Key Points
The HHT-specific QoL instrument is short, easy to administer, and reliable.
The HHT-QoL correlates well with patient-reported HHT severity and PROMIS QoL. It will be a valuable component of future clinical research.
Abstract
Hereditary hemorrhagic telangiectasia (HHT) is characterized by arteriovenous malformations and telangiectasia, with primary clinical manifestations of epistaxis and gastrointestinal bleeding and resultant anemia. HHT negatively affects health-related quality of life (HR-QoL); however, existing tools to measure HR-QoL are not HHT specific. Our objective was to develop an HHT-specific HR-QoL (HHT-QoL) instrument and evaluate its performance in a cross-sectional survey of individuals with HHT. Four HHT-specific questions were developed to evaluate the impact of HHT on productivity and social and personal interactions. An anonymous e-mail survey was conducted through Cure HHT. Participants also indicated their perceived HHT severity and completed 3 Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires: Discretionary Social Activities, Social Roles, and Emotional Distress. Complete data were available for 290 participants who self-identified their HHT severity as mild (29%), moderate (46%), or severe (25%). The HHT-QoL scale was reliable (Cronbach’s-α, 0.83). Principal components analysis indicated the instrument was unidimensional. Participants had low levels of QoL with their ability to participate in discretionary social activities (PROMIS mean 36.4 [standard deviation 14.3]) and perform in social roles (41.5 [17.2]), and the presence of a high level of emotional distress (64.8 [24.2]). The HHT-QoL score correlated negatively with PROMIS Discretionary Social Activities (r = −0.65) and Social Roles (r = −0.68) and positively correlated with PROMIS Emotional Distress (r = 0.51). In conclusion, the 4-item HHT-QoL instrument provides valuable insight and may be a useful addition to future clinical research in HHT.
Introduction
Hereditary hemorrhagic telangiectasia (HHT; Osler-Weber-Rendu syndrome) is an underrecognized, autosomal dominant disorder that affects 1 in 5 000 to 1 in 10 000 persons worldwide.1 Mutations in 3 genes encoding proteins that modulate transforming growth factor-β superfamily signaling in vascular endothelial cells are associated with most cases of HHT. These include the endoglin gene on chromosome 9, the activin receptor–like kinase gene on chromosome 12, and the SMAD4 gene on chromosome 18 (HHT with juvenile polyposis).2 HHT-related gene mutations are thought to underlie the development of vascular malformations, ranging from mucocutaneous telangiectasias to large arteriovenous malformations (AVMs) in the pulmonary, hepatic, cerebral, and spinal circulations. The penetrance of symptoms is nearly complete by 40 years of age; the type and severity of manifestations are variable, with significant intrafamilial and interfamilial variability, suggesting the presence of modulatory genes and the impact of environmental factors.3 Recurrent, severe epistaxis is the most common manifestation of HHT and leads to anemia in 50% of affected individuals, with severe anemia requiring blood transfusions and iron infusions occurring in one-third of patients.4 Gastrointestinal bleeding is also common and increases with age. Up to 50% of patients experience life-threatening or disabling complications, such as stroke, cerebral abscess, or heart failure resulting from cerebral, pulmonary, or hepatic AVMs.5
Patient-reported outcomes (PROs) are increasingly recognized as a measure of well-being, and health-related quality of life (HR-QoL) has emerged as an important outcome measure, especially in patients living with chronic diseases such as HHT, where symptoms such as recurrent epistaxis and anemia interfere with daily activities and social functioning.6 Several studies have reported that HHT negatively impacts HR-QoL.7-10 Importantly, HR-QoL measures are responsive to clinical changes and can be a useful complement to biological measures of health status and well-being.11-13 Three independent studies have reported that individuals with HHT had lower scores compared with normative data on all domains of the short form 36 (SF-36) except bodily pain.7-10 Severe epistaxis is the most significant predictor of poor HR-QoL in HHT.14 In 2 studies in which a symptom-specific HHT questionnaire was administered along with the SF-36, recurrent nosebleed was the major factor found to negatively affect work, social activities, and HR-QoL.8 In a small study, treatment of HHT with thalidomide led to improvements in the epistaxis severity score and multiple dimensions of the SF-36, including physical functioning, physical component summary, and mental component summary.15
A limitation of the existing studies evaluating HR-QoL in HHT is that they used the SF-36, which is an extensively validated but generic tool that is not specific to HHT. Whereas generic HR-QoL measures allow for comparisons across disease states, disease-specific tools have the ability to focus on health aspects that are relevant to a specific patient group. They can also help determine the contribution of specific symptoms to limitations in physical functioning and may be more responsive to disease-specific therapies and interventions, making them more effective PROs for clinical trials and effectiveness research.16 The objective of this study was to develop an HHT-specific HR-QoL instrument and to evaluate its performance in a cross-sectional survey of individuals with HHT. We also evaluated its correlation with self-reported HHT severity and Patient-Reported Outcomes Measurement Information System (PROMIS) HR-QoL measures.
