TO THE EDITOR:
Normal hemoglobin (Hb) is responsible for carrying oxygen in the blood. It is composed of heme plus 4 polypeptide globin chains whose chemical structure is genetically controlled. The normal adult hemoglobin molecule (Hb A) consists of a pair of α chains and a pair of β chains.1 The symptoms of sickle cell disease (SCD) were first described in 1670 in Africa.2 In 1910, James Herrick noted, “peculiar, elongated sickle shaped red blood cells (RBCs)” in the blood of an anemic medical student. The sickling phenomenon was demonstrated in vitro by Emeel, who was able to show the sickling cells in the deoxygenated RBC in family members with sickle cell anemia.3 In 1949, Pauling and his team, using electrophoresis techniques, found that hemoglobin from sickle shaped RBC’s had abnormal electrophoretic movement in comparison with other Hb when deoxygenated.4 The sickle cell mutation of the β globin gene (HbS) is inherited in an autosomal recessive fashion. When present in the homozygous state (HbSS), the problems of sickle cell anemia (SCA) manifest.5,6 In the heterozygous state known as sickle cell trait, there are few, if any, clinical consequences under normal physiologic conditions, and patients are normally asymptomatic. SCD can also result from a compound heterozygous state of HbS in combination with other abnormal Hb such as β thalassemia, hemoglobin C, and others.7 Such patients have variable phenotypes that can be as severe as the homozygous HbSS state.5,6 HbS is caused by a single mutation in the β-globin gene, substituting a valine amino acid for glutamic acid at position 6 of the β-chain, making HbS molecules more likely to polymerize under conditions such as hypoxia, dehydration, and acidosis, into the unique sickle shape leading to anemia, vaso-occlusion, adhesion, and vasoconstriction. The net result is frequent painful crises and/or end-organ damage in the form of cerebrovascular disease, retinopathy, acute chest syndrome, skin ulcers, and avascular necrosis, among others. Thus, the management is directed at reducing the risk of sickling by suppressing HbS levels (eg, with hydroxyurea), addressing complications, and providing supportive care as required. Hematopoietic stem cell transplantation may also be offered.8 The prevalence of sickle cell varies significantly between populations. SCD is most common in black populations, particularly in consanguineous marriage. Also, it is common among other countries such as Italy, Greece, Turkey, Saudi Arabia, India, Pakistan, Bangladesh, China, and Cyprus.5,9,10 Several studies discussed health care costs; as an example, 1 study suggested that the average total cost of care per patient-month was $1946 ± $2889, with substantial variation across age groups peaking at $2853 ± $4352) per patient-month for the 30- to 39-year age group compared with just $892 ± $2058) per patient-month for patients at birth through age 9 years.11
One complication of SCD is a substantially increased risk of venous thromboembolism (VTE), which in turn increases the risk of mortality.12 RBCs contribute to both hemostasis and thrombosis and play a key role in VTE and clot contraction. Furthermore, hemolytic events contribute to thrombosis, first through the production of free hemoglobin, which ultimately leads to damage to the endothelium through oxygen radicals when it interacts with nitric oxide, as well as induction of endothelial tissue factor expression.13 Moreover Pakbaz et al14 noticed that SCD alters all the components of hemostasis predisposing to VTE. These changes include activation of the coagulation cascade during both clinically normal states and vaso-occlusive crises, as evidenced by markers of increased thrombin generation including lower levels of factors V, VII, and VIIa, as well as increased D-dimer. They also include reduction in natural anticoagulant levels, such as protein C, protein S, and antithrombin III, along with impaired fibrinolysis characterized by increased levels of plasminogen activator inhibitor and plasmin-antiplasmin complexes, and finally platelet abnormalities leading to decreased survival and expanded consumption. All 3 parts of Virchow’s triad (stasis of blood flow, hypercoagulability, and vascular wall injury) have been implicated in the pathogenesis of venous thrombosis among patients with SCD.15 First, RBCs in patients with SCD show increased adherence to the vessel endothelium, which leads to stasis of venous flow and subsequently predisposes to the formation of a venous thrombus. Second, as described above, there is a procoagulant state with an increase in tissue factor expression. Third, free heme, released during intravascular hemolysis in SCD, can damage the vessel endothelium through different mechanisms, primarily through induction of oxidative stress by the formation of oxygen free radicals.15,16
Oral anticoagulants are commonly used for the treatment of patients with a confirmed diagnosis of VTE, including deep vein thrombosis (DVT) and pulmonary embolism (PE), and for stroke prevention among patients with atrial fibrillation who are at high risk of stroke.17,18 For several decades, vitamin K antagonists (VKAs), primarily warfarin, were the only available oral anticoagulants, and hence the experience of using them is significant. However, VKA use has drawbacks including a narrow therapeutic window that warrants frequent monitoring of the international normalized ratio, drug interactions with numerous medications, and food interaction with vitamin K–rich food.19 Meanwhile, direct oral anticoagulants (DOACs) were developed to overcome these limitations. DOACs, including dabigatran, rivaroxaban, apixaban, and edoxaban, are oral anticoagulants approved for use for the prevention and treatment of thrombosis in several cardiovascular indications.18 VTE among patients with SCD is associated with a 2 to 4 times increase in mortality risk compared with patients with SCD without VTE.11 Nevertheless, the evidence guiding the management of VTE in SCD specifically in terms of the anticoagulant of choice is scarce. Therefore, we conducted a systematic review and meta-analysis to address this important question. The aim of this systematic review and meta-analysis was to evaluate DOAC effectiveness and safety in SCD.
