unless otherwise specified); MRDneg CR, MRD-negative complete response; N, no; ND, not done; Neg, negative; NGS, next-generation sequencing; NR, no response; PET, positron emission tomography; Pos, positive; Y, yes.
Data presented as patient number unless otherwise specified; dose level 1 = 1 × 106 CAR+ cells per kg; dose level 2 = 3 × 106 CAR+ cells per kg.
Disease status was assessed within 2 weeks of start of lymphodepleting chemotherapy and after any bridging therapy.
Positive PET defined as presence of hypermetabolic activity outside of BM and/or lymph nodes.
Genetic alteration categorized as Ph-like.
NGS testing was available and resulted as <10−5.
Generation and characterization of CD19-CAR T cells
CD19-CAR T cells were successfully generated from CD4/CD8-purified leukapheresis products for all patients. The median time to manufacture cell products was 7 (range: 7-8) days. All products had >90% viability, with >98% of cells being CD45+/CD3+ (supplemental Figure 1A,B). The median transduction efficiency was 37.1% (range: 29.5-67.7) as judged by flow cytometric analysis, and the median vector copy number was 1.17 (range: 0.85-3.39); all products passed functional release criteria with a CD19-specific cytolytic activity of >20% (supplemental Figure 1C-E). Within the CAR-positive T-cell population, there was a significantly higher percentage of CD4+ vs CD8+ T cells (supplemental Figure 2A). Phenotypically, CAR-positive T cells had a predominantly central memory (CD45RO+/CCR7−) or effector memory (TEM; CD45RO+/CCR7−) phenotype (supplemental Figure 2B). Within the TEM compartment, >95% of T cells were transitional memory (CD28+/CD95+), which include CD27+ (EM1) as well as CD27− (EM4) subsets (supplemental Figure 2C,D). Phenotypic analysis of CAR-negative T cells within the CAR T-cell product revealed a similar phenotype (supplemental Figure 2B-D). CAR+/CD4+ T cells had a significantly higher percentage of PD1+/TIM3+ cells than CAR−/CD4+ T cells (supplemental Figure 2E). Likewise, CAR-positive T cells had a significantly higher percentage of CD39+ T cells than their CAR-negative counterparts (supplemental Figure 2F).
Administration and safety of CD19-CAR T cells
Twelve patients received protocol-defined lymphodepletion followed by CAR T-cell infusion (DL1: 1 × 106 CAR-positive T cells per kg [n = 6] and DL2: 3 × 106 CAR-positive T cells per kg [n = 6]). Patients remained inpatient after CAR T-cell infusion for a median of 11.5 (range: 8-44) days (1 discharge was patient transfer back to the referring hospital). Two patients required escalation of care to the intensive care unit due to high-grade CRS requiring either high-flow nasal cannula oxygen or vasopressor support at 7 and 9 days after CAR T-cell infusion, respectively. No patients required readmission within 30 days of infusion.
Toxicities were minimal (Table 2). Hematologic toxicities included 11 patients with grade 4 neutropenia (7 of which were preexisting) and 6 with concurrent grade 3 thrombocytopenia. Six patients developed CRS (grade 3 to 4; n = 2). Three patients developed NTX, including 1 patient at grade 3 (Table 2). The median time to onset of CRS and NTX was 5 (range: 0-11) days and 6 (range: 5-7) days, respectively. Two patients showed initial improvement of CRS (resolution of fever and down-trending CRP) and then developed a subsequent hyperinflammatory syndrome consistent with CAR-associated hemophagocytic lymphohistiocytosis (carHLH),21 diagnosed at 9 and 10 days after CAR T-cell infusion (Table 2).22 Treatment of immune-mediated side effects included tocilizumab (n = 5; grade ≥3 CRS [n = 2] and carHLH [n = 1]), corticosteroids (methylprednisolone and/or dexamethasone; n = 2; grade ≥3 CRS [n = 1] and carHLH [n = 1]), siltuximab (n = 1; grade ≥3 NTX), and/or anakinra (n = 2; carHLH). Three patients received tocilizumab for grade 1 CRS due to ongoing fevers and clinical concern for the risk of rapid CRS progression. One patient later developed carHLH, while the other 2 had resolution of CRS without symptom progression. Patients recovered from CRS and NTX without lasting complications. The patients with carHLH showed clinical stability in response to immunomodulatory agents.22 Two dose limiting toxicities (DLTs) were observed: 1 on DL1 (grade 3 acute kidney injury in the setting of carHLH and presumed renal leukemic infiltrates as judged by metabolic activity on pretherapy PET), and 1 on DL2 (grade 4 CRS that failed to resolve ≤72 hours of onset). Though patients treated on DL2 had higher rates of grade ≥3 CRS/NTX and carHLH (Table 2), only 1 patient on DL2 experienced a DLT. Therefore, DL2 was determined to be the maximum tolerated dose (MTD) as per the study design and was selected for the phase 2 portion of the study.
