TO THE EDITOR:
CPX-351 (United States: Vyxeos; Europe: Vyxeos liposomal) is a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar ratio.1 CPX-351 is approved for newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes in adults and pediatric patients aged ≥1 year in the United States and in adults in Europe.2,3 In a phase 3 study in older adults with newly diagnosed, high-risk/secondary AML, CPX-351 improved overall survival (OS), complete remission (CR) rate, allogeneic hematopoietic cell transplantation (alloHCT) rate, and OS landmarked from the alloHCT date vs conventional 7 + 3 (cytarabine/daunorubicin).4
In older adults and high-risk AML, alloHCT is the preferred postremission strategy, owing to high relapse and poor OS with conventional chemotherapy.5,6 Here, we performed detailed analyses of outcomes after alloHCT following CPX-351 vs 7 + 3 in the phase 3 study, with 5 years of follow-up.
Details of this multicenter, randomized, open-label, phase 3 study have been described previously.4 Briefly, patients aged 60 to 75 years with newly diagnosed, high-risk/secondary AML were randomized 1:1 to receive up to 2 induction cycles of CPX-351 (100 U/m2 [daunorubicin 44 mg/m2 plus cytarabine 100 mg/m2] via 90-minute infusion on days 1, 3, and 5 [second induction: days 1 and 3]) or 7 + 3 (cytarabine 100 mg/m2 per day continuous infusion for 7 days plus daunorubicin 60 mg/m2 on days 1-3 [second induction: 5 + 2 schedule]) followed by up to 2 postremission consolidation cycles with CPX-351 65 U/m2 or 5 + 2, respectively. Patients were stratified by age and AML subtype (Table 1) and followed until death or up to 5 years after randomization. alloHCT was allowed at the physician’s discretion. The protocol was amended to collect additional alloHCT-specific information and outcomes, including cumulative incidence of relapse, nonrelapse mortality (NRM), and acute and chronic graft-versus-host disease (GVHD).
Baseline patient characteristics
| Characteristic, n (%) . | CPX-351 (n = 53) . | 7 + 3 (n = 39) . | Nominal P value* . |
|---|---|---|---|
| Age, y | |||
| 60-69 | 37 (70) | 33 (85) | .10 |
| 70-75 | 16 (30) | 6 (15) | |
| Male | 34 (64) | 23 (59) | .61 |
| ECOG performance status | |||
| 0 | 18 (34) | 17 (44) | .35 |
| 1 | 31 (58) | 20 (51) | .49 |
| 2 | 4 (8) | 2 (5) | .30 |
| AML subtype | |||
| Therapy-related AML | 11 (21) | 9 (23) | .79 |
| AML with antecedent MDS | |||
| With prior HMA | 14 (26) | 14 (36) | .33 |
| Without prior HMA | 8 (15) | 5 (13) | .23 |
| AML with antecedent CMML | 3 (6) | 0 | .19 |
| de novo AML with MDS karyotype | 17 (32) | 11 (28) | .69 |
| Cytogenetic risk by NCCN | |||
| Favorable | 3/49 (6) | 0 | .18 |
| Intermediate | 25/49 (51) | 18/37 (49) | .83 |
| Poor | 21/49 (43) | 19/37 (51) | .43 |
| Genetic risk by ELN 2017 | |||
| Favorable | 5/51 (10) | 0 | .06 |
| Intermediate | 14/51 (27) | 14/38 (37) | .35 |
| Adverse | 32/51 (63) | 24/38 (63) | .97 |
| Median bone marrow blasts (range), % | 30 (4.5, 87) | 28 (7, 68) | .24 |
| WBC count <20 000/μL | 48 (91) | 35 (90) | .80 |
| Last response prior to alloHCT | |||
| CR + CRi | 40 (75) | 24 (62) | .15 |
| CR | 30 (57) | 19 (49) | .45 |
| CRi | 10 (19) | 5 (13) | .17 |
| No response | 13 (25) | 15 (38) | .15 |
| Median HCT comorbidity index (range) | 4 (0, 8) | 3 (0, 8) | .65 |
| Transplant donor | |||
| HLA-identical sibling | 11 (21) | 3 (8) | .06 |
| Haploidentical | 4 (8) | 5 (13) | .19 |
| Matched unrelated | 26 (49) | 19 (49) | .97 |
| Mismatched unrelated | 2 (4) | 2 (5) | .37 |
| Unknown/missing | 7 (13) | 9 (23) | .22 |
| Graft source | |||
| Bone marrow | 4 (8) | 1 (3) | .23 |
| Cord blood | 1 (2) | 1 (3) | .49 |
| Peripheral blood | 40 (75) | 27 (69) | .51 |