We included all studies evaluating the use of DOACs for VTE treatment among adult patients with SCD in the systematic review, regardless of the study design. We limited our review to articles published in English language only.
In the meta-analysis, we included only comparative studies evaluating VKAs vs DOACs in SCD for VTE.
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We searched the English literature (Google scholar, PubMed, and SCOPUS) for studies, reviews, case series, and case reports about the use of DOAC for treating thromboembolic disease in patients with SCA. We used the terms in combination: “Sickle cell disease” or “Sickle cell anemia,” “direct oral anticoagulant,” “novel oral anticoagulant,” “DOAC,” “NOAC,” “rivaroxaban,” “apixaban,” “dabigatran,” and “edoxaban.” The review included patients with SCA with various phenotypes who were treated with any of the DOACs. The reference lists of the included studies were scanned for any additional articles. The search included all articles published up to 20 April 2021. Two independent reviewers screened the titles and abstracts of the records independently, and papers unrelated to our inclusion criteria were excluded. Outcomes assessed were recurrent VTE and major bleeding.
Two reviewers (WR and AR) independently performed the quality and risk of bias assessment for each included study. The Risk of Bias In Non-randomized Studies of Interventions assessment tool was used as all included studies were observational nonrandomized studies.20 Disagreements were resolved through discussions and consensus. Using 7 domains, the overall risk of bias of each evaluated study was judged as low if the risk of bias is low in all domains, as moderate if the risk of bias is low or moderate for all domains, as serious if the risk of bias is serious in at least 1 domain, as critical if the risk of bias is critical in at least 1 domain, or as no information when there is a lack of information in 1 or more domains and the study is not at serious or critical risk of bias in any of the 7 domains.
The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for the desired outcomes, including recurrent VTE and major bleeding. Data were combined in systematic review, forest plots, and meta-analysis. The meta-analysis was carried out using the random-effects model. The analysis included the study of potential covariates, overall effect size, and the existence of heterogeneity. The Q test and I2 were used to examine heterogeneity, with I2 > 50% indicating marked heterogeneity. Statistically significant results were identified with P < .05 and CIs excluding a null effect. We planned and executed a sensitivity analysis. The effect of each study on the overall effect size was assessed by sensitivity analysis using the leave-one-out approach. Assessment for publication bias was not done as only 3 studies could be included in each analysis. Meta-analyses were performed using Review Manager software (version 5.4; The Cochrane Collaboration, Software Update, Oxford, UK).
This systematic review identified a total of 7 articles: 4 observational studies and 3 case series addressing this matter with a total of 236 patients as shown in Table 1,21-27 of which, 3 observational studies that compared VKAs and DOACs were included in the meta-analysis to evaluate the effectiveness and safety of DOACs in SCD, whereas the other studies were not included in the analysis as 3 were case series and 1 was a single arm study of rivaroxaban use for VTE among patient with SCD. Patel et al21 found that the use of DOACs, including rivaroxaban, dabigatran, and apixaban, in comparison with VKAs and low-molecular-weight heparin (LMWH) for the treatment of VTE in SCD among adults was associated with a similar VTE recurrence rate and a better safety profile in terms of a significant reduction in major bleeding events. Similarly, Roberts et al22 reported that the use of DOACs for VTE treatment in SCD compared with VKAs resulted in similar effectiveness in terms of VTE recurrence, and the use of DOACs was associated with similar safety in comparison with VKAs. Additionally, in a single-arm prospective observational study of 12 patients with SCD and VTE who received rivaroxaban, its use was well tolerated without major bleeding events, and only 2 patients developed recurrent VTE, including a patient with 2 positive antiphospholipid antibody tests (lupus anticoagulant and anticardiolipin immunoglobulin G).23 With regards to the lower risk of major hemorrhagic events associated with the use of non-VKAs, Gupta et al24 showed that among 55 patients with SCD treated with VKAs, DOACs, or injectable anticoagulants, only the use of VKAs resulted in major bleeding.