Notable toxicities after CD19-CAR T cell infusion∗
| Adverse event . | Total . | Dose level 1 . | Dose level 2 . |
|---|---|---|---|
| CRS | |||
| Any grade | 6 | 3 | 3 |
| Grade 3-4 | 2 | 0 | 2 |
| NTX | |||
| Any Grade | 3 | 2 | 1 |
| Grade 3-4 | 1 | 0 | 1 |
| carHLH | 2 | 1 | 1 |
| Infection | Total | ||
| Bacteremia | 2 | ||
| Clostridioides difficile | 1 | ||
| Viremia | 2 | ||
| Invasive fungal | 0 | ||
| Other nonmetabolic or hematologic† | |||
| Acute kidney injury | 1 | ||
| Hepatitis | 1 | ||
| Nausea/vomiting/anorexia | 5 | ||
| Pain | 1 | ||
| Headache | 1 | ||
| Ejection fraction decreased | 1 | ||
| Hypertension | 2 | ||
| Hypotension (without fever) | 1 | ||
| Any Grade 5 | 0 | ||
| Adverse event . | Total . | Dose level 1 . | Dose level 2 . |
|---|---|---|---|
| CRS | |||
| Any grade | 6 | 3 | 3 |
| Grade 3-4 | 2 | 0 | 2 |
| NTX | |||
| Any Grade | 3 | 2 | 1 |
| Grade 3-4 | 1 | 0 | 1 |
| carHLH | 2 | 1 | 1 |
| Infection | Total | ||
| Bacteremia | 2 | ||
| Clostridioides difficile | 1 | ||
| Viremia | 2 | ||
| Invasive fungal | 0 | ||
| Other nonmetabolic or hematologic† | |||
| Acute kidney injury | 1 | ||
| Hepatitis | 1 | ||
| Nausea/vomiting/anorexia | 5 | ||
| Pain | 1 | ||
| Headache | 1 | ||
| Ejection fraction decreased | 1 | ||
| Hypertension | 2 | ||
| Hypotension (without fever) | 1 | ||
| Any Grade 5 | 0 | ||
Patients were counted once per adverse event.
Occurring within 4 weeks of CAR T-cell infusion.
Grade ≥3, possibly/probably/definitely attributed to lymphodepletion and/or CAR T-cell product and not associated with CRS/NTX.
Antileukemia activity of CD19-CAR T cells and clinical outcome
At 4 weeks after infusion, 9/12 patients (75%) achieved an MRD-negative CR (MRDneg CR) in the BM, of which 7 were confirmed negative by NGS (Table 1). No patients had progressive CNS disease. PET reimaging of 3 MRDneg CR patients with abnormal pretherapy findings showed mixed metabolic responses at 4 weeks after infusion (patients 4, 6, and 11). Two patients had subsequent resolution of metabolic uptake on serial imaging (patients 6 and 11). The third patient, patient 4, had progressive disease with a tibial chloroma, which was later biopsied, and immunohistochemistry demonstrated CD19-positivity of tumor cells and presence of infiltrating CD19-CAR T cells as judged by qPCR (data not shown). Thus, 8/12 patients (67%) achieved a CR based on the National Comprehensive Cancer Network (NCCN) pediatric B-ALL response criteria.
The clinical course of all patients is summarized in Figure 1A. With a median follow-up of 15.2 months (range: 1.7-22.4), the median EFS and OS for the entire cohort were 7.2 and 15.2 months, respectively (Figure 1B). Among the 9 patients who achieved an MRDneg CR, 5 patients proceeded to a planned consolidative AlloHCT at a median of 2.7 months (range: 1.9-3) after CAR T-cell infusion. For these 5 patients, this was their first AlloHCT, and all remained in remission at the time of the last follow-up (median: 19.1 months after AlloHCT; range: 7.0-30.7), with 1 patient dying of transplant-related complications. The remaining 4 patients all experienced disease reoccurrence at a median of 4.4 months (range: 2.3-9.0) after CAR T-cell infusion. Three relapsed with CD19-positive B-ALL (sites: EM, BM, and BM/EM) and 1 with CD19-negative B-ALL (site: BM). The patients with CD19-positive relapse all had a history of prior AlloHCT, and 2 patients also had a history of extensive EM disease before CAR T-cell therapy, making them less desirable candidates for a planned consolidative AlloHCT after CAR. The patient with CD19-negative relapse experienced relapse before planned AlloHCT; this patient received further leukemia-directed therapy, attained remission, proceeded to AlloHCT, and remains alive and in remission. Additionally, loss of B-cell aplasia (BCA) was noted in 4 of the 9 CR patients at a median of 3.6 months after T-cell infusion (range: 2.4-5.8). Loss of BCA occurred just before planned AlloHCT (n = 2) or coincided with CD19-positive marrow relapse (n = 2). The remaining 5 patients had ongoing BCA at time of relapse (CD19-negative BM, n = 1; CD19-positive EM, n = 1) or at last evaluation before HCT (n = 3) (Figure 1C). These data suggest that mechanisms of CAR T-cell failure included limited persistence (loss of BCA) and leukemic evasion (antigen loss and EM disease). Evaluation of employed immune evasion mechanisms of leukemic blasts is ongoing.
Clinical outcomes after infusion of CD19-CAR T cells. (A) Swimmers plot depicting the clinical course for each patient, with each lane representing a single patient. Patient 6: the indication for a third CAR T-cell reinfusion was a loss of BCA; the patient achieved BCA again after infusion. (B) EFS and OS of the entire patient cohort (n = 12). EFS was defined as the time from CAR T-cell infusion to NR at 4 weeks after CAR T-cell infusion, relapse, or death, with censoring at the time of the last follow-up. OS was defined by the time from CAR T-cell infusion to death, censoring at the time of the last follow-up. EFS and OS rates were estimated by the Kaplan-Meier method and compared by the log-rank test. (C) Duration of BCA among patients achieving a CR after CAR T-cell therapy. (D) Graphical presentation of outcome and observed toxicities, including disease response in the BM at 4 weeks after CAR T-cell infusion, the highest grade of CRS/NTX, and presence of carHLH. Patients are grouped based on the level of leukemic disease in the BM before CAR T-cell infusion (high: ≥40% morphologic blasts; low: <40% morphologic blasts).