Methods
Development of an HHT-specific QoL instrument
QoL working group.
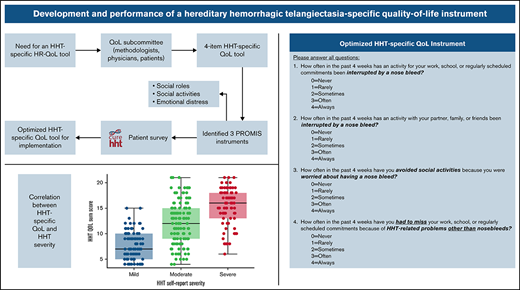
A QoL working group was assembled consisting of physicians with expertise in HHT, individuals with HHT, representatives from the advocacy group Cure HHT, and statisticians with expertise in QoL instruments. The objectives of the working group were (1) to develop an easy-to-administer QoL instrument that comprised a few questions that specifically evaluated QoL-related issues encountered by individuals with HHT and that could be piloted in a prospective trial of pomalidomide in HHT and (2) to identify an existing QoL measure that was best suited to evaluate the performance of the HHT-specific QoL instrument.
HHT-specific QoL items.
Based on published data7-10 and expert opinion, the working group identified epistaxis as the most common HHT-related cause of disruption in day-to-day activities and QoL. Therefore, the impact of epistaxis-related events was a particular focus when developing the questionnaire. The impact of epistaxis could be a disruption in activities at work or during leisure time. Depending on the severity of the epistaxis episodes, some individuals have also reported avoiding social interactions because of the fear that epistaxis would occur during such interactions. To effectively capture all of these situations, separate questions pertaining to work, leisure, and avoiding social interactions were developed. These are questions 1, 2, and 3 in the HHT-specific QoL pilot instrument (supplemental Figure 1). Other disease-related complications, such as those related to visceral AVMs, could also affect an individual’s QoL. Examples include bleeding from gastrointestinal AVMs and exacerbation of heart failure resulting from liver AVMs, among others. Absence from scheduled activities related to such complications was the objective of question 4 of the HHT QoL pilot instrument. It was the working group’s impression that these 4 questions provided the appropriate balance of having the scope to encompass nearly all avenues by which HHT affects an individual’s QoL while being simple and easy to administer.
Evaluation of the HHT-specific QoL instrument
Patient perceived severity of HHT.
A useful measure to evaluate the performance of the HHT-specific QoL instrument would be the severity of HHT, as poor QoL would be expected to correlate with more severe disease. There are currently no guidelines on classification of disease severity in HHT. The closest to such an assessment is the Epistaxis Severity Score,17 which classifies the severity of epistaxis in HHT into mild, moderate, and severe, and uses an online tool to calculate a weighted score based on the patient’s responses to 6 epistaxis-related questions. It was the working group’s impression that incorporating the Epistaxis Severity Score into an online survey to assess the HHT-QoL instrument would make the survey too complex. Therefore, a final question was included in the survey that aimed at determining the patient’s perceived severity of HHT, with response options of mild, moderate, and severe. The response could be used to assess the correlation between disease severity and responses to the QoL instrument.
Comparator HR-QoL measures.
