Key Points
Participants who undergo gene therapy for ADA-SCID may harbor underlying germline alterations that increase risk of malignancy.
Participants who undergo gene therapy for ADA-SCID may have decreased telomere length after busulfan conditioning.
Abstract
Autologous stem cell transplant with gene therapy (ASCT-GT) provides curative therapy while reducing pretransplant immune-suppressive conditioning and eliminating posttransplant immune suppression. Clonal hematopoiesis of indeterminate potential (CHIP)–associated mutations increase and telomere lengths (TLs) shorten with natural aging and DNA damaging processes. It is possible that, if CHIP is present before ASCT-GT or mutagenesis occurs after busulfan exposure, the hematopoietic stem cells carrying these somatic variants may survive the conditioning chemotherapy and have a selective reconstitution advantage, increasing the risk of hematologic malignancy and overall mortality. Seventy-four peripheral blood samples (ranging from baseline to 120 months after ASCT-GT) from 10 pediatric participants who underwent ASCT-GT for adenosine deaminase–deficient severe combined immune deficiency (ADA-SCID) after reduced-intensity conditioning with busulfan and 16 healthy controls were analyzed for TL and CHIP. One participant had a significant decrease in TL. There were no CHIP-associated mutations identified by the next-generation sequencing in any of the ADA-SCID participants. This suggests that further studies are needed to determine the utility of germline analyses in revealing the underlying genetic risk of malignancy in participants who undergo gene therapy. Although these results are promising, larger scale studies are needed to corroborate the effect of ASCT-GT on TL and CHIP. This trial was registered at www.clinicaltrials.gov as #NCT00794508.
Introduction
Gene therapy (GT) for severe combined immune deficiency (SCID) uses autologous hematopoietic stem cell transplant (ASCT), in which a participant’s stem cells are genetically corrected ex vivo before being reinfused into the participant. The key potential advantage of ASCT with GT (ASCT-GT), compared with allogeneic stem cell transplant, is that it may have a better safety profile because it eliminates pretransplant immune-suppressive conditioning and posttransplant immune suppression and eliminates the risk of graft-versus-host disease. Since the mid-2000s, most ASCT-GT studies have used self-inactivating lentiviral vectors that lack the potent long terminal repeat enhancers of γ-retroviral vectors (gRV) and overall have an excellent safety record.1 However, concerns regarding potential development of myelodysplastic syndrome (MDS) and leukemia after GT have resurfaced recently because of 2 cases of MDS and acute leukemia after ASCT-GT with lentiviral vectors for sickle cell disease.2,3
ASCT-GT is typically preceded by cytoreductive conditioning with busulfan, an alkylating agent, and potential mutagen. When reduced-intensity conditioning (RIC) is used with lower doses of busulfan, recipient hematopoietic stem cells are not completely eradicated, and that may lead to clonal expansion in 2 ways: (1) preexisting clones with somatic mutations may be resistant to busulfan and therefore survive to expand, or (2) busulfan may directly cause somatic mutations in cells that remain after RIC. With the use of ASCT-GT in young participants, there may be a risk of developing clonal hematopoiesis of indeterminate potential (CHIP) decades earlier than would be expected as a result of physiologic aging.4,5
Somatic mutations in leukemia-associated genes have been found to drive CHIP, and many studies to date have focused on establishing risk of hematologic malignancy in the presence of CHIP.6-8 Participants who have CHIP before ASCT have increasing variant allele frequencies after transplant.9 Hematopoietic stem cells carrying somatic variants may survive conditioning chemotherapy and have a subsequent selective reconstitution advantage, increasing the risk of hematologic malignancy and overall mortality.
Another established marker of cellular aging is telomere length (TL). Telomeres protect the DNA from degradation and damage by providing a protective cap of repetitive DNA-protein components on the ends of chromosomes.10,11 It is recognized that these telomere caps shorten with each cell division and lead to senescence. TL has been shown to predict incidence and severity of cardiovascular disease and cancer and risk of mortality risk.11,12
This study was conducted to evaluate the presence of clonal hematopoiesis and telomere shortening in circulating granulocytes in pediatric participants with adenosine deaminase deficiency and severe combined immune deficiency (ADA-SCID) who underwent ASCT-GT with the myeloproliferative sarcoma virus enhancer, negative control region deleted, dl587rev primer-binding site substituted-ADA gRV after RIC with busulfan.13,14 To our knowledge, this is the first study to address CHIP and TL in the pediatric population and was undertaken because of the recent and growing concerns for MDS and leukemia in the post-GT patient population. Samples from participants were evaluated at various time points, ranging from before ASCT-GT to up to 10 years after treatment for CHIP mutations and TLs.