| Unknown/missing | 8 (15) | 10 (26) | .21 |
| Conditioning regimen† | |||
| Myeloablative | 9/45 (20) | 5/31 (16) | .22 |
| Reduced intensity | 23/45 (51) | 18/31 (58) | .55 |
| Total lines of treatment for patients who were nonresponders or relapsed before alloHCT‡ | 13 | 14 | .24 |
| 1 | 5 | 2 | |
| 2 | 3 | 6 | |
| 3 | 4 | 2 | |
| 4 | 1 | 3 | |
| 5 | 0 | 0 | |
| 6 | 0 | 1 |
| Characteristic, n (%) . | CPX-351 (n = 53) . | 7 + 3 (n = 39) . | Nominal P value* . |
|---|---|---|---|
| Age, y | |||
| 60-69 | 37 (70) | 33 (85) | .10 |
| 70-75 | 16 (30) | 6 (15) | |
| Male | 34 (64) | 23 (59) | .61 |
| ECOG performance status | |||
| 0 | 18 (34) | 17 (44) | .35 |
| 1 | 31 (58) | 20 (51) | .49 |
| 2 | 4 (8) | 2 (5) | .30 |
| AML subtype | |||
| Therapy-related AML | 11 (21) | 9 (23) | .79 |
| AML with antecedent MDS | |||
| With prior HMA | 14 (26) | 14 (36) | .33 |
| Without prior HMA | 8 (15) | 5 (13) | .23 |
| AML with antecedent CMML | 3 (6) | 0 | .19 |
| de novo AML with MDS karyotype | 17 (32) | 11 (28) | .69 |
| Cytogenetic risk by NCCN | |||
| Favorable | 3/49 (6) | 0 | .18 |
| Intermediate | 25/49 (51) | 18/37 (49) | .83 |
| Poor | 21/49 (43) | 19/37 (51) | .43 |
| Genetic risk by ELN 2017 | |||
| Favorable | 5/51 (10) | 0 | .06 |
| Intermediate | 14/51 (27) | 14/38 (37) | .35 |
| Adverse | 32/51 (63) | 24/38 (63) | .97 |
| Median bone marrow blasts (range), % | 30 (4.5, 87) | 28 (7, 68) | .24 |
| WBC count <20 000/μL | 48 (91) | 35 (90) | .80 |
| Last response prior to alloHCT | |||
| CR + CRi | 40 (75) | 24 (62) | .15 |
| CR | 30 (57) | 19 (49) | .45 |
| CRi | 10 (19) | 5 (13) | .17 |
| No response | 13 (25) | 15 (38) | .15 |
| Median HCT comorbidity index (range) | 4 (0, 8) | 3 (0, 8) | .65 |
| Transplant donor | |||
| HLA-identical sibling | 11 (21) | 3 (8) | .06 |
| Haploidentical | 4 (8) | 5 (13) | .19 |
| Matched unrelated | 26 (49) | 19 (49) | .97 |
| Mismatched unrelated | 2 (4) | 2 (5) | .37 |
| Unknown/missing | 7 (13) | 9 (23) | .22 |
| Graft source | |||
| Bone marrow | 4 (8) | 1 (3) | .23 |
| Cord blood | 1 (2) | 1 (3) | .49 |
| Peripheral blood | 40 (75) | 27 (69) | .51 |
| Unknown/missing | 8 (15) | 10 (26) | .21 |
| Conditioning regimen† | |||
| Myeloablative | 9/45 (20) | 5/31 (16) | .22 |
| Reduced intensity | 23/45 (51) | 18/31 (58) | .55 |
| Total lines of treatment for patients who were nonresponders or relapsed before alloHCT‡ | 13 | 14 | .24 |
| 1 | 5 | 2 | |
| 2 | 3 | 6 | |
| 3 | 4 | 2 | |
| 4 | 1 | 3 | |
| 5 | 0 | 0 | |
| 6 | 0 | 1 |
CIBMTR, Center for International Blood and Marrow Transplant Research; CMML, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group; HMA, hypomethylating agent; NCCN, National Comprehensive Cancer Network; WBC, white blood cell.
All P values are nominal and do not imply statistical significance.
Conditioning regimen intensity classified by CIBMTR criteria.21
Total lines of treatment, including study treatment and additional treatment (≥second line) received after completion of study treatment and before alloHCT.
The distribution of time-to-event end points was estimated using the Kaplan-Meier method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using a Cox proportional hazards regression model stratified by age and AML subtype. OS was estimated by the Kaplan-Meier method. Relapse and GVHD were estimated using the cumulative incidence with competing risk method (death as a competing event). All P values are nominal and do not imply statistical significance.
The study protocol and all amendments were approved by the institutional review board/ethics committee at each site. All patients provided written informed consent before study participation. This trial was registered at clinicaltrials.gov as #NCT01696084.