Clinical outcomes after infusion of CD19-CAR T cells. (A) Swimmers plot depicting the clinical course for each patient, with each lane representing a single patient. Patient 6: the indication for a third CAR T-cell reinfusion was a loss of BCA; the patient achieved BCA again after infusion. (B) EFS and OS of the entire patient cohort (n = 12). EFS was defined as the time from CAR T-cell infusion to NR at 4 weeks after CAR T-cell infusion, relapse, or death, with censoring at the time of the last follow-up. OS was defined by the time from CAR T-cell infusion to death, censoring at the time of the last follow-up. EFS and OS rates were estimated by the Kaplan-Meier method and compared by the log-rank test. (C) Duration of BCA among patients achieving a CR after CAR T-cell therapy. (D) Graphical presentation of outcome and observed toxicities, including disease response in the BM at 4 weeks after CAR T-cell infusion, the highest grade of CRS/NTX, and presence of carHLH. Patients are grouped based on the level of leukemic disease in the BM before CAR T-cell infusion (high: ≥40% morphologic blasts; low: <40% morphologic blasts).
The 3 patients with CD19-positive relapse received treatment with repeat lymphodepletion and CD19-CAR T-cell infusions using the previously manufactured products at a median of 6.4 months (range: 5.8-10.4) from initial infusion (Table 3; supplemental Figure 3). While these patients initially were treated on DL1 with reinfusion, they were treated at DL2 (the determined MTD) and received intensified lymphodepletion (fludarabine [30 mg/m2, days −4 to −2] and cyclophosphamide [500 mg/m2, days −3 to −2]). The one exception to this was the second infusion for patient 6, who received protocol-defined lymphodepletion chemotherapy, CAR T-cell infusion per DL1, and concurrent pembrolizumab. With reinfusion, the 2 patients with loss of BCA/relapse achieved an MRDneg CR, and the third patient (ongoing BCA/EM relapse; patient 4) had NR. Of the 2 patients with MRDneg CR, 1 proceeded to a second AlloHCT and died due to transplant-related complications (patient 5). The other (patient 6) again developed recurrent CD19-positive disease (EM), received a third CD19-CAR T-cell infusion (intensified LD; DL2), and again achieved MRDneg CR. The patient subsequently had a loss of BCA, received a fourth infusion, and again achieved BCA. This patient remains alive and in remission at the time of data cutoff (Table 3; supplemental Figure 3).
Outcome of patients who received a repeat CD19-CAR T cell infusion using a previously manufactured cellular product
| Patient no. . | Prior CAR+ T cell per kg dose . | Days from first infusion to first relapse . | CD19- expression . | Sites of recurrent detectable disease . | Subsequent therapies/response . | CAR T-cell reinfusion treatment characteristics . |
|---|---|---|---|---|---|---|
| 4 | 1 × 106 | 70 | Positive | EM | Focal RT to EM site and CD3-depleted DLI → improved PET → BM relapse∗ → CAR infusion #2 → NR (NGS = 16 clones per million) → progressive disease (combined BM∗/EM; 57 d after infusion) → palliative therapy → died secondary to disease | Preinfusion marrow MRD 0.052%; intensified† LD; dose: 3 × 106 CAR+ T cells per kg |
| 5 | 1 × 106 | 271 | Positive | BM | Methotrexate/mercaptopurine → CAR infusion #2 → MRDneg CR (NGS = 0 clones per million) → HCT #2 → died secondary to transplant related toxicity | Preinfusion marrow MRD 0.165%; intensified† LD; dose: 3 × 106 CAR+ T cells per kg; grade 2 CRS (tocilizumab ×2) |
| 6 | 1 × 106 | 160 | Positive | BM∗/EM | Chloroma excision → CAR infusion #2 with pembrolizumab → MRDneg CR (NGS = 0 clones per million) and improved PET → consolidative RT to prior EM site → relapse (combined BM [NGS = 189 clones per million]/EM; 183 d after infusion) → CAR infusion #3 → MRDneg CR (NGS = 0 clones per million) and improved PET → consolidative RT to prior EM site → loss of BCA → CAR infusion #4 → BCA with continued disease remission | CAR Infusion #2: before infusion marrow MRD 0.006%; nonintensified LD; dose: 1 × 106 CAR+ T cells per kg; immune mediated side effects (felt to be pembrolizumab-related) CAR Infusion #3: before infusion marrow MRD 7.605%; intensified LD; dose: 3 × 106 CAR+ T cells per kg; grade 1 CRS (tocilizumab ×1) CAR Infusion #4: before infusion marrow MRD negative (NGS 0); intensified LD; dose: 3 × 106 CAR+ T cells per kg; grade 1 CRS |
| Patient no. . | Prior CAR+ T cell per kg dose . | Days from first infusion to first relapse . | CD19- expression . | Sites of recurrent detectable disease . | Subsequent therapies/response . | CAR T-cell reinfusion treatment characteristics . |
|---|---|---|---|---|---|---|
| 4 | 1 × 106 | 70 | Positive | EM | Focal RT to EM site and CD3-depleted DLI → improved PET → BM relapse∗ → CAR infusion #2 → NR (NGS = 16 clones per million) → progressive disease (combined BM∗/EM; 57 d after infusion) → palliative therapy → died secondary to disease | Preinfusion marrow MRD 0.052%; intensified† LD; dose: 3 × 106 CAR+ T cells per kg |
| 5 | 1 × 106 | 271 | Positive | BM | Methotrexate/mercaptopurine → CAR infusion #2 → MRDneg CR (NGS = 0 clones per million) → HCT #2 → died secondary to transplant related toxicity | Preinfusion marrow MRD 0.165%; intensified† LD; dose: 3 × 106 CAR+ T cells per kg; grade 2 CRS (tocilizumab ×2) |
| 6 | 1 × 106 | 160 | Positive | BM∗/EM | Chloroma excision → CAR infusion #2 with pembrolizumab → MRDneg CR (NGS = 0 clones per million) and improved PET → consolidative RT to prior EM site → relapse (combined BM [NGS = 189 clones per million]/EM; 183 d after infusion) → CAR infusion #3 → MRDneg CR (NGS = 0 clones per million) and improved PET → consolidative RT to prior EM site → loss of BCA → CAR infusion #4 → BCA with continued disease remission | CAR Infusion #2: before infusion marrow MRD 0.006%; nonintensified LD; dose: 1 × 106 CAR+ T cells per kg; immune mediated side effects (felt to be pembrolizumab-related) CAR Infusion #3: before infusion marrow MRD 7.605%; intensified LD; dose: 3 × 106 CAR+ T cells per kg; grade 1 CRS (tocilizumab ×1) CAR Infusion #4: before infusion marrow MRD negative (NGS 0); intensified LD; dose: 3 × 106 CAR+ T cells per kg; grade 1 CRS |
DLI, donor lymphocyte infusion; EM, extramedullary disease (PET- and biopsy-proven CD19-positive disease); HCT, hematopoietic cell transplant; LD, lymphodepletion; RT, radiation therapy.