All included studies had an overall critical or serious risk of bias as demonstrated in (Figure 1A). Publication bias was not assessed as only 3 studies could be included in the analysis of outcomes.
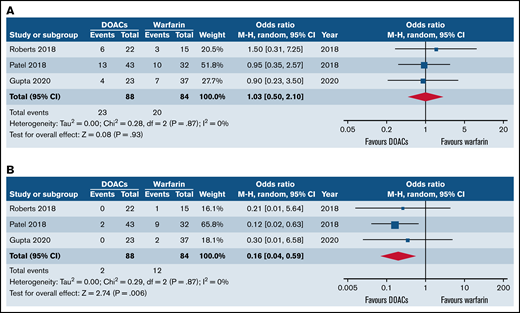
Forest plots of clinical outcomes of DOACs versus warfarin in SCD. (A) Forest plot of recurrent VTE between DOACs and warfarin. (B) Forest plot of major bleeding of DOACs compared with warfarin.
Forest plots of clinical outcomes of DOACs versus warfarin in SCD. (A) Forest plot of recurrent VTE between DOACs and warfarin. (B) Forest plot of major bleeding of DOACs compared with warfarin.
All studies reported VTE recurrence. Three studies (n = 172) were included in the meta-analysis with 88 patients with SCD receiving DOACs and 84 patients receiving warfarin. The use of DOACs was associated with similar rate of VTE recurrence compared with warfarin (OR = 1.03; 95% CI, 0.5-2.10; I2 = 0%) as shown in Figure 1B.
Three studies (n = 172) were included in the meta-analysis with 88 patients with SCD receiving DOACs and 84 patients receiving warfarin. Overall, the use of DOACs was associated with significantly reduced odds of major bleeding in comparison with warfarin (OR = 0.16; 95% CI, 0.04-0.59; I2 = 0%) as demonstrated in Figure 1B).
The leave-one-out approach was carried out as a tool for sensitivity analysis for the bleeding outcome to assess the effect of each study on the overall effect size. By removing the study of Roberts et al,22 the overall OR remained significant (OR = 0.15; 95% CI, 0.04-0.63; P = .01). Similarly, by removing the study of Gupta et al,24 the overall effect remained statistically significant (OR = 0.14; 95% CI, 0.03-0.59; P = .007). Nevertheless, when the analysis was carried out without the work by Patel et al,21 the statistical significance of the overall OR did not persist (OR = 0.26; 95% CI, 0.03-2.42; P = .24), indicating that the latter was the major drive of the statistical significance for the bleeding outcome.
The sensitivity analysis of the VTE outcome showed a persistent lack of statistical significance of the overall OR.