Of 309 randomized patients, 92 (30%) underwent alloHCT (CPX-351: 53 of 153 [35%]; 7 + 3: 39 of 156 [25%]; Table 1). Patient characteristics were generally balanced between arms except a greater proportion of patients receiving CPX-351 were aged >70 years (30% vs 15%). Numerically more patients who achieved CR or CR with incomplete neutrophil or platelet recovery (CRi) with CPX-351 than 7 + 3 proceeded to alloHCT (41 of 73 [56%] vs 24 of 52 [46%]).
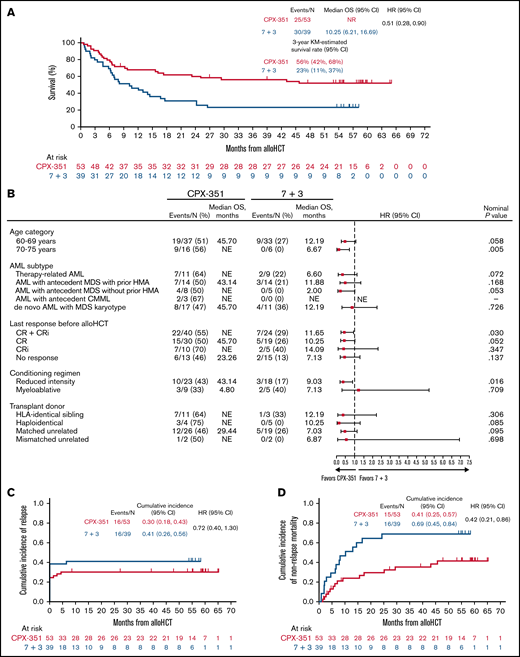
At a median follow-up of 61 months with CPX-351 and 60 months with 7 + 3, median OS landmarked from the alloHCT date was not reached with CPX-351 vs 10.3 months with 7 + 3 (HR = 0.51; 95% CI: 0.28, 0.90), and 3-year OS was 56% vs 23%, respectively (Figure 1A). Subgroup analyses indicated the OS difference consistently favored CPX-351 across age groups, AML subtypes, disease status, donor types, and conditioning intensities (Figure 1B). The Kaplan-Meier–estimated 5-year OS from randomization was also higher for CPX-351 vs 7 + 3 and was >50% at 5 years for patients treated with CPX-351. The most common causes of death were progressive leukemia (CPX-351: 9 of 53 [17%]; 7 + 3: 9 of 38 [24%]), GVHD complications (5 of 53 [9%]; 3 of 38 [8%]), and sepsis (3 of 53 [9%]; 2 of 38 [5%]).
Post-alloHCT Outcomes. (A) OS landmarked from the alloHCT date. Reprinted from Lancet Hematology7 with permission from Elsevier. (B) Subgroup analyses of OS landmarked from the alloHCT date. “N” denotes the number of patients who proceeded to alloHCT. One patient who achieved a best response of CR relapsed before alloHCT. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM. CMML, chronic myelomonocytic leukemia; HMA, hypomethylating agent.
Post-alloHCT Outcomes. (A) OS landmarked from the alloHCT date. Reprinted from Lancet Hematology7 with permission from Elsevier. (B) Subgroup analyses of OS landmarked from the alloHCT date. “N” denotes the number of patients who proceeded to alloHCT. One patient who achieved a best response of CR relapsed before alloHCT. (C) Cumulative incidence of relapse. (D) Cumulative incidence of NRM. CMML, chronic myelomonocytic leukemia; HMA, hypomethylating agent.
The cumulative incidence of relapse was 0.30 vs 0.41 with CPX-351 vs 7 + 3, respectively (HR = 0.72; 95% CI: 0.40, 1.30; Figure 1C). The difference in OS was primarily because of lower post-alloHCT NRM in the CPX-351 arm (HR = 0.42; 95% CI: 0.21, 0.86; Figure 1D). Among patients who were nonresponders or relapsed before alloHCT, additional treatment (≥second line) after completion of study treatment and before alloHCT was received by 8 of 13 (62%) patients in the CPX-351 arm and 12 of 14 (86%) in the 7 + 3 arm. Median time from the most recent therapy to alloHCT was 104 days (range: 6, 645 days) for CPX-351 and 92 days (range: 22, 312 days) for 7 + 3.
The cumulative incidence of acute GVHD (death as a competing event) was .49 with CPX-351 vs 0.38 with 7 + 3 at 6 months from the alloHCT date and 0.55 vs 0.44 overall (HR = 1.35 95% CI: 0.74, 2.44). The cumulative incidence of chronic GVHD was similar between arms (0.12 vs 0.08, respectively; HR = 1.47; 95% CI: 0.37, 5.88; supplemental Figure S1).