MRD testing positive by flow cytometry.
Intensified LD regimen administrated of fludarabine 40mg/m2 on days −4, −3, and −2, and cyclophosphamide 600 mg/m2 on days −3 and −2.
Disease burden correlates with therapeutic response and incidence of CRS, NTX, and carHLH but has no effect on CAR T-cell expansion
Based on prior reports,8 we classified high disease burden as having ≥40% morphologic blasts on pre-CAR T-cell disease evaluation. Five out of 12 patients had a high disease burden before treatment, and 4 of these were treated at DL2 (Figure 1D). We then focused our analysis on evaluating the influence of disease burden and dose level on clinical response, toxicity, plasma cytokines/chemokines, and CD19-CAR T-cell expansion. All 7 patients with low disease burden had MRDneg CRs, in contrast to only 2 of the 5 patients with high disease burden (Figure 1D). Likewise, severe CRS/NTX (grade 3 to 4) or carHLH occurred only in patients with a high disease burden (Figure 1D).
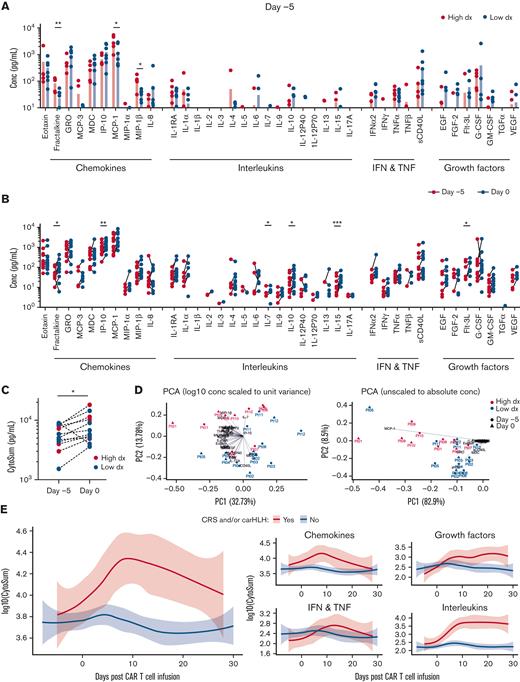
We determined the concentration of 38 plasma cytokines, chemokines, and growth factors by multiplex analysis before and after lymphodepletion and after CAR T-cell infusion (at 2 to 4 hours and 1, 2, 3, and 4 weeks after infusion). Before lymphodepletion, the concentration of fractalkine, MCP-1, and MIP-1β was significantly higher in high disease-burden patients (Figure 2A), with no significant differences observed after lymphodepletion (supplemental Figure 4). Lymphodepletion resulted in a significant increase in individual (fractalkine, IP-10, IL-7, IL-15, and Flt-3L), as well as the sum of all cytokines, chemokines, and growth factors (Figure 2B,C). Low and high disease-burden patients generally clustered into separate groups by principal component analysis both when scaling to unit variance or analyzing absolute concentrations, with segregation in the latter case largely driven by MCP-1, GRO, and IP-10 (Figure 2D). The 4 patients who developed severe CRS or carHLH after CAR T-cell infusion all had high disease burden before infusion and exhibited elevation of total cytokines, chemokines, and growth factors consistently throughout the study observation period (Figure 2E), including cytokines associated with CRS or carHLH (IL-6, IL-1RA, and IL-1) (supplemental Figure 5).
Plasma chemokine, cytokine, and growth factor levels are influenced by disease burden, lymphodepleting chemotherapy, and CRS/ICANS. (A) Day −5 (before lymphodepleting chemotherapy), t test, ∗P < .05 and ∗∗P < .01. (B and C) Change in plasma chemokine, cytokine, and growth factor levels before and after lymphodepleting chemotherapy. (B) Individual chemokine, cytokine, and growth factor levels, t test, ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. (C) Sum of all chemokine, cytokine, and growth factor levels (CytoSum), t test, ∗P < .05. (D) Principal component analysis. (E) Loess plots comparing plasma chemokine, cytokine, and growth factor levels in patients with or without CRS and/or carHLH.
Plasma chemokine, cytokine, and growth factor levels are influenced by disease burden, lymphodepleting chemotherapy, and CRS/ICANS. (A) Day −5 (before lymphodepleting chemotherapy), t test, ∗P < .05 and ∗∗P < .01. (B and C) Change in plasma chemokine, cytokine, and growth factor levels before and after lymphodepleting chemotherapy. (B) Individual chemokine, cytokine, and growth factor levels, t test, ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. (C) Sum of all chemokine, cytokine, and growth factor levels (CytoSum), t test, ∗P < .05. (D) Principal component analysis. (E) Loess plots comparing plasma chemokine, cytokine, and growth factor levels in patients with or without CRS and/or carHLH.