Methods
All study participants provided informed consent for the protocol, which was approved by the UCLA Institutional Review Board.
Samples analyzed
Seventy-four peripheral blood samples from 10 pediatric participants who underwent ASCT-GT for ADA-SCID after RIC with busulfan and 16 healthy controls were analyzed. Five participants with ADA-SCID had baseline samples available from before busulfan treatment. Participant samples were collected up to 120 months after GT (mean, 44 months). Control samples were obtained through the California Center for Rare Diseases and were from subjects 7 to 10 years of age with no hematologic abnormalities.
Sample processing
Ficoll hypaque (GE Healthcare, Chicago, IL) was used to separate the peripheral blood mononuclear cells from heparinized peripheral blood samples. The granulocyte fraction was depleted of lymphocytes with immunomagnetic beads to CD3, CD19, and CD56 (Miltenyi Biotech, Auburn, CA). Cell pellets were stored at −80°C until genomic DNA was isolated from cells by the PureLink Genomic DNA Mini Kit (Invitrogen Corp, Waltham, MA).
Next-generation sequencing methods and analysis pipeline
The DNA samples were evaluated for somatic mutations in the CLIA (Clinical Laboratory Improvement Amendments) certified UCLA molecular diagnostics laboratory. Targeted amplification of multiple coding regions of 54 genes recurrently mutated in hematologic malignancies was performed with the Illumina TruSight Myeloid Sequencing Panel (Illumina, Inc, San Diego, CA). After next-generation sequencing on Illumina Miseq, raw sequencing data were processed with the Illumina Real-Time Analysis Pipeline to identify and annotate sequence alterations. The resultant bases were aligned to targeted regions (GRCh37/hg19). Alterations were filtered and reviewed manually to determine pathogenicity and reportability. The established limit of detection for this methodology is ∼5% allele frequency (mutant reads/total reads) for single-nucleotide variants and 10% for insertion/deletion (indel) variants.
TL testing method
TLs were measured by using real-time quantitative polymerase chain reaction on the Bio-Rad iCycler thermal cycler (Bio-Rad Laboratories, Irvine, CA), as previously described.15 This method amplifies the DNA sequence of the telomere and a single-copy gene. In brief, samples were diluted to 5 ng/μL and plated in triplicate alongside a standard curve with concentrations ranging from 1 to 0.94 ng. After amplification, cycle threshold values were converted to nanograms of DNA by using the standard curve. TL is expressed as the T/S ratio of estimated concentration of telomere DNA (T) repeats divided by single-copy (S) hemoglobin gene. Linear regression analyses were performed and, for participants who had baseline samples available, analyses were constrained through the baseline time point. All intraassay and interassay coefficient of variance were <1.05% (mean, 0.68%).
Results and discussion
Eight participants with ADA-SCID were evaluated for CHIP at multiple time points (baseline to 120 months after GT). Sequencing mean coverage was 4574×. No somatic mutations in coding regions of the 54 genes covered by the panel were identified in any of the ADA-SCID participants or 5 controls at any time point (Table 1). A KIT p.N567S (NM_000222.2) variant of uncertain significance (ClinVar 458888) was identified in a pair of ADA-SCID siblings (participants 402 and 404) and a TP53 p.E339K (NM_000546.5) likely benign variant (ClinVar 142846) in ADA-SCID participant 410, with allele frequencies near 50%, suggestive of heterozygous germline origin and reported interpretations of uncertain significance and most likely benign, respectively, in ClinVar (Table 1).