We wished to evaluate consistency of the HHT-QoL instrument with validated nonspecific QoL measures. The few studies that have evaluated QoL in HHT thus far have used the generic SF-36 QoL instrument. In recent years, the validated PROMIS tool has become widely used.18-20 PROMIS is a set of person-centered measures developed with the support of the National Institutes of Health (NIH) to evaluate and monitor physical, mental, and social health. Further, the PROMIS tool can be used in individuals with chronic illnesses, for both the adult and pediatric age groups. PROMIS was chosen by the working group as the QoL instrument to use as a comparator for the HHT-specific QoL instrument. There are many items within the adult PROMIS system, and they are grouped into scales/pools, short forms, domains, and profiles. To maintain simplicity, short-form PROMIS questionnaires were chosen. All available PROMIS forms were evaluated and, through a process of discussion and elimination, the following were chosen by the working group as being the most appropriate to evaluate HR-QoL in HHT: (1) Satisfaction With Participation in Discretionary Social Activities, item bank v1.0 (12 questions); (2) Satisfaction With Participation in Social Roles, item bank v1.0 (14 questions); and (3) Emotional Distress-Depression, item bank v1.0 (30 questions).21-23 PROMIS has standardized response categories and scoring systems that make the tool user friendly. PROMIS has since released a new instrument consolidating forms 1 and 2 called Satisfaction with Social Roles and Activities, item bank v2.0.21
Patient survey.
A cross-sectional survey of individuals with an established diagnosis of HHT was conducted to evaluate the HHT-specific QoL. Participants were recruited via e-mail using the e-mail contact list (includes 8000 individual addresses) of members of Cure HHT, an international nonprofit advocacy organization dedicated to supporting patients and families affected by HHT, educating medical professionals, and supporting research. The survey was available only in English and was therefore limited to English-speaking participants. The survey was conducted from 15 to 25 January 2016. Online consent was obtained before the survey was administered. Only individuals who were ≥18 years of age were included. Survey responses were collected in REDCap, a secure, Web-based application for building and managing online research surveys and databases, which is hosted at the Cleveland Clinic. This study was approved by the Institutional Review Board at the Cleveland Clinic Foundation and was conducted according to the Declaration of Helsinki.
Scoring of survey instruments.
Each of the 4 HHT-QoL pilot items was assessed on an ordinal scale, with 5 levels ranging from 1, least impacted, to 5, most impacted. For item 1 (work), an additional sixth category was provided for not working. HHT severity was scored as 1, mild; 2, moderate, 3, severe. Summary T scores for PROMIS Discretionary Social Activities, PROMIS Social Roles, and PROMIS Emotional Distress-Depression were obtained from the PROMIS Web site and are relative to a normative sample with a mean of 50 and standard deviation (SD) of 10. For all scores other than PROMIS Discretionary social activities and PROMIS Social roles, larger values indicate greater severity A response for the HHT-specific QoL questionnaire, including the HHT severity item was considered missing if any of the 5 items were missing. A response for each PROMIS T score was considered missing if the instruments could not be scored via the PROMIS Web site.
Statistical analysis.
Survey responses were characterized by using frequency distributions and means and standard deviations. The reliability, or internal consistency, of the HHT QoL items was assessed by Pearson correlation among the items and Cronbach’s α. The Cronbach’s α described, on a scale of 0 to 1, the extent to which multiple items on a test measure the same construct or, in other words, their degree of correlation.24 Scale validity comprises 3 components: (1) construct validity (does it measure what it purports to measure?), (2) predictive validity (does it predict some criterion?), and (3) content validity (does it measure sample adequately from the content space?).25 Construct validity was addressed by assessing the dimensionality of the HHT-QoL items by principal components analysis.26 Predictive validity was assessed by Pearson correlation of the HHT-QoL scores with disease severity and the PROMIS T scores. Content validity, which cannot be directly measured, was addressed through the expertise of the members of the working group.
In the final step, results of the statistical analysis were used to guide refinement of the 4 HHT items and define a summary score to produce the final version of the HHT-QoL scale.