CAR T cells expanded in all patients, except for 1 patient with NR (patient 7), as judged by qPCR with no difference between low and high disease burden or dose level (Figure 3A,B; supplemental Figure 6). Peak expansion occurred between 1 and 2 weeks after infusion with subsequent CAR T-cell contraction. We observed no significant difference in peak expansion or area under the curve analysis for the first 4 weeks after CAR T-cell infusion when stratifying by disease burden (Figure 3C,E) or dose level (Figure 3D,F). At 4 weeks after infusion, we detected CD19-CAR T cells in all evaluated BM samples (n = 11) and in 9/10 CSF samples. In the CSF, there was significant enrichment of CD19-CAR T cells as judged by a high CD19-CAR/TRAC locus qPCR ratio (Figure 3G,H). Data on long-term CAR T-cell persistence (qPCR) is limited since 5/9 patients who achieved an MRDneg CR proceeded to consolidative AlloHCT (supplemental Figure 6). At peak expansion, we performed the same phenotypic analysis that was performed on the CAR T-cell product. Compared with the infused product, there was a significant increase in the percentage of CD8+ CD19-CAR T cells, consistent with a preferential expansion of CD8+ vs CD4+ CD19-CAR T cells (Figure 4A). CD19-CAR T-cell subset analysis revealed predominantly effector memory T-cell subsets in both CD4+ and CD8+ CAR T-cell compartments (Figure 4B). Within the effector memory compartment, there was a significant decrease in transitional memory CD8+ CAR T cells (Figure 4C). In regard to exhaustion markers, the frequency of PD1+/TIM3+ cells was significantly increased in the CD8+ CAR T-cell compartment in comparison with the infused CD8+ CAR T cells, with no significant change in CD39 expression (Figure 4D, supplemental Figure 7A). Comparing phenotype and PD1, TIM3, and CD39 expression at peak expansion between CAR-positive and CAR-negative T-cells, we observed a significantly higher percentage of effector memory T cells within the CAR-positive T-cell compartment (Figure 4E,F; supplemental Figure 7B). In addition, the frequency of PD1+/TIM3+ cells within the CAR-positive T-cell compartment was increased (Figure 4G). Subgroup analyses based on low and high disease burden for patients who had achieved a CR did not reveal significant differences in the performed phenotypic analysis (data not shown).
Expansion and persistence of infused CD19-CAR T cells does not correlate with disease burden or cell dose. CAR T-cell expansion was determined by qPCR for the CD19-CAR transgene. CAR expansion based on (A) high (≥40% morphologic blasts in the BM) and low disease burden or (B) cell dose (DL1, dose level 1 [1 × 106 CAR+ T cells per kg]; DL2, dose level 2, 3 × 106 CAR+ T cells per kg]). (C and D) Peak expansion is stratified for disease burden or cell dose. (E and F) The area under the curve analysis for the first 28 days after the infusion was stratified for disease burden or cell dose. (G and H) The ratio of qPCR for the CD19-CAR transgene and the TRAC locus at 4 weeks after infusion in PB, BM, and CSF stratified for disease burden or cell dose. For patient 12 (low disease burden), qPCR data were only available until day 7 after CAR T cell infusion due to coronavirus disease 2019 (COVID-19); qPCR data are shown in panels (A and B) but not included in the analysis; t test. ns, not significant. ∗∗P < .01 and ∗∗∗P < .001.
Expansion and persistence of infused CD19-CAR T cells does not correlate with disease burden or cell dose. CAR T-cell expansion was determined by qPCR for the CD19-CAR transgene. CAR expansion based on (A) high (≥40% morphologic blasts in the BM) and low disease burden or (B) cell dose (DL1, dose level 1 [1 × 106 CAR+ T cells per kg]; DL2, dose level 2, 3 × 106 CAR+ T cells per kg]). (C and D) Peak expansion is stratified for disease burden or cell dose. (E and F) The area under the curve analysis for the first 28 days after the infusion was stratified for disease burden or cell dose. (G and H) The ratio of qPCR for the CD19-CAR transgene and the TRAC locus at 4 weeks after infusion in PB, BM, and CSF stratified for disease burden or cell dose. For patient 12 (low disease burden), qPCR data were only available until day 7 after CAR T cell infusion due to coronavirus disease 2019 (COVID-19); qPCR data are shown in panels (A and B) but not included in the analysis; t test. ns, not significant. ∗∗P < .01 and ∗∗∗P < .001.
CD8-positive CD19-CAR T cells preferentially expand in vivo and have an effector memory phenotype. The phenotype of CAR-positive and CAR-negative T cells was determined by flow cytometry in the infused cell product and at peak expansion (week [wk] 1 or 2 after infusion); n = 9 (patients 2 to 6, and 8 to 11). (A-D) Comparison of CAR-positive T-cell phenotype in cell product and at peak expansion (paired t test for CD4+/CAR+ GMP vs Peak and CD8+/CAR− GMP vs Peak. ns: not significant. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .01). (A) Percent CD4-positive and CD8-positive CAR T cells. (B) Percent naïve (TN), central memory (TCM), effector memory (TEM), and effector memory T cells reexpressing CD45RA (TEMRA) T cells. (C) Percent of transitional memory (Ttm) T cells. (D) Percent of PD1+ and TIM3+ CAR-positive T cells. (E-G) Comparison of CAR-positive and CAR-negative T-cell phenotype at peak expansion (paired t test for CD4+/CAR+ vs CD4+/CAR− and CD8+/CAR+ vs CD8+/CAR−. ∗P < .05 and ∗∗P < .01): (E) TN, TCM, TEM, and effector memory T cells reexpressing CD45RA (TEMRA) T cells. (F) Percent of Ttm T cells. (G) Percent PD1- and/or TIM3-positive T cells.