Results from the TruSight Myeloid Sequencing Panel for each participant at each time point evaluated after GT
| Patient ID . | Age at GT (mo) . | Time point after GT (mo) . | Variant detected (pos/neg) . | Variant/description . | VAF, % . |
|---|---|---|---|---|---|
| 401 | 184 | 0∗ | Negative | — | — |
| 12 | Negative | — | — | ||
| 48 | Negative | — | — | ||
| 72 | Negative | — | — | ||
| 402† | 4 | 0∗ | Positive | KIT- VUS c.1700A>G (p.N567S) | 50 |
| 120 | Positive | KIT- VUS c.1700A>G (p.N567S) | 50 | ||
| 404† | 3 | 0∗ | Positive | KIT- VUS c.1700A>G (p.N567S) | 49 |
| 96 | Positive | KIT- VUS c.1700A>G (p.N567S) | 49 | ||
| 405 | 8 | 0∗ | Negative | — | — |
| 48 | Negative | — | — | ||
| 96 | Negative | — | — | ||
| 407 | 14 | 60 | Negative | — | — |
| 408 | 3 | 48 | Negative | — | — |
| 409 | 20 | 0∗ | Negative | — | — |
| 84 | Negative | — | — | ||
| 410 | 3 | 36 | Positive | TP53- LB c.1015G>A (p.E339K) | 48 |
| Controls ×5 | N/A | N/A | Negative | — | — |
| Patient ID . | Age at GT (mo) . | Time point after GT (mo) . | Variant detected (pos/neg) . | Variant/description . | VAF, % . |
|---|---|---|---|---|---|
| 401 | 184 | 0∗ | Negative | — | — |
| 12 | Negative | — | — | ||
| 48 | Negative | — | — | ||
| 72 | Negative | — | — | ||
| 402† | 4 | 0∗ | Positive | KIT- VUS c.1700A>G (p.N567S) | 50 |
| 120 | Positive | KIT- VUS c.1700A>G (p.N567S) | 50 | ||
| 404† | 3 | 0∗ | Positive | KIT- VUS c.1700A>G (p.N567S) | 49 |
| 96 | Positive | KIT- VUS c.1700A>G (p.N567S) | 49 | ||
| 405 | 8 | 0∗ | Negative | — | — |
| 48 | Negative | — | — | ||
| 96 | Negative | — | — | ||
| 407 | 14 | 60 | Negative | — | — |
| 408 | 3 | 48 | Negative | — | — |
| 409 | 20 | 0∗ | Negative | — | — |
| 84 | Negative | — | — | ||
| 410 | 3 | 36 | Positive | TP53- LB c.1015G>A (p.E339K) | 48 |
| Controls ×5 | N/A | N/A | Negative | — | — |
LB, likely benign; VUS, variant of uncertain significance; N/A, not applicable.
0, baseline sample before exposure to busulfan.
Sibling donor.
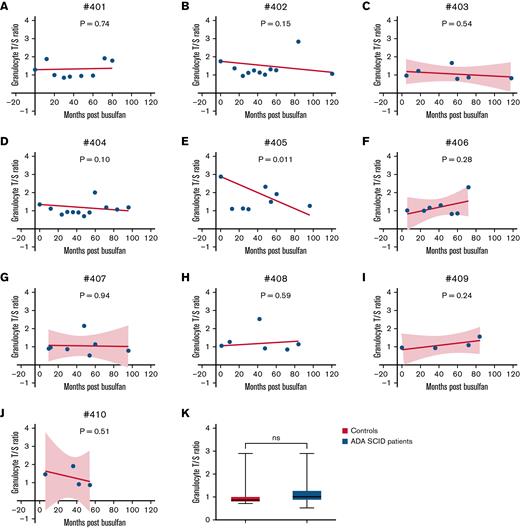
All 10 participants and 16 controls had TL T/S ratios analyzed from granulocytes (Figure 1). Granulocytes were selected as the cell population for this study because they are most reflective of current hematopoietic stem cells and therefore are likely to reflect the cells engrafted after busulfan conditioning. Furthermore, granulocytes have been shown to have telomere shortening rates similar to those of T cells.16 Participant 405 had a significant decrease in TL when linear regression was forced through the baseline sample T/S ratio (P = .011). Shortening of TLs has not been reported in patients who undergo GT after busulfan exposure. No significant differences in TL were detected when overall baseline samples were compared to samples at 12 to 36 months and 80 to 120 months after busulfan administration (P = .5 and P = .54, respectively). Overall, no significant differences in mean TL were detected when ADA-SCID participant samples were compared to control samples (Figure 1K).