Results
Survey responses
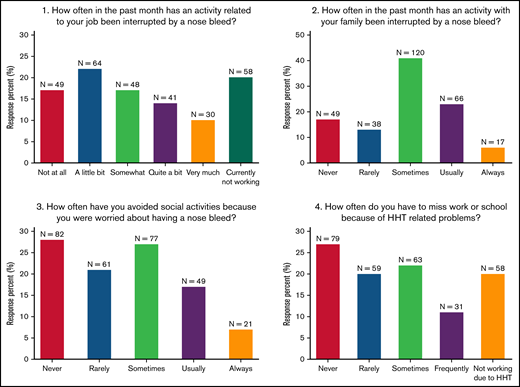
A total of 492 responses were received for the study survey. Of these, 290 responses contained complete data for the 4 HHT QoL questions, HHT severity, and the PROMIS measures, and these were selected for data analysis. Overall, 45% of respondents indicated that HHT interrupted their job “quite a bit” or more, 29% indicated that HHT interrupted family activities “usually” or “always,” 24% avoided social activities “usually” or “always”, and 31% missed work or school “frequently” or did not work because of HHT (Figure 1). Twenty-nine percent of respondents reported their HHT as “mild”, 46% as “moderate,” and 25% as “severe” (Table 1). The PROMIS Satisfaction With Participation in Discretionary Social Activities (mean 36.4; standard deviation [SD] 14.3), Satisfaction with Participation in Social Roles (41.5 [17.2]), and Emotional Distress-Depression (64.8 [24.2]) indicated that respondents had lower levels of QoL with the ability to participate in discretionary social activities and social roles and a higher level of depression compared with normal healthy subjects (Table 1). HHT self-reported severity correlated with each of the PROMIS measures (correlation strength ranged from 0.42 to −0.59; Table 2).
Distribution of responses for HHT QoL pilot items. Responses to the 4 items in the HHT QoL pilot instrument. N = 290.
Distribution of responses for HHT QoL pilot items. Responses to the 4 items in the HHT QoL pilot instrument. N = 290.
Internal consistency and construct validity
The 4 HHT-QoL questions correlated moderately with each other (1 pairwise correlation of 0.40, all others ranged from 0.52 to −0.64; Table 2). The Cronbach’s α for the 4 HHT-QoL items was 0.83, indicating that the items form a reliable scale.24 A principal components analysis of the HHT QoL items assessed whether the scale was unidimensional. The first principal component (PC1) had an Eigen value of 2.7 and explained 67% of the variance, whereas all other principal components had Eigen values >1 and added little variance explanation (Figure 2A), which suggests that a single-component solution is appropriate and that PC1 scores could serve as HHT-QoL scale scores. A scatterplot of the first 2 principal components (Figure 2B) showed no pattern, providing further evidence for a unidimensional scale.
Principal components analysis of HHT-QoL pilot items. (A) Scree plot for principal components analysis of HHT-QoL pilot items showing a single principal component with eigen value >1 that explains most of the variance in the items. (B) Scatterplot of the first 2 principal components (PC1 × PC2) shows no pattern. N = 290.
Principal components analysis of HHT-QoL pilot items. (A) Scree plot for principal components analysis of HHT-QoL pilot items showing a single principal component with eigen value >1 that explains most of the variance in the items. (B) Scatterplot of the first 2 principal components (PC1 × PC2) shows no pattern. N = 290.
Predictive validity
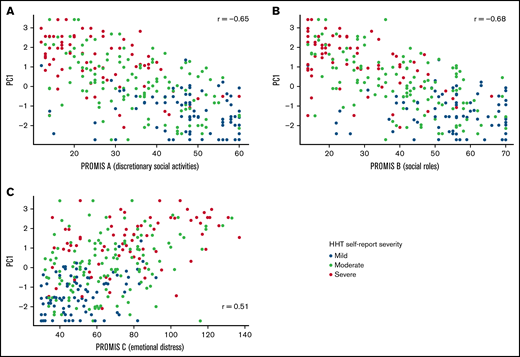
The 4 HHT-QoL questions correlated moderately with self-reported HHT severity and the 3 PROMIS measures (correlation strength ranged from 0.35 to 0.71; Table 2). As expected, because of the scale directions, correlations with the PROMIS QoL scales were all negative. Correlation between PC1 and the PROMIS measures (Figure 3) were also moderately strong (correlation strength ranged from 0.51 to 0.68). All correlations were in the predicted direction, indicating that the HHT-QoL scale shows predictive validity by correlation with the validated PROMIS measures.
Correlation of PC1 with PROMIS scales. Scatterplot and Pearson correlation for HHT QoL first principal component (PC1) by PROMIS QoL with participation in discretionary social activities (A), QoL with participation in social roles (B), and emotional distress-depression (C). N = 290.
Correlation of PC1 with PROMIS scales. Scatterplot and Pearson correlation for HHT QoL first principal component (PC1) by PROMIS QoL with participation in discretionary social activities (A), QoL with participation in social roles (B), and emotional distress-depression (C). N = 290.