In this randomized phase 3 study in older adults with newly diagnosed, high-risk/secondary AML, the OS benefit with CPX-351 vs 7 + 3 in the overall study cohort was maintained after 5 years of follow-up (median OS: 9.33 vs 5.95 months; HR = 0.70; 95% CI: 0.55, 0.91).7 In this report, we show CPX-351 treatment resulted in greater proportions of patients undergoing alloHCT overall and in CR + CRi vs 7 + 3, as well as improved post-alloHCT OS. It is notable that among transplanted patients, a reduction in NRM was observed with CPX-351 despite a higher proportion of patients aged >70 years in the CPX-351 arm, potentially indicating the importance of treatment tolerability and better overall health in this older population. The difference in NRM did not appear to be driven by differences in GVHD; however, patients in the CPX-351 arm received fewer subsequent therapy lines before alloHCT and had a longer interval after their most recent therapy, allowing more time for recovery before alloHCT.
The long-term OS rates landmarked from the alloHCT date with CPX-351 in this study (>50%) compare favorably with historical rates for intensive chemotherapy.8,9 Furthermore, few studies have demonstrated an impact of pre-HCT therapy on alloHCT outcomes in AML. In the RATIFY study in FLT3-mutated AML, patients randomized to midostaurin plus chemotherapy had higher alloHCT rates than those randomized to placebo plus chemotherapy, with the best outcomes in patients receiving midostaurin followed by an alloHCT in first CR.10 Although gemtuzumab ozogamicin is associated with veno-occlusive disease after alloHCT when combined with myeloablative conditioning, recent data suggest no impact on survival after alloHCT.11,12 Analysis of alloHCT outcomes in patients with AML or myelodysplastic syndrome found comparable outcomes after induction with standard chemotherapy vs hypomethylating agents.13,14 In an analysis from the European Society for Blood and Marrow Transplantation, the addition of postremission chemotherapy did not impact alloHCT outcomes after reduced-intensity conditioning in AML.15
The lack of a standardized assessment of measurable residual disease (MRD) was a limitation in this analysis. MRD positivity before alloHCT has a powerful negative impact on alloHCT outcomes, primarily by identifying individuals at high risk of relapse after RIC, and likely influences the choice of conditioning regimen or decision to perform alloHCT.16,17 Unfortunately, at the time our study was initiated (2012), MRD testing in AML was limited and used disparate platforms, precluding formal analysis. Two recent real-world studies of CPX-351 have reported MRD-negative CR rates of 64% and 57%,18,19 which appear higher than what has been reported for 7 + 3 in similar patient populations.20 Additionally, because of the post hoc nature of this analysis, chronic GVHD was likely underreported.
The pattern of alloHCT outcomes in this study suggests improved disease control with CPX-351, allowing for higher alloHCT rates and, importantly, improved tolerability with lower NRM. These data provide the basis for planned randomized studies with CPX-351 in high-risk AML populations in which alloHCT is the preferred postremission strategy.
Acknowledgments: The authors thank all of the patients who participated in the study and their families, as well as the investigators, nurses, coordinators, and other research staff at each study site. Editorial assistance was provided by Senem Kurtoglu Lubin, of Cello Health/SciFluent Communications, Inc., under the direction of the authors, and was financially supported by Jazz Pharmaceuticals.
This study was supported by research funding from Jazz Pharmaceuticals.
Contribution: S.F. and J.E.L. participated in conception and design of the study; G.L.U., L.F.N., T.L.L., S.L.G., M.J.W., and J.E.L. treated patients and participated in the clinical data collection and assembly; all authors participated in data analysis and interpretation; R.J.R. provided statistical analysis; G.L.U. wrote the manuscript; L.F.N., T.L.L., S.L.G., M.J.W., R.J.R., S.F., and J.E.L. critically revised the manuscript; all authors provided final approval of the submitted version for publication; and all authors had access to primary clinical trial data.
Conflict-of-interest disclosure: G.L.U. has received consulting fees from AbbVie, Agios, Genentech, GlaxoSmithKline, Jazz Pharmaceuticals, and Novartis and is a clinical research scholar of the Leukemia & Lymphoma Society. T.L.L. has received institutional research funding from AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech/Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero Pharmaceuticals, and Trovagene. M.J.W. has received consulting fees from Daiichi Sankyo and holds stock ownership in Reata Pharmaceuticals. R.J.R is a former employee of Jazz Pharmaceuticals. S.F. is an employee of and holds stock ownership/options in Jazz Pharmaceuticals. J.E.L. has received consulting fees from Agios, Daiichi Sankyo, Jazz Pharmaceuticals, and Pfizer. All remaining authors declare no competing financial interests.
Correspondence: Geoffrey L. Uy, Division of Oncology, Washington University School of Medicine, 660 S. Euclid Ave, CB 8007, St. Louis, MO 63110; e-mail: guy@wustl.edu.
References
Author notes
All relevant data are provided within this manuscript and supporting files or within the files for the previous study publications.4,7
The full-text version of this article contains a data supplement.