CD8-positive CD19-CAR T cells preferentially expand in vivo and have an effector memory phenotype. The phenotype of CAR-positive and CAR-negative T cells was determined by flow cytometry in the infused cell product and at peak expansion (week [wk] 1 or 2 after infusion); n = 9 (patients 2 to 6, and 8 to 11). (A-D) Comparison of CAR-positive T-cell phenotype in cell product and at peak expansion (paired t test for CD4+/CAR+ GMP vs Peak and CD8+/CAR− GMP vs Peak. ns: not significant. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .01). (A) Percent CD4-positive and CD8-positive CAR T cells. (B) Percent naïve (TN), central memory (TCM), effector memory (TEM), and effector memory T cells reexpressing CD45RA (TEMRA) T cells. (C) Percent of transitional memory (Ttm) T cells. (D) Percent of PD1+ and TIM3+ CAR-positive T cells. (E-G) Comparison of CAR-positive and CAR-negative T-cell phenotype at peak expansion (paired t test for CD4+/CAR+ vs CD4+/CAR− and CD8+/CAR+ vs CD8+/CAR−. ∗P < .05 and ∗∗P < .01): (E) TN, TCM, TEM, and effector memory T cells reexpressing CD45RA (TEMRA) T cells. (F) Percent of Ttm T cells. (G) Percent PD1- and/or TIM3-positive T cells.
Discussion
In this phase 1 study of CD19-CAR T-cells with a 41BBζ signaling domain, we observed a high initial response rate, with 9/12 of patients (75%) achieving an MRDneg CR in the BM and 8/12 a CR based on the NCCN pediatric B-ALL response criteria. Notably, approximately half of this patient cohort would not have met inclusion criteria on the tisagenlecleucel registration trial,9 including (1) age ≤3 years old, (2) prior treatment with CD19-directed therapies, and/or (3) low preinfusion disease burden in the BM. High disease burden (≥40% morphologic blasts) before CAR T-cell infusion correlated with increased side effects and lower response rate, but not with CD19-CAR T-cell expansion. Additionally, cell dose did not impact CAR T-cell expansion. While patients treated at DL2 did experience more side effects, this dose level was also enriched for patients with a high disease burden. Furthermore, our phenotypic analysis revealed that CD8+ CAR T cells had a proliferative advantage over CD4+ CAR T cells, and that expanded CAR T cells had an effector memory phenotype with evidence of antigen-driven differentiation.
The incidence of high grade (grade 3 to 4) CRS (16.6%) or NTX (8.3%) was lower than reported in the pivotal trial of tisagenlecleucel in a similar patient population,9 but is in line with recently reported data using the same product.7 As highlighted by other investigators,23 our use of the ASTCT consensus grading for CRS and NTX may partially account for such differences. Additionally, consistent with previous reports of early intervention for CRS,8,24 3 of our 4 patients with low-grade CRS received therapy with tocilizumab, and none had symptom progression. Furthermore, carHLH is increasingly described in the literature as a complication of CD19-CAR T-cell therapy, with varying diagnostic criteria employed.21,25-29 In our cohort, 2 patients developed carHLH, and their detailed clinical course is described elsewhere.22 Further studies are needed to inform upon uniform diagnostic criteria and treatment strategies.
Our experience highlights the critically important question regarding the role of consolidative HCT after CD19-CAR T-cell therapy. Similar to others,16 in our cohort, patients with no prior history of AlloHCT who underwent a planned consolidative AlloHCT had improved leukemia-free survival. Consolidative AlloHCT was well tolerated with no treatment-related mortality within the initial 100 days after HCT. Developing an algorithm to decide which patients would benefit from a consolidative AlloHCT after successful CD19-CAR T-cell therapy remains a major challenge. Pretreatment antigen burden is increasingly recognized as an important predictor of both short and long-term response after CD19-CAR T-cell therapy.3,8,14 We also found that a high disease burden correlated with a higher risk of early treatment failure. However, the definition of “high disease burden” is not consistent across studies,6,8,10,30-32 and thus needs to be interpreted in the context of each study.
NGS testing is a promising approach to assess the risk of relapse after CAR T-cell therapy, particularly when used in conjunction with close monitoring for loss of BCA.12,13,33 In our study, 7 patients with MRDneg CR had available NGS testing, and all were considered negative per study definitions (<10 clones per million). While 4 of these patients proceeded to a planned consolidative AlloHCT, recent data suggest that pediatric and AYA patients with B-ALL who achieve an NGS-negative MRDneg CR and have persistent BCA after CD19-CAR T-cell therapy have excellent outcomes33 and may require only close monitoring. Clearly, future prospective clinical studies are needed to further inform on clinically relevant definitions of MRD negativity and determine the best management of these patients.
Before lymphodepleting chemotherapy, patients with a high disease burden had significantly higher levels of fractalkine, MCP-1, and MIP-1β. Elevated levels of MCP-1 have been reported in pediatric and adult patients with B-ALL,34,35 whereas no data are currently available for fractalkine and MIP-1β. Given the role of these chemokines in inflammation and their impact on numerous immune cells, including NK cells, monocytes, and macrophages, their preinfusion elevations suggest a proinflammatory state. However, we observed no correlation with the development of CRS, ICANS, and/or carHLH. Clearly, larger studies are needed to explore if elevated levels of fractalkine, MCP-1, and/or MIP-1β before infusion correlate with side effects after CAR T-cell infusion. After lymphodepleting chemotherapy, we saw a significant increase in circulating chemokines and cytokines, including IL-15, which is consistent with reports in adult patients who received lymphodepleting chemotherapy before the adoptive transfer of CD19-CAR T cells.36 We used a standard qPCR assay to track CD19-CAR T cells, which we combined with qPCR analysis for the TRAC locus. Based on our analysis, we found a striking enrichment of CD19-CAR T cells within the CSF, highlighting their ability to traffic to the CNS even in the absence of active disease.