DOACs exhibit their pharmacologic activity by either inhibiting factor Xa or thrombin. Rivaroxaban, apixaban, and edoxaban are direct factor Xa inhibitors, whereas dabigatran is a direct thrombin inhibitor.19 DOACs are relatively new agents that have demonstrated superiority or noninferiority to VKAs or LMWHs in reducing the risk of thromboembolic complications with similar or reduced risk of bleeding.28,29 The US Food and Drug Administration approved the first DOAC (dabigatran) in 2010, and rivaroxaban, apixaban, and edoxaban were approved later. Dabigatran, rivaroxaban, apixaban, and edoxaban are approved for the prophylaxis and treatment of DVT and PE, as well as for reducing the risk of stroke in nonvalvular atrial fibrillation defined as atrial fibrillation in the absence of mechanical heart valves and moderate or severe mitral stenosis.30-33
SCD is considered a hypercoagulable state increasing the risk of VTE with an estimated incidence rate of 11% to 12% by the age of 40 years among adults with SCD.11 VTE among patients with SCD is associated with a 2 to 4 times increase in mortality risk compared with patients with SCD without VTE.11 Despite this, there is scant specific evidence guiding the choice of anticoagulant in the management of VTE in SCD. Therefore, we conducted a systematic review and meta-analysis to address this important question. In this systematic review and meta-analysis, we identified a total of 7 relevant publications: 4 observational studies and 3 case series addressing this matter. The current meta-analysis of these studies showed that the use of DOACs for VTE in SCD resulted in similar rates of VTE recurrence in comparison with other anticoagulants, including VKAs and injectable anticoagulants but with a better safety profile. As a result, although there are no specific clinical practice guidelines for the treatment of VTE among patients with SCD, it is reasonable to apply the general clinical practice guideline recommendations for VTE treatment to patients with SCD. According to the CHEST guidelines (2016 and 2021) for the treatment of VTE in adults, the use of DOACs (dabigatran, rivaroxaban, apixaban, or edoxaban) is recommended in patients with VTE over VKAs.34,35 Similarly, the latest American Society of Hematology (2020) guidelines for VTE suggests DOACs over VKAs, except among patients with renal insufficiency (creatinine clearance < 30 mL/min), moderate to severe liver disease, or those with antiphospholipid syndrome.36
No new evidence was found specifically directed toward the management of SCD-associated VTE in special populations such as pregnancy, pediatrics, or chronic kidney disease. DOACs are not approved for use in pregnant women because of limited safety data.30-33 This systematic review and meta-analysis did not identify any new information on the management of pregnant women with SCD who develop VTE. Current American Society of Hematology (2018) guidelines recommend that pregnant women who develop VTE during pregnancy should be treated with therapeutic-dose LMWH rather than unfractionated heparin, with a planned delivery with prior discontinuation of LMWH.37 Women with SCD who are being treated with a DOAC for VTE prior to pregnancy should be switched to LMWH once pregnancy is confirmed. Women with SCD at the preconceptual stage who are planning for pregnancy can be switched from a DOAC to a VKA until pregnancy is confirmed and then switched again to LMWH once pregnancy is confirmed.38
For pediatric patients with SCD who develop symptomatic VTE, LMWH or VKAs can used in accordance with the American Society of Hematology guidelines for VTE among pediatric patients.39 DOACs safety and effectiveness in the pediatric population has not yet been established and their use should be limited to clinical trials.30-33
This systematic review and meta-analysis has some limitations. The included studies were observational studies with their inherent methodologic limitations of bias and confounding. Although a random effect model was used in an attempt to overcome this limitation and reduce the potential bias in estimates, we acknowledge that the studies included had serious or critical risk of bias. Additionally, the studies had diverse definitions of outcomes, especially bleeding and different follow-up durations that made results comparison between studies hard. However, the results of our meta-analysis showed 0% heterogeneity for both VTE and major bleeding outcomes, which should be cautiously interpreted in small meta-analyses, as I2 is prone to bias and imprecision in small meta-analysis. Our review included only studies in adult of both genders, and we excluded pregnant women, which limits its generalizability to special population such as pregnancy and pediatrics. In addition, our review included only studies published in English, which might have resulted in publication bias. In addition, gray literature including conference abstracts was not included in this review. Nevertheless, this systematic review and meta-analysis tried to answer a crucial question in terms of anticoagulation choice for SCD.
In conclusion, the results of this systematic review and meta-analysis showed lower major bleeding rates and similar VTE recurrence rates with DOACs use compared with VKA in patients with SCD, making DOACs a reasonable alternative to VKAs for VTE in patients with SCD.
For special SCD populations, including pregnancy, pediatrics, and chronic kidney disease, general recommendations provided by clinical practice guidelines for VTE should be followed. Extending the anticoagulation duration beyond 3 months among patients with SCD should be determined by balancing the risk of recurrent VTE and major bleeding.
Contribution: M.A.Y. and W.R. conceived the idea; W.R., A.R., E.A.A., A.A.-M., Y.H., and I.K. reviewed the literature and extracted data; W.R. and A.R. assessed risk of bias; A.R. performed data analysis; W.R., A.R., M.A.Y., and L.J.F. reviewed and interpreted the results; W.R., A.R., E.A.A., A.A.-M., Y.H., and I.K. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Waail Rozi, Department of Internal Medicine, Hamad Medical Corporation, PO Box 3050, Doha, Qatar; e-mail: waail.rozi@gmail.com.
References
Author notes
Presented in abstract form at the virtual 63rd annual meeting of the American Society of Hematology, 11 December 2022, Atlanta, GA.
Data are available upon request from the corresponding author at waail.rozi@gmail.com.