Linear regression analysis of TL measurements. Relative TL (T/S ratio), an estimated concentration of the telomere DNA (T) repeats divided by the single-copy (S) hemoglobin gene in participant 401, forced through baseline time point (ranging from baseline to 80 months after GT) (A); participant 402 forced through baseline time point (ranging from baseline to 120 months after GT) (B); participant 403 (ranging from 5 to 118 months after GT) (C); participant 404 forced through baseline time point (ranging from baseline to 96 months after GT) (D); participant 405 intercept forced through baseline time point (ranging from baseline to 96 months after GT) (E); participant 406 (ranging from 6 to 72 months after GT) (F); participant 407 (ranging from 10 to 96 months after GT) (G); participant 408 forced through baseline time point (ranging from 1 to 84 months after GT) (H); participant 409 (ranging from baseline to 84 months after GT) (I); participant 410 (ranging from 6 to 54 months after GT) (J); and unpaired t test of TLs in 16 controls and all time points (ranging from baseline to 120 months after GT) in patients with ADA-SCID (K). Dotted lines represent 95% confidence bands of the best-fit line (only for those patients without a forced baseline intercept). P-values calculated to represent significant slope deviation from 0.
Linear regression analysis of TL measurements. Relative TL (T/S ratio), an estimated concentration of the telomere DNA (T) repeats divided by the single-copy (S) hemoglobin gene in participant 401, forced through baseline time point (ranging from baseline to 80 months after GT) (A); participant 402 forced through baseline time point (ranging from baseline to 120 months after GT) (B); participant 403 (ranging from 5 to 118 months after GT) (C); participant 404 forced through baseline time point (ranging from baseline to 96 months after GT) (D); participant 405 intercept forced through baseline time point (ranging from baseline to 96 months after GT) (E); participant 406 (ranging from 6 to 72 months after GT) (F); participant 407 (ranging from 10 to 96 months after GT) (G); participant 408 forced through baseline time point (ranging from 1 to 84 months after GT) (H); participant 409 (ranging from baseline to 84 months after GT) (I); participant 410 (ranging from 6 to 54 months after GT) (J); and unpaired t test of TLs in 16 controls and all time points (ranging from baseline to 120 months after GT) in patients with ADA-SCID (K). Dotted lines represent 95% confidence bands of the best-fit line (only for those patients without a forced baseline intercept). P-values calculated to represent significant slope deviation from 0.
Although the participants in this study did not demonstrate detectable CHIP, a likely germline variant of uncertain significance was identified in a gene associated with hematologic malignancy. Further studies are needed to determine the utility of comprehensive germline evaluation before ASCT-GT to fully assess cancer risk. In addition, 1 participant had significant telomere shortening of granulocytes after ASCT-GT, whereas there were no significant differences in the other participants or as an overall group. Additional studies investigating TL in different subpopulations, such as T cells, are necessary to fully understand the effect of ASCT-GT on telomere length and potential cancer risk.
GT remains a curative approach as an alternative to allogeneic bone marrow transplant for those with a genetic blood disorder who do not have a matched sibling or other suitable donor. Further study of the role of CHIP, telomere degradation, and identification of additional predictive biomarkers is warranted to prevent occurrence of hematologic malignancy.
Acknowledgments
The authors thank Stan Nelson and Genecee V. Renteria (California Center for Rare Disease) for assistance and collecting normal control samples.
This work was supported in part by the UCLA Cousins Center for Psychoneuroimmunology and the George F. Solomon Endowment. The CHIP and TL studies were supported by an endowment from the UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research (D.B.K.). The ADA-SCID gene therapy trial was supported by R01 FD003005 from the U.S. Food and Drug Administration, Office of Orphan Products Development (OOPD) (D.B.K.). V.Y.C. is supported by National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) grant K08 HL138305. S.L.W. is supported by NIH NHLBI grant T32 HL086345 training grant in Developmental Hematology.
Authorship
Contribution: D.B.K. and V.Y.C. designed the research; S.L.W., T.D.L., T.T., L.P., B.C.F., and A.D. performed the research; S.W., T.D.L., J.E.C., and V.Y.C. analyzed data; and all authors contributed to writing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vivian Y. Chang, Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail: vchang@mednet.ucla.edu.
References
Author notes
Requests for any data should be sent by e-mail to the corresponding author (vchang@mednet.ucla.edu).