The investigated CD19.41BBζ-CAR T-cell product had a high percentage of CD4+ CAR T cells with an effector and central memory phenotype. This is in contrast to 2 other studies, which reported that CAR T-cell products generated with IL-7 and IL-15, or no cytokines, resulted in T-cell products that contain a significant number of naïve-like T cells.37,38 Both of these studies used unselected peripheral blood mononuclear cells as starting material, whereas we used CD4/CD8-selected T cells from leukapheresis products. A recent integrative and single-cell RNA sequencing (scRNA-seq) analysis of pediatric autologous leukapheresis products used to generate CD19-CAR T-cell products highlighted the role of transcriptional networks driven by TCF7 in promoting CAR T-cell persistence and the negative impact of IRF7-regulated networks.39 Additional studies are needed, and we are conducting scRNA-seq and methylome analyses40 of CD19-CAR T-cell products and sorted CAR T cells after infusion to gain additional insights. Comparing CAR-negative and CAR-positive T-cell populations within the infused cell product revealed significant phenotypic differences, including a higher frequency of CD4+ T cells in the CAR-positive compartment and a higher frequency of PD1+/TIM3+ T cells. This finding is most likely explained by baseline signaling (aka tonic signaling)41 of the CD19.41BBζ-CAR within CD4+ T cells. Recent studies highlighted that CAR signaling can be blocked with the abl/src family tyrosine kinase inhibitor dasatinib during CAR T-cell production, improving their effector function.42-44
After infusion, CD8+ CAR T cells expanded to a greater extent than CD4+ CAR T cells. This finding is consistent with a previous study in which a CD19-CAR T-cell product with a defined CD4/CD8 ratio was infused into adults with ALL,45 highlighting that this observation does not depend on the separate manufacturing of CD4 and CD8 CD19-CAR T cells. Both CAR T-cell populations at peak expansion had a predominantly effector memory phenotype. Similar results have been reported for CD19.CD28ζ-CAR T cells in pediatric patients with B-ALL.5 Of note, without performing lymph node biopsies, it is impossible to assess if the decrease of central memory CAR T cells at the peak of expansion is due to differentiation or merely reflects the trafficking of central memory T cells to lymphoid tissues.46 At peak expansion, we observed evidence of antigen-driven differentiation consistent with a loss of developmental potential, with a significantly greater percentage of CAR-positive T cells expressing PD1 in comparison with the infused CAR T-cell product. In addition, at peak expansion, the frequency of PD1+/TIM3+ T cells was significantly higher in the CAR-positive vs CAR-negative compartment. A recent study demonstrated that murine and human effector memory T-cell populations are heterogenous,47 highlighting the need to perform additional studies to further resolve the functional state of infused CAR T cells. These necessary studies include scRNA-seq and whole-genome bisulfite sequencing,48-50 which are currently in progress for our patient cohort. In particular, scRNA-seq analysis of CD19.41BBζ-CAR T cells in adult patients suggests that at early time points after infusion, CD19-CAR T cells have a transcriptional profile that is consistent with an effector response and lack the coexpression of PD1 and TIM3.51
In summary, our phase 1 study shows a favorable toxicity and efficacy profile of our institutional autologous CD19.41BBζ-CAR T-cell product. Our correlative studies highlight the role of disease burden on the outcome and the preferential expansion of CD8+ CAR T cells after infusion. Likewise, we demonstrate antigen-driven CAR T-cell differentiation, providing impetus to evaluate approaches to modulate the effector function of CD19-CAR T cells before and/or after infusion.
Acknowledgments
The authors thank the referring physicians, the staff of the clinical research office for assisting with conducting the clinical study, the staff of the Human Applications Laboratory and GMP facility for assisting in CAR T-cell production and analysis, and the staff of the Department of Bone Marrow Transplantation and Cellular Therapy for their excellent patient care. The authors also thank the patients who participated in this study and their caregivers, who entrusted the care of their children to us.
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute grants P30CA021765, R01CA237311 (B.Y.), the American Society of Transplantation and Cellular Therapy (A.C.T.), the American Society of Hematology (A.C.T. and A. Sharma), the Key for a Cure Foundation (B.Y. and P.G.T.), the Mark Foundation ASPIRE Award (P.G.T.), and the American Lebanese Syrian Associated Charities. Part of the laboratory studies were performed by the Center for Translational Immunology and Immunotherapy (CeTI2), which is supported by St. Jude. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: M.P.V., C.W., W.Z., S.Z., T.L., T.L.G., M.M., and S.G. performed preclinical work related to clinical study development; A.C.T., B.M.T., and S.G. conceived the clinical study; A.C.T., A.Q., E.M., C.H., R.M., A. Sharma, A. Suliman, A. Srinivasan, M.P.V., E.A.O., S.E.K., H.I., A.B., C.P., B.M.T., and S.G. conducted the clinical study; J.M., D.L., J.C.C., C.W., S.A., W.Z., S.Z., S.S., M.T., D.C., S.L.P., C.Z., and T.L. performed laboratory tests and/or cell processing of study samples; J.C.C., S.H., C.C., and S.G. performed statistical analysis; A.C.T., J.M., D.L., J.C.C., C.W., Y.L., P.G.T., B.Y., T.L., B.M.T., and S.G. analyzed and interpreted data; A.C.T., B.M.T., and S.G. wrote the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: A. Sharma consults/consulted for Spotlight Therapeutics and Medexus Inc. B.Y. consults/consulted for ElevateBio. S.G. consults/consulted for TESSA Therapeutics, TIDAL, Catamaran, and Novartis. S.G. is a Data Safety and Monitoring Board (DSMB) member of Immatics. M.P.V. is a member of the Medical Advisory Board for the Rally Foundation. M.P.V., B.Y., C.Z., J.C.C., P.G.T., and S.G. have patents/patent applications in he fields of T-cell and/or gene therapy for cancer. The remaining authors declare no competing financial interests.