Choice of summary score
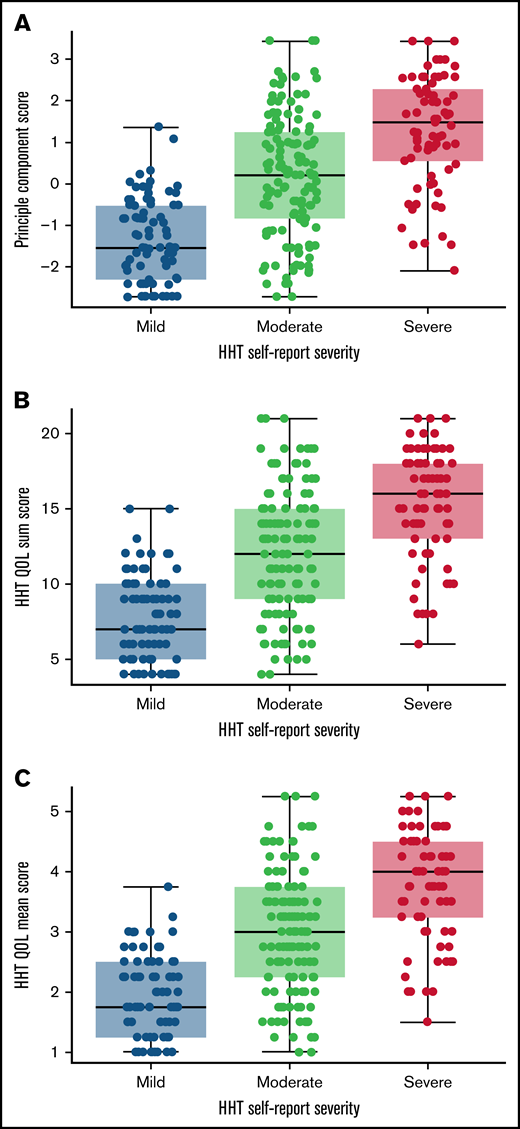
The distribution of 3 scoring methods: PC1, sum of the 4 HHT-QoL items, and average of the 4 HHT-QoL items indicated similar distributions (Figure 4). A total sum score was selected as the single overall score measure because of its ease of calculation.
Distribution of potential HHT QoL scoring methods. Distribution plots of the HHT QoL first principal component (A), sum of the 4 items (B), and mean of the 4 items (C), by HHT self-reported severity indicate similarity among the 3 potential scoring methods. N = 290.
Distribution of potential HHT QoL scoring methods. Distribution plots of the HHT QoL first principal component (A), sum of the 4 items (B), and mean of the 4 items (C), by HHT self-reported severity indicate similarity among the 3 potential scoring methods. N = 290.
Instrument refinement
Item review during the data analysis led to the following modifications for the final HHT-QoL instrument (Figure 5): item 1 (work) was changed from a 6-point to a 5-point scale, and the 5 response choices across all 4 items were standardized by using the same response options as the PROMIS measures: never, rarely, sometimes, often, and always. For items 1 and 4, “work” was broadened to “work, school, or regularly scheduled commitments” so that the items focused on impact on prescheduled commitments and would be addressable by all individuals. It was felt that employment status (employed, student, homemaker, retired, unemployed, and unable to work because of HHT) could be assessed separately from QoL in a demographic question. Similarly, for item 2, “family” was broadened to “partner, family, or friends” so it would be addressable by all individuals. The wording of item 4 was modified to more clearly state the focus on impact of HHT other than nosebleeds, as intended by the working group. The total score of the final HHT-QoL instrument is obtained by summing the responses from the 4 items each scored on a scale from 0 (never) to 4 (always). The total score ranges from 0 to 16. If any items are not answered, the total score should not be calculated. This instrument is currently in use in the ongoing NIH, National Heart Lung and Blood Institute (NHLBI)–funded clinical trial of pomalidomide for control of bleeding in HHT (PATH-HHT; registered on clinicaltrials.gov as #NCT03910244).
HHT: QoL final instrument. The total score is calculated by summing the items (each scored from 0 to 4). The total score ranges from 0 (no limitations) to 16 (severe limitations). If any items are missing, the total score is not calculated.
HHT: QoL final instrument. The total score is calculated by summing the items (each scored from 0 to 4). The total score ranges from 0 (no limitations) to 16 (severe limitations). If any items are missing, the total score is not calculated.