Correspondence: Aimee C. Talleur, Department of Bone Marrow Transplantation & Cellular Therapy, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS1130, Memphis, TN; e-mail: aimee.talleur@stjude.org; and Stephen Gottschalk, Department of Bone Marrow Transplantation & Cellular Therapy, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS321, Memphis, TN; e-mail: stephen.gottschalk@stjude.org.
References
Author notes
For data sharing, contact the corresponding authors at aimee.talleur@stjude.org or stephen.gottschalk@stjude.org.
The full-text version of this article contains a data supplement.


![Expansion and persistence of infused CD19-CAR T cells does not correlate with disease burden or cell dose. CAR T-cell expansion was determined by qPCR for the CD19-CAR transgene. CAR expansion based on (A) high (≥40% morphologic blasts in the BM) and low disease burden or (B) cell dose (DL1, dose level 1 [1 × 106 CAR+ T cells per kg]; DL2, dose level 2, 3 × 106 CAR+ T cells per kg]). (C and D) Peak expansion is stratified for disease burden or cell dose. (E and F) The area under the curve analysis for the first 28 days after the infusion was stratified for disease burden or cell dose. (G and H) The ratio of qPCR for the CD19-CAR transgene and the TRAC locus at 4 weeks after infusion in PB, BM, and CSF stratified for disease burden or cell dose. For patient 12 (low disease burden), qPCR data were only available until day 7 after CAR T cell infusion due to coronavirus disease 2019 (COVID-19); qPCR data are shown in panels (A and B) but not included in the analysis; t test. ns, not significant. ∗∗P < .01 and ∗∗∗P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/21/10.1182_bloodadvances.2021006293/3/m_blooda_adv-2021-006293-gr3.jpeg?Expires=1768460332&Signature=N1lvn1mva~uK5I3BEgd9a6NdyinzmlptMS7Ev4gdD8vV4e24kYNHCbbm6SQpu8wGCQNi3RqYNOh17w0g9Qv8hS7js7VN~WFr2x0r7xNEcwfw2jBDdoOeJdorxO0LK4WGOrNjV6MQghLTtQ-HAOvxJ3oGeR1PA9Pt7q871rsjH~fkyxiPPoG5T7uoXkuLREENV2PKgAQ1Sh5z1GjsqDvVXIJOgxblBpYwU2DpPN1N~bGYq2-if8cQoiUzyJweTlOD4VD6x1r5J9FoADmEP8f6ymCGOafJrpAIGj4hiBOJ~FAio~JMPCRSAB8G6OLf1xCOuc~OhX2okYbSPMXSRx-pKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD8-positive CD19-CAR T cells preferentially expand in vivo and have an effector memory phenotype. The phenotype of CAR-positive and CAR-negative T cells was determined by flow cytometry in the infused cell product and at peak expansion (week [wk] 1 or 2 after infusion); n = 9 (patients 2 to 6, and 8 to 11). (A-D) Comparison of CAR-positive T-cell phenotype in cell product and at peak expansion (paired t test for CD4+/CAR+ GMP vs Peak and CD8+/CAR− GMP vs Peak. ns: not significant. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .01). (A) Percent CD4-positive and CD8-positive CAR T cells. (B) Percent naïve (TN), central memory (TCM), effector memory (TEM), and effector memory T cells reexpressing CD45RA (TEMRA) T cells. (C) Percent of transitional memory (Ttm) T cells. (D) Percent of PD1+ and TIM3+ CAR-positive T cells. (E-G) Comparison of CAR-positive and CAR-negative T-cell phenotype at peak expansion (paired t test for CD4+/CAR+ vs CD4+/CAR− and CD8+/CAR+ vs CD8+/CAR−. ∗P < .05 and ∗∗P < .01): (E) TN, TCM, TEM, and effector memory T cells reexpressing CD45RA (TEMRA) T cells. (F) Percent of Ttm T cells. (G) Percent PD1- and/or TIM3-positive T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/21/10.1182_bloodadvances.2021006293/3/m_blooda_adv-2021-006293-gr4.jpeg?Expires=1768460332&Signature=1j6MehsSAGfEIP0YAw1UuxaL6vULRgA-sU4z4MId4PyUhDiw2VBcH31GXL2LgwOubbXW3t8IPpfbtJJWzaKEQPp6TxZ6lB84NZsHLbzEOEXoQ7UFf89biQ6GzZLqBumjIkxsRtVSzQVuMUt9vGC3NAzdvpgLGsSrhgk5zG0OmOjgrZ050r8cHWxJJP1dp8Fjdvx-UVuGS1cmoZ0lGFG9LG6kE7aBXoUzLrFrNu~1w29tvsexXGM9rRBQcJyH4XnMH4qrqFaK8LrjZPUZP1XwH7DIHSOgKRqJfh0VRRls77U8uajQeFSPgZvc18sFtKhpEeNcuJoWyeRwmFiu3~bltQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)