Discussion
HHT is a multisystem hereditary bleeding disorder, and affected individuals develop a wide range of disease-related manifestations, ranging from epistaxis to gastrointestinal bleeding to symptoms secondary to untreated visceral AVMs, such as heart failure from liver AVMs, and paradoxical embolism/stroke and systemic infections caused by pulmonary AVMs. Iron deficiency anemia is also a common complication of bleeding in affected individuals, with an estimated prevalence of 50%. Collectively, disease-related manifestations result in decreased QoL in affected individuals. Past studies on HR QoL have found that epistaxis has the greatest impact on QoL in HHT. As the QoL instruments used thus far, such as the SF-36, are nonspecific, our objective was to develop a simple, yet clinically useful, HHT-specific QoL instrument.
The QoL working group developed an HHT-specific QoL instrument that consisted of 4 questions that assessed whether HHT-related symptoms prevented or interfered with an individual’s ability to perform work or engage in social activities and/or affected their social interactions. This instrument was then evaluated through a cross-sectional survey of individuals with HHT. As part of this study, the HHT-specific QoL instrument was compared with select PROMIS HRQoL measures (Satisfaction With Participation in Discretionary Social Activities, Satisfaction With Participation in Social Roles, and Emotional Distress-Depression) and patient’s self-report of HHT severity that was also assessed as part of the survey.
Almost half of the survey respondents rated their HHT severity as moderate (46%), and a quarter of them rated it as severe (25%). This self-reported severity of HHT correlated with all 3 of the PROMIS measures, with patients reporting greater severity of HHT having lower QoL when participating in discretionary social activities and roles and also having higher emotional distress. The HHT-specific QoL instrument was reliable (Cronbach’s-α, 0.83) and principal components analysis indicated that the instrument was unidimensional, indicating that the scale items represent a common variable, QoL. Scoring the HHT-specific QoL instrument as principal component score, sum score or an average score revealed similar distributions. The HHT-specific QoL instrument correlated well with patient-reported HHT severity.
The QoL working group revised the HHT-specific QoL instrument after completion of the study to optimize it and simplify implementation in the future. This revision entailed standardizing the response choices and number of response options for all 4 questions. The working group also recommended a standardized scoring system for the QoL instrument using sum scores. The revised HHT-specific QoL instrument is presented in Figure 5.
In summary, HHT-related manifestations have a considerable adverse impact on HR-QoL of affected individuals, the magnitude of which remains underappreciated, at least in part because of the lack of a specific QoL instrument to reliably measure such outcomes. We have developed and evaluated a simple, 4-question HHT-specific QoL instrument that correlates well with patient self-reported HHT severity and select PROMIS HRQoL measures. Inclusion of HHT-specific QoL measures in current and future clinical trials and validation of this QoL instrument will advance our understanding and the care of individuals with HHT.
Acknowledgments
The authors thank Paul Elson (retired) for statistical assistance.
This study was supported by funding from the National Institutes of Health, National Heart, Lung, and Blood Institute grant U34HL123416 and NIH cooperative grant UG3-140097-01A1 (K.R.M.).
Authorship
Contribution: K.R.M. and R.S.K. contributed to study design, data collection, data interpretation, and drafting of the manuscript; S.C. contributed to data interpretation and drafting of the manuscript; S.T. and N.V. contributed to statistical analysis, data interpretation, and drafting of the manuscript; C.B. contributed to study design; and N.S., M.S.C., and R.P. contributed to the study design and data collection.
Conflict-of-interest disclosure: K.R.M. has served on advisory boards for Sobi, Novartis, Momenta, and Sanofi-Genzy. S.C. has served on advisory boards for Sanofi Genzyme, Alexion, Takeda, and Dova Pharmaceuticals, and her institution has received research support from Takeda on her behalf. N.S., M.S.C. are employed by CureHHT. The remaining authors report no competing financial interests.
Correspondence: Raj S. Kasthuri, Division of Hematology, University of North Carolina at Chapel Hill, 8206B, Mary Ellen Jones Bldg, 116 Manning Dr, CB 7035, Chapel Hill, NC 27599-7035; e-mail: raj_kasthuri@med.unc.edu.
References
Author notes
Presented in abstract form at the Thirteenth International HHT Scientific Conference, Rio Grande, Puerto Rico, 12-16 June 2019.
The original data set is available by e-mail request to the corresponding author (raj_kasthuri@med.unc.edu).
The full-text version of this article contains a data